Abstract
Background
Both serum uric acid (SUA) levels and body mass index (BMI) are recognized as important risk factors for hypertension. The current study aimed to investigate the interaction effects between SUA levels and overweight (defined as BMI ≥ 24 kg/m2 in Chinese) on the incidence of hypertension among Chinese adults.
Methods
1124 hypertensive participants and 7283 non-hypertensive participants, extracted from the China Health and Nutrition Survey (CHNS), were analyzed. Participants were categorized based on their SUA levels and BMI, to investigate the interaction effects between SUA levels and overweight on hypertension.
Results
In comparison with the reference group (BMI < 24 kg/m2 and in the 1st quintile of SUA), multivariable adjusted analysis demonstrated that the odds ratio (OR) (95% confidence interval, 95% CI) of hypertension for participants with overweight alone was 2.18 (1.41–3.37); for elevated SUA levels alone, the ORs (95% CIs) were 1.57 (1.08–2.30), 1.84 (1.24–2.74), 2.21 (1.47–3.32), and 2.48 (1.55–3.96) across SUA quintiles; and for the combined effect of higher SUA levels and overweight, the ORs (95% CIs) were 3.25 (2.19–4.82), 3.73 (2.51–5.55), 5.17 (3.42–7.80), and 6.21 (4.01–9.60). The relative excess risk due to interaction (RERI) was 3.26 (1.43–5.09) at the 5th quintile of SUA, indicating the presence of additive interaction between overweight and SUA levels on hypertension.
Conclusion
Interaction between SUA levels and overweight on hypertension exists specifically at the highest quintile (Q5, > 6.39 mg/dL) of SUA among Chinese adults. Therefore, strategies to lower SUA levels could be considered as a potential approach to mitigate hypertension risk in overweight individuals within this specific subgroup.
Keywords: Serum uric acid, Body mass index, Overweight, Hypertension, Interaction, China Health and Nutrition Survey, Chinese adults
Introduction
Hypertension stands as the predominant chronic condition worldwide, marking itself as the principal contributor to both disability and premature mortality. It affects nearly 1 billion adults globally, and is associated with over 9 million deaths annually [1]. Particularly in China, the prevalence of hypertension has witnessed a consistent rise over the last two to three decades, a trend primarily attributed to enhanced life expectancy and shifts in lifestyle habits [2]. According to the global report on hypertension, unhealthy diet, harmful use of alcohol, lack of physical activities, excess weight, and exposure to persistent stress are strongly related to hypertension [3]. Additionally, emerging evidence increasingly underscores serum uric acid (SUA) as a significant risk factor for hypertension, further broadening our understanding of its etiology [4–6].
Obesity and overweight have emerged as significant public health challenges in China, witnessing a rapid escalation over the past four decades [7]. Overweight, characterized by elevated body mass index (BMI), is firmly recognized as a crucial risk factor for hypertension [8–11]. Particularly, excess weight gain, notably associated with increased visceral adiposity, serves as a primary catalyst for hypertension, contributing to 65–75% of the risk for primary hypertension in humans [12]. Furthermore, evidence from Mendelian randomization studies corroborates the causal linkage between BMI and hypertension, reinforcing the critical nature of weight management in hypertension prevention and control [13].
Uric acid, the end product of purine metabolism, was first linked to primary hypertension in the 1870s by Frederick Akbar Mahomed [14]. Since Mahomed’s pioneering work, thousands of studies have explored the relationship between uric acid and hypertension [15]. Epidemiological studies showed that hyperuricemia is common in people who present with primary hypertension, and SUA could predict subsequent blood pressure elevation [16, 17]. Experimental research in mice and rats demonstrated that inhibiting or knocking down uricase leads to the development of hyperuricemia, kidney disease, and hypertension [18, 19]. Furthermore, a randomized controlled trial has provided evidence that reducing SUA levels can effectively lower blood pressure in hyperuricemic patients with hypertension and normal renal function [20].
Considering that both SUA and BMI are pivotal risk factors for hypertension, our study aimed to evaluate the interaction between overweight (defined as BMI ≥ 24 kg/m2 in Chinese) and SUA on hypertension among Chinese adults [4, 8–11, 16, 17, 21, 22].
Methods
Study population
All study data were obtained from the China Health and Nutrition Survey (CHNS), initiated in 1989 as a collaborative endeavor between the University of North Carolina at Chapel Hill and the China Center for Disease Control and Prevention (CCDC). The survey has been conducted in multiple waves: 1991, 1993, 1997, 2000, 2004, 2006, 2009, 2011, and 2015 [23]. Its primary aim is to assess the impact of health, nutrition, and family planning policies and programs by national and local governments and to understand the influence of China’s social and economic changes on the health and nutritional status of its population. The sampling strategy employed a multistage, random cluster process across 15 provinces, with detailed methodologies and findings reported in prior publications [23, 24]. For data access, please visit the CHNS website (https://www.cpc.unc.edu/projects/china/data/datasets/data_downloads).
In the current study, we included participants from the CHNS who had available blood assay results. Participants with the following criteria were excluded: absence of data for hypertension diagnosis, BMI, and SUA levels; age under 18 years; and pregnancy at the time of the investigation.
Definitions
Hypertension was diagnosed based on self-reports and blood pressure measurements. In order to reduce the influence of measurement errors, the analysis utilized the average of three separate readings. Hypertension was subsequently identified when diastolic blood pressure (DBP) ≥ 90 mmHg and/or systolic blood pressure (SBP) ≥ 140 mmHg [25, 26]. According to the criteria recommended by Working Group on Obesity in China, overweight was defined as BMI ≥ 24 kg/m2 [22].
Measurements
Weight, height, and blood pressure (BP) were measured following standardized protocols from the World Health Organization (WHO). BP was assessed by trained professionals employing a mercury sphygmomanometer, recorded at three distinct intervals of 3–5 minutes during a single visit. Weight measurements were taken with participants in light clothing using a calibrated beam scale, and height was measured without shoes using a portable stadiometer. BMI was calculated by dividing weight in kilograms by the square of height in meters.
Details on laboratory measurements are available elsewhere (https://www.cpc.unc.edu/projects/china/data/datasets/biomarker-data). Briefly, participants were instructed to follow their normal routines for at least three days and fast for 8–12 h before blood sample collection. SUA levels were measured by enzymatic colorimetric method using reagents from Randox Laboratories Ltd., UK. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as [fasting insulin (µU/mL) * fasting blood glucose (mmol/L) / 22.5] [27].
Statistical analysis
Continuous variables were assessed for normality using the Shapiro-Wilk test. Given that all continuous variables in this study deviated from normal distribution, they were presented as median and interquartile range (IQR) and analyzed using the Kruskal-Wallis rank test. Categorical variables were reported as frequency (percentage) and compared using Pearson’s chi-square test or Fisher’s exact test, depending on suitability. Missing values were imputed using multiple imputation.
Participants were grouped according to the quintile partitioning (Q1-Q5) of the SUA levels. Overweight was defined as BMI ≥ 24 kg/m2. Incorporating both the SUA quintiles and BMI, we categorized participants into four distinct groups: (1) Reference group: participants with SUA levels in Q1 and with BMI < 24 kg/m2; (2) Group for higher SUA levels alone: participants with higher SUA levels in Q2-Q5, but with BMI < 24 kg/m2; (3) Group for overweight alone: participants with BMI ≥ 24 kg/m2, but with SUA levels in Q1; and (4) Group for both overweight and higher SUA levels: BMI ≥ 24 kg/m2 and SUA levels in Q2-Q5.
Unconditional logistic regression analysis was employed to calculate odds ratios (ORs) and 95% confidence intervals (CIs). The interactions involving measures of effect modification were evaluated on both the additive and multiplicative scales. Considering the presence (A and B) and absence (A and B) of two risk factors, and using the terms R for risk and RR for relative risk, we defined RERI as follows [28]:
 |
Statistical analyses were conducted using STATA 15.1 (Stata Corp, TX, US). Two-tailed P-value < 0.05 was considered to be statistically significant.
Results
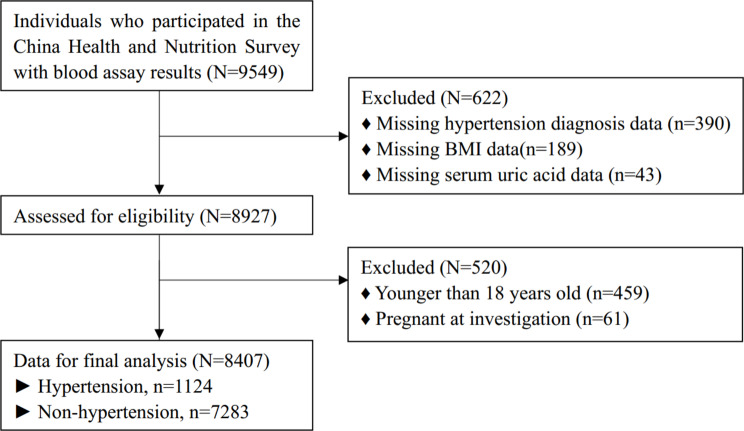
A total of 9549 participants were initially considered for analysis. Of these, 390 were excluded due to missing data on hypertension diagnosis; 189 for missing BMI data; 43 for missing SUA data; 459 for being younger than 18 years; and 61 due to pregnancy at the time of investigation. Consequently, 8407 participants were included in the final analysis, comprising 1124 participants with hypertension and 7283 without hypertension (Fig. 1).
Fig. 1.
Flow chart of participants selection
Baseline characteristics
Baseline characteristics of the participants were shown in Table 1. Compared with non-hypertensive participants, participants with hypertension were significantly older (62 years vs. 48 years, P < 0.001), and more likely to reside in urban areas (43.5% vs. 31.3%, P < 0.001). The gender distribution was relatively balanced across both groups (P = 0.126). The hypertension group exhibited notably higher BMI and blood pressure compared with their non-hypertensive counterparts (all P < 0.001). Regarding lifestyle factors, participants with hypertension consumed fewer daily calories (1984.74 kcal vs. 2091.38 kcal, P < 0.001), and were less likely to be current drinkers (18.4% vs. 21.4%, P = 0.022) and current smokers (26.4% vs. 29.4%, P = 0.045) compared with participants without hypertension. The hypertensive group also exhibited a higher prevalence of stroke (6.5% vs. 0.6%, P < 0.001) and myocardial infarction (MI) (4.6% vs. 0.4%, P < 0.001). As expected, compared with non-hypertensive participants, participants with hypertension showed elevated levels of urea, uric acid, apolipoprotein B, lipoprotein (a), creatinine, low density lipoprotein cholesterol, triglycerides, total cholesterol, and HOMA-IR (5.69 mmol/L vs. 5.24 mmol/L, 5.60 mg/dL vs. 4.89 mg/dL, 0.98 g/L vs. 0.87 g/L, 84 mg/L vs. 78 mg/L, 89 µmol/L vs. 84 µmol/L, 3.13 mmol/L vs. 2.87 mmol/L, 1.63 mmol/L vs. 1.21 mmol/L, 5.04 mmol/L vs. 4.73 mmol/L, 3.04 vs. 2.30, respectively), and lower levels of apolipoprotein A1 and high density lipoprotein cholesterol (1.08 g/L vs. 1.10 g/L, 1.30 mmol/L vs. 1.39 mmol/L, respectively).
Table 1.
Baseline characteristics
| Characteristics | Total | Missing | Non-hypertension | Hypertension | P value |
|---|---|---|---|---|---|
| (n = 8407) | (n) | (n = 7283) | (n = 1124) | ||
| Age, years | 51 (40, 61) | 48 (38, 59) | 62 (54, 70) | < 0.001 | |
| Sex, % | 0.126 | ||||
| Male | 3948 (47.0) | 3444 (47.3) | 504 (44.8) | ||
| Female | 4459 (53.0) | 3839 (52.7) | 620 (55.2) | ||
| Residence, % | < 0.001 | ||||
| Urban | 2765 (32.9) | 2276 (31.3) | 489 (43.5) | ||
| Rural | 5642 (67.1) | 5007 (68.7) | 635 (56.5) | ||
| Education, % | 13 | < 0.001 | |||
| None | 1982 (23.6) | 1595 (21.9) | 387 (34.5) | ||
| Primary school | 1654 (19.7) | 1410 (19.4) | 244 (21.7) | ||
| Lower middle school | 2765 (32.9) | 2487 (34.2) | 278 (24.8) | ||
| Upper middle school | 983 (11.7) | 891 (12.3) | 92 (8.2) | ||
| Technical or vocational school | 592 (7.1) | 510 (7.0) | 82 (7.3) | ||
| College or higher | 418 (5.0) | 379 (5.2) | 39 (3.5) | ||
| Marital status, % | 38 | < 0.001 | |||
| Never married | 486 (5.8) | 484 (6.7) | 2 (0.2) | ||
| Married | 7091 (84.7) | 6170 (85.1) | 921 (82.3) | ||
| Divorced | 83 (1.0) | 80 (1.1) | 3 (0.3) | ||
| Widowed | 651 (7.8) | 461 (6.4) | 190 (17.0) | ||
| Separated | 58 (0.7) | 55 (0.8) | 3 (0.3) | ||
| Height, cm | 160.7 (155.00, 167.40) | 161.0 (155.2, 167.4) | 159.5 (154.0, 167.0) | < 0.001 | |
| Weight, kg | 60.0 (52.9, 68.0) | 59.5 (52.5, 67.0) | 63.9 (56.0, 72.0) | < 0.001 | |
| BMI, kg/m2 | 23.1 (20.9, 25.6) | 22.9 (20.7, 25.2) | 24.8 (22.4, 27.3) | < 0.001 | |
| SBP, mmHg | 120.7 (110.7, 136.0) | 120.0 (110.0, 130.0) | 142.7 (131.3, 158.7) | < 0.001 | |
| DBP, mmHg | 80.0 (72.0, 88.0) | 80.0 (70.0, 85.0) | 90.0 (80.0, 100.0) | < 0.001 | |
| Total calorie intake, kcal | 2074.82 (1677.83, 2511.08) | 110 | 2091.38 (1689.39, 2530.11) | 1984.74 (1610.89, 2386.51) | < 0.001 |
| Current smoking, % | 2367 (29.0) | 253 | 2088 (29.4) | 279 (26.4) | 0.045 |
| Current drinking, % | 1752 (21.0) | 51 | 1546 (21.4) | 206 (18.4) | 0.022 |
| Stroke, % | 118 (1.4) | 5 | 45 (0.6) | 73 (6.5) | < 0.001 |
| MI, % | 82 (1.0) | 8 | 30 (0.4) | 52 (4.6) | < 0.001 |
| Urea, mmol/L | 5.30 (4.40, 6.34) | 5.24 (4.34, 6.29) | 5.69 (4.79, 6.72) | < 0.001 | |
| Uric acid, mg/dL | 4.97 (4.00, 6.07) | 4.89 (3.93, 5.97) | 5.60 (4.59, 6.66) | < 0.001 | |
| Apo A1, g/L | 1.10 (0.95, 1.29) | 1.10 (0.95, 1.29) | 1.08 (0.93, 1.27) | 0.012 | |
| Apo B, g/L | 0.88 (0.72, 1.07) | 0.87 (0.71, 1.05) | 0.98 (0.81, 1.17) | < 0.001 | |
| Lp(a), mg/L | 79 (40, 168) | 78 (40, 167) | 84 (43, 181) | 0.019 | |
| Creatinine, µmol/L | 85 (76, 96) | 84 (75, 95) | 89 (79, 102) | < 0.001 | |
| HDL-C, mmol/L | 1.38 (1.16, 1.63) | 2 | 1.39 (1.17, 1.64) | 1.30 (1.09, 1.57) | < 0.001 |
| LDL-C, mmol/L | 2.91 (2.33, 3.53) | 2.87 (2.31, 3.49) | 3.13 (2.54, 3.83) | < 0.001 | |
| Albumin, g/L | 47.30 (45.20, 49.50) | 47.30 (45.30, 49.50) | 47.25 (45.20, 49.65) | 0.909 | |
| Triglycerides, mmol/L | 1.26 (0.86, 1.96) | 1.21 (0.83, 1.86) | 1.63 (1.07, 2.46) | < 0.001 | |
| Total cholesterol, mmol/L | 4.77 (4.16, 5.46) | 4.73 (4.13, 5.40) | 5.04 (4.43, 5.75) | < 0.001 | |
| HOMA-IR | 2.37 (1.61, 3.65) | 16 | 2.30 (1.58, 3.50) | 3.04 (1.91, 4.96) | < 0.001 |
The continuous variables were expressed as median (interquartile ranges), and categorical variables as number (n) and percentage (%); P values were calculated by Wilcoxon test for continuous variables and Chi-square test for categorical variables. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MI, myocardial infarction; Lp(a), lipoprotein (a); Apo A1, apolipoprotein A1; Apo B, apolipoprotein B; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance.
ORs of overweight alone for hypertension
Compared with the reference group, crude OR for overweight alone was 2.27(1.52–3.39), and this association persisted after multivariable adjustments. ORs(95%CI) for overweight were all statistically significant within SUA strata (Table 2).
Table 2.
Odds ratios of hypertension for elevated SUA levels and elevated BMI
| SUA | BMI < 24 kg/m2 | BMI ≥ 24 kg/m2 | ORs for higher BMI within strata of SUA | |||||
|---|---|---|---|---|---|---|---|---|
| N(HT/non-HT) | Crude *OR (95%CI) | Adjusted *OR (95%CI) | N(HT/non-HT) | Crude *OR (95%CI) | Adjusted *OR (95%CI) | Crude #OR (95%CI) | Adjusted #OR (95%CI) | |
| Q1 (≤ 3.78 mg/dL) | 54/1128 | 1 | 1 | 49/451 | 2.27(1.52–3.39) | 2.18(1.41–3.37) | 2.27(1.52–3.39) | 2.18(1.41–3.37) |
| Q2 (≤ 4.55 mg/dL) | 87/1018 | 1.79(1.26–2.53) | 1.57(1.08–2.30) | 88/488 | 3.77(2.64–5.37) | 3.25(2.19–4.82) | 2.11(1.54–2.89) | 2.04(1.44–2.90) |
| Q3 (≤ 5.34 mg/dL) | 93/921 | 2.11(1.49–2.98) | 1.84(1.24–2.74) | 125/542 | 4.82(3.45–6.74) | 3.73(2.51–5.55) | 2.28(1.71–3.05) | 2.19(1.59–3.03) |
| Q4 (≤ 6.39 mg/dL) | 126/829 | 3.17(2.28–4.42) | 2.21(1.47–3.32) | 160/566 | 5.91(4.27–8.17) | 5.17(3.42–7.80) | 1.86(1.44–2.40) | 1.84(1.39–2.45) |
| Q5 (≤ 39.83 mg/dL) | 103/666 | 3.23(2.29–4.55) | 2.48(1.55–3.96) | 239/674 | 7.41(5.43–10.10) | 6.21(4.01–9.60) | 2.29(1.78–2.96) | 2.86(2.14–3.84) |
| †ORs(95%CI) for higher SUA within strata of BMI | 1.57(1.08–2.30) | 1.44(0.95–2.17) | ||||||
| 1.84(1.24–2.74) | 1.66(1.12–2.48) | |||||||
| 2.21(1.47–3.32) | 2.36(1.55–3.60) | |||||||
| 2.48(1.55–3.96) | 2.71(1.72–4.28) | |||||||
HT/non-HT, hypertension/non-hypertension
*, comparing with BMI < 24 kg/m2 and SUA ≤ 3.78 mg/dL
#, ORs of BMI ≥ 24 kg/m2 comparing with BMI < 24 kg/m2 at corresponding SUA quintiles
†, ORs of SUA in Q2-Q5 comparing with Q1 in the same BMI level
Adjustment: age, sex, smoking, total calorie intake, low-density lipoprotein cholesterol, triglyceride, creatinine, HOMA-IR
ORs of higher SUA alone for hypertension
Compared with the reference group, crude ORs for elevated SUA levels alone in Q2-Q5 were 1.79(1.26–2.53), 2.11(1.49–2.98), 3.17(2.28–4.42), and 3.23(2.29–4.55), respectively. These associations persisted after multivariable adjustments (Table 2).
ORs of both overweight and higher SUA levels for hypertension
Compared with the reference group, crude ORs for both higher SUA levels and overweight were 3.77(2.64–5.37), 4.82(3.45–6.74), 5.91(4.27–8.17), and 7.41(5.43–10.10), respectively. These associations were maintained after multivariable adjustments (Table 2).
Interaction of SUA with overweight on hypertension
According to the bottom (Q1) and the higher (Q2-Q5) quartiles of SUA, and considering the BMI categories (overweight as BMI ≥ 24 kg/m2 or not), 4 relative excess risks due to interaction (RERIs) (95%CI) at Q2-Q5 were calculated 0.46 (-0.67–1.59), 0.90 (-0.24–2.04), 1.04 (-0.22–2.29), and 3.26 (1.43–5.09). Notably, the RERI at Q5 did not cover zero, highlighting significant interactions between SUA levels and overweight. The P values of multiplicative were all > 0.05 (Table 3).
Table 3.
Interaction of UA with overweight on hypertension on additive and multiplicative scale
| SUA | RERI (95%CI) | P | Product term OR (95%CI) | P |
|---|---|---|---|---|
| Q2 | 0.46 (-0.67–1.59) | 0.43 | 0.93 (0.54–1.59) | 0.78 |
| Q3 | 0.90 (-0.24–2.04) | 0.12 | 1.00 (0.60–1.69) | 0.99 |
| Q4 | 1.04 (-0.22–2.29) | 0.11 | 0.88 (0.53–1.45) | 0.62 |
| Q5 | 3.26 (1.43–5.09) | < 0.01 | 1.32 (0.80–2.18) | 0.28 |
RERI, relative excess risk due to interaction; SUA, serum uric acid. Adjustment as in Table 2
Discussion
To the best of our knowledge, this study is the first to evaluate the interaction between overweight and SUA levels on hypertension with the measures of interaction on both additive and multiplicative scales. In the current study, RERI was 3.26 at Q5 of SUA and the 95% CI did not cover 0, which means interaction does exist on additive scale. 95% CIs of product term ORs at Q2-Q5 all contain 1, which means interaction does not exist on multiplicative scale.
Associations of overweight with hypertension
Overweight is a well-established risk factor for hypertension [8–11]. In the current study, across all SUA stratifications, adjusted odds ratios (ORs) for overweight were consistently significant, affirming overweight as a risk factor for hypertension irrespective of SUA levels.
Associations of elevated SUA with hypertension
SUA is another risk factor for hypertension [16, 17, 21]. Supporting this, our findings align with a cross-sectional study from a rural Beijing district, which reported a J-shaped relationship between SUA levels and hypertension risk [29]. Our study further confirmed that elevated SUA levels are associated with an increased risk of hypertension, regardless of BMI status.
Associations of both overweight and elevated SUA levels with hypertension
Both higher BMI and elevated SUA levels were independent predict factors for new one-set hypertension [30]. A cohort study involving obese adolescents with type 2 diabetes indicated that higher baseline SUA levels significantly increased the risk of developing hypertension [31]. Furthermore, a study involving participants not on antihypertensive medication showed that the relationship between SUA levels and blood pressure intensified in those with higher BMI compared with those with BMI under 25 kg/m2, although the interaction did not reach statistical significance [32]. However, the interaction of SUA with overweight on hypertension with additive or multiplicative model had not been assessed previously. A study demonstrated that in hypertensive patients, elevated SUA levels and insulin resistance had an additive interaction on arterial stiffness [33]. Rothman et al. proposed that the interaction should be classified as either a statistical or a biologic interaction and that the biologic interaction should be measured using an additive model [34]. In the current study, the risk of hypertension for participants in the highest SUA quintile (Q5) with BMI ≥ 24 kg/m2 was 6.21 times greater than that for participants in the lowest SUA quintile (Q1) with BMI < 24 kg/m2. The RERI (95% CI) at Q5 of SUA was 3.26 (1.43–5.09), indicating that the additional risk due to the interaction between Q5 of SUA and overweight was 3.26 times greater than the sum of the risks associated with Q5 of SUA and overweight alone. Our study revealed a synergistic effect between overweight and elevated SUA levels on hypertension.
Possible mechanisms for interaction between overweight and SUA on hypertension
Experimental findings indicated that uric acid plays a causal role in hypertension development, closely interacting with both neurohumoral and vascular mechanisms [4]. Initially, uric acid prompts reversible vascular constriction through mechanisms such as demodulation of nitric oxide (NO) availability, activation of the renin-angiotensin system (RAS), and production of reactive oxygen species (ROS), thromboxane (Tx), and cyclooxygenase (COX). In the later stages of hypertension development, there is sustained vascular remodeling characterized by proliferation of vascular smooth muscle cells, thickening of the vascular wall, alteration in pressure natriuresis, culminating in permanent sodium-sensitive hypertension [4]. In vivo, hypertension may also be associated with endothelial cell senescence and death regulated by uric acid through local activation of oxidative stress and the renin-angiotensin system [35].
The activation of the renin-angiotensin-aldosterone system is crucial in the development of obese hypertension [12]. Adipose tissue plays a significant role at the cellular level, impairing endothelial function through the secretion of various hormones and paracrine signals, collectively known as adipokines. In the case of obesity, there is excessive secretion of pro-inflammatory and vasoactive adipokines such as angiotensinogen, angiotensin II, aldosterone, accompanied by increased plasma renin activity [36]. In addition, there is an upregulation of renin receptor expression in human visceral adipose tissue [37].
Overweight and elevated SUA may interact synergistically and cause hypertension eventually through activation of the renin-angiotensin-aldosterone system. Kidney injury, insulin resistance, sympathetic nervous system activation, and inflammation are closely related to hypertension, with overweight and elevated SUA levels potentially interacting through these pathways to exert a synergistic effect on its development [38, 39]. Evidence from a randomized controlled trial indicates that reducing SUA levels can effectively decrease blood pressure in patients with hyperuricemia and hypertension [20]. Therefore, lowering SUA levels may be an option for reducing the risk of hypertension in overweight persons.
Limitations
There are several limitations to the current study. First, the CHNS 2009 survey did not include data on participants’ cancer histories, preventing us from excluding individuals with a history of cancer. Considering that cancer-related cell death can induce hyperuricemia and that weight loss may occur due to cancer-induced cachexia, this omission may affect the study’s comprehensiveness [40, 41]. Second, the CHNS did not specify the types of antihypertensive medications used, and the use of thiazide diuretics, which can elevate serum uric acid levels, may had influenced the results. Third, in the current study, overweight was defined as BMI ≥ 24 kg/m2. While BMI is a common tool in obesity research, it simplifies the assumption of adipose tissue distribution, not accounting for individual variations in body fat distribution. This variability could contribute to the observed disparities in the relationship between BMI and cardiovascular disease (CVD) across studies [42]. Additionally, missing data in the covariates of the current study required imputation, which may have introduced bias into the statistical analysis.
Conclusions
Interaction between SUA levels and overweight on hypertension exists specifically at the highest quintile (Q5, > 6.39 mg/dL) of SUA among Chinese adults. Therefore, strategies to lower SUA levels could be considered as a potential approach to mitigate hypertension risk in overweight individuals within this specific subgroup.
Acknowledgements
The authors thank all volunteers and staff involved in the CHNS project.
Author contributions
All authors participated in the design and coordination of the study. HW performed the statistical analysis and wrote the manuscript. JLF was involved in the study design and critically revised the manuscript. All authors read and approved the final version of the manuscript.
Funding
None.
Data availability
https://www.cpc.unc.edu/projects/china/data/datasets/data_downloads.
Declarations
Ethics approval and consent to participate
Due to the use of secondary de-identified publicly available data, this study was deemed exempt from ethical review by the Ethics Committee of the First Affiliated Hospital of Soochow University.
Consent for publication
Not applicable.
Clinical trial number
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lamirault G, Artifoni M, Daniel M, Barber-Chamoux N. Nantes University Hospital Working Group on H: resistant hypertension: Novel insights. Curr Hypertens Reviews. 2020;16(1):61–72. [DOI] [PubMed] [Google Scholar]
- 2.Wang JG, Zhang W, Li Y, Liu L. Hypertension in China: epidemiology and treatment initiatives. Nat Reviews Cardiol. 2023;20(8):531–45. [DOI] [PubMed] [Google Scholar]
- 3.Su L, Sun L, Xu L. Review on the prevalence, risk factors and disease management of hypertension among floating population in China during 1990–2016. Global Health Res Policy. 2018;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borghi C, Agnoletti D, Cicero AFG, Lurbe E, Virdis A. Uric acid and hypertension: a review of evidence and future perspectives for the Management of Cardiovascular Risk. Hypertens (Dallas Tex: 1979). 2022;79(9):1927–36. [DOI] [PubMed] [Google Scholar]
- 5.Kuwabara M, Kodama T, Ae R, Kanbay M, Andres-Hernando A, Borghi C, Hisatome I, Lanaspa MA. Update in uric acid, hypertension, and cardiovascular diseases. Hypertens Research: Official J Japanese Soc Hypertens. 2023;46(7):1714–26. [DOI] [PubMed] [Google Scholar]
- 6.Saito Y, Tanaka A, Node K, Kobayashi Y. Uric acid and cardiovascular disease: a clinical review. J Cardiol. 2021;78(1):51–7. [DOI] [PubMed] [Google Scholar]
- 7.Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373–92. [DOI] [PubMed] [Google Scholar]
- 8.Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, Obarzanek E, Ernst ND, Horan M. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8(9):605–19. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Min C, Song X, Zhang H, Yuan C, Chen L, Zhang H. The dose-response relationship between BMI and hypertension based on restricted cubic spline functions in children and adolescents: a cross-sectional study. Front Public Health. 2022;10:870568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanna D, Peltzer C, Kahar P, Parmar MS. Body Mass Index (BMI): a Screening Tool Analysis. Cureus. 2022;14(2):e22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linderman GC, Lu J, Lu Y, Sun X, Xu W, Nasir K, Schulz W, Jiang L, Krumholz HM. Association of Body Mass Index with blood pressure among 1.7 million Chinese adults. JAMA Netw open. 2018;1(4):e181271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circul Res. 2015;116(6):991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MS, Kim WJ, Khera AV, Kim JY, Yon DK, Lee SW, Shin JI, Won HH. Association between adiposity and cardiovascular outcomes: an umbrella review and meta-analysis of observational and mendelian randomization studies. Eur Heart J. 2021;42(34):3388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piani F, Cicero AFG, Borghi C. Uric acid and hypertension: Prognostic Role and Guide for treatment. J Clin Med. 2021;10(3). [DOI] [PMC free article] [PubMed]
- 15.Sanchez-Lozada LG, Rodriguez-Iturbe B, Kelley EE, Nakagawa T, Madero M, Feig DI, Borghi C, Piani F, Cara-Fuentes G, Bjornstad P, et al. Uric acid and hypertension: an Update with recommendations. Am J Hypertens. 2020;33(7):583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertens (Dallas Tex: 1979). 2003;42(4):474–80. [DOI] [PubMed] [Google Scholar]
- 17.Alper AB Jr., Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertens (Dallas Tex: 1979). 2005;45(1):34–8. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Hou X, Yuan X, Cui L, Liu Z, Li X, Ma L, Cheng X, Xin Y, Wang C, et al. Knockout of the urate oxidase gene provides a stable mouse model of hyperuricemia associated with metabolic disorders. Kidney Int. 2018;93(1):69–80. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez-Lozada LG, Soto V, Tapia E, Avila-Casado C, Sautin YY, Nakagawa T, Franco M, Rodríguez-Iturbe B, Johnson RJ. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Ren Physiol. 2008;295(4):F1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunawardhana L, McLean L, Punzi HA, Hunt B, Palmer RN, Whelton A, Feig DI. Effect of Febuxostat on ambulatory blood pressure in subjects with hyperuricemia and hypertension: a phase 2 Randomized Placebo-controlled study. J Am Heart Association. 2017;6(11). [DOI] [PMC free article] [PubMed]
- 21.Kuwabara M, Niwa K, Nishi Y, Mizuno A, Asano T, Masuda K, Komatsu I, Yamazoe M, Takahashi O, Hisatome I. Relationship between serum uric acid levels and hypertension among Japanese individuals not treated for hyperuricemia and hypertension. Hypertens Research: Official J Japanese Soc Hypertens. 2014;37(8):785–9. [DOI] [PubMed] [Google Scholar]
- 22.Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci: BES. 2002;15(1):83–96. [PubMed] [Google Scholar]
- 23.Zhang B, Zhai FY, Du SF, Popkin BM. The China Health and Nutrition Survey, 1989–2011. Obes Reviews: Official J Int Association Study Obes. 2014;15(Suppl 1):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popkin BM, Du S, Zhai F, Zhang B. Cohort Profile: the China Health and Nutrition Survey–monitoring and understanding socio-economic and health change in China, 1989–2011. Int J Epidemiol. 2010;39(6):1435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du T, Sun X, Yin P, Huo R, Ni C, Yu X. Increasing trends in central obesity among Chinese adults with normal body mass index, 1993–2009. BMC Public Health. 2013;13:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng Q, Sun L, Zeng Q. Trajectories of mid-life to elderly adulthood BMI and incident hypertension: the China Health and Nutrition Survey. BMJ open. 2021;11(7):e047920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song S, Zhang Y, Qiao X, Duo Y, Xu J, Peng Z, Zhang J, Chen Y, Nie X, Sun Q, et al. HOMA-IR as a risk factor of gestational diabetes mellitus and a novel simple surrogate index in early pregnancy. Int J Gynaecol Obstet. 2022;157(3):694–701. [DOI] [PubMed] [Google Scholar]
- 28.Hu Y, Tao JY, Cai DP, He YM. Interaction of lipoprotein(a) with low-density lipoprotein cholesterol on first incident acute myocardial infarction. Clin Chim Acta. 2020;501:1–5. [DOI] [PubMed] [Google Scholar]
- 29.Wu L, He Y, Jiang B, Liu M, Wang J, Zhang D, Wang Y, Zeng J. Association between serum uric acid level and hypertension in a Chinese elderly rural population. Clin Experimental Hypertens (New York NY: 1993). 2017;39(6):505–12. [DOI] [PubMed] [Google Scholar]
- 30.Kuriyama S, Maruyama Y, Nishio S, Takahashi Y, Kidoguchi S, Kobayashi C, Takahashi D, Sugano N, Hosoya T, Yokoo T. Serum uric acid and the incidence of CKD and hypertension. Clin Exp Nephrol. 2015;19(6):1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjornstad P, Laffel L, Lynch J, El Ghormli L, Weinstock RS, Tollefsen SE, Nadeau KJ. Elevated serum uric acid is Associated with Greater Risk for Hypertension and Diabetic kidney diseases in obese adolescents with type 2 diabetes: an observational analysis from the Treatment options for type 2 diabetes in adolescents and youth (TODAY) study. Diabetes Care. 2019;42(6):1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding N, He L, Li C, Su Y. Uric acid and blood pressure in NHANES dated from 2009 to 2018: a cross-sectional research. Nutr Metabolism Cardiovasc Diseases: NMCD. 2022;32(11):2568–78. [DOI] [PubMed] [Google Scholar]
- 33.Cassano V, Crescibene D, Hribal ML, Pelaia C, Armentaro G, Magurno M, Toscani A, Miceli S, Andreozzi F, Maio R et al. Uric acid and vascular damage in essential hypertension: role of insulin resistance. Nutrients. 2020;12(9). [DOI] [PMC free article] [PubMed]
- 34.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Volume 3. Wolters Kluwer Health/Lippincott Williams & Wilkins Philadelphia. 2008.
- 35.Yu MA, Sánchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28(6):1234–42. [PubMed] [Google Scholar]
- 36.El Meouchy P, Wahoud M, Allam S, Chedid R, Karam W, Karam S. Hypertension related to obesity: Pathogenesis, characteristics and factors for control. Int J Mol Sci. 2022;23(20). [DOI] [PMC free article] [PubMed]
- 37.Achard V, Boullu-Ciocca S, Desbriere R, Nguyen G, Grino M. Renin receptor expression in human adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R274–282. [DOI] [PubMed] [Google Scholar]
- 38.Hwu CM, Lin KH. Uric acid and the development of hypertension. Med Sci Monitor: Int Med J Experimental Clin Res. 2010;16(10):Ra224–230. [PubMed] [Google Scholar]
- 39.Hall JE, Mouton AJ, da Silva AA, Omoto ACM, Wang Z, Li X, do Carmo JM. Obesity, kidney dysfunction, and inflammation: interactions in hypertension. Cardiovascular Res. 2021;117(8):1859–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allegrini S, Garcia-Gil M, Pesi R, Camici M, Tozzi MG. The Good, the Bad and the New about Uric Acid in Cancer. Cancers. 2022;14(19). [DOI] [PMC free article] [PubMed]
- 41.Yuan Q, Du M, Loehrer E, Johnson BE, Gainor JF, Lanuti M, Li Y, Christiani DC. Postdiagnosis BMI change is Associated with Non-small Cell Lung Cancer Survival. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research. Cosponsored Am Soc Prev Oncol. 2022;31(1):262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359–404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
https://www.cpc.unc.edu/projects/china/data/datasets/data_downloads.