Abstract
Background
WNT7B is a glycoprotein that plays a crucial role in tumorigenesis. This study aimed to investigate the role of WNT7B in oral squamous cell carcinoma (OSCC).
Methods
Bioinformatic databases, immunohistochemistry, a real-time polymerase chain reaction, western blot, and enzyme-linked immunosorbent assay were used to detect WNT7B expression in OSCC. The clinical and prognostic importance of WNT7B expression was evaluated. WNT7B expression was examined in oral leukoplakia and carcinoma induced by 4-nitroquinoline 1-oxide in mice. Loss- and gain-of-function analyses were performed to elucidate the role of WNT7B in OSCC cells. Subcutaneous tumor model was established to observe the effects of WNT7B on tumor growth. Co-Immunoprecipitation was used to explore the Frizzled receptors that WNT7B may bind to.
Results
WNT7B upregulated in OSCC and associated with lymph node metastasis, perineural invasion, and an unfavorable prognosis in patients with OSCC. A gradual increased in WNT7B expression during the malignant progression of OSCC. WNT7B promoted cell proliferation, migration, invasion, while silencing WNT7B abolished these effects. Knocking down the expression of WNT7B inhibits tumor growth in vivo. WNT7B functions by binding to the Frizzled 7 receptor and facilitates the nuclear translocation of β-catenin.
Conclusions
WNT7B contributes to the progression of OSCC by modulating the WNT/β-catenin signaling pathway. These findings highlight the potential of WNT7B as a novel prognostic biomarker and promising therapeutic target for OSCC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-024-05113-9.
Keywords: Oral squamous cell carcinoma, WNT7B, WNT/β-catenin, epithelial-mesenchymal transition, prognosis
Background
Cancer remains one of the leading causes of mortality worldwide, with approximately 10 million deaths attributed to it in 2020 [1]. More than 90% of oral cancers are oral squamous cell carcinoma. Despite improvements in therapeutic approaches, such as surgery, radiotherapy, and chemotherapy, the 5-year survival rate for OSCC remains low at only 50% due to challenges in early diagnosis [2]. The main cause of death in patients with OSCC is the high incidence of lymph nodes and distant metastasis [3]. Currently, conventional clinicopathological parameters are insufficient to accurately predict the behavior and clinical outcomes of OSCC. Therefore, there is an urgent need to develop prognostic models and to gain a better understanding of the molecular mechanisms underlying OSCC tumorigenesis. This will help improve therapeutic efficacy and explore more effective treatment strategies for OSCC.
WNTs are secreted proteins that are involved in embryogenesis, cell differentiation, proliferation, and cancer development [4–8]. WNT signaling is mainly transmitted through two distinct pathways: the canonical WNT/β-catenin pathway, the non-canonical WNT/Ca2+pathway and the WNT/Planar cell polarity (PCP) pathway [4]. In the WNT/β-catenin signaling pathway, WNT ligands bind to transmembrane receptors, including Frizzled (FZD) receptors and LRP5/6 co-receptors, inhibiting the formation of β-catenin destruction complexes. This leads to accumulation of β-catenin in the cytoplasm, followed by its translocation to the nucleus where it binds to TCF/LEF transcription factors to activate downstream target genes [9]. In the non-canonical WNT/PCP pathway, WNT ligands bind to Frizzled receptors. Subsequently, they activate Ras homolog gene family member A (RhoA), Ras-related C3 botulinum toxin substrate (RAC) cell division control protein 42 (Cdc42), small Guanosine Triphosphatases through Dishevelled. This activation influencing the cellular cytoskeleton and initiating the transcriptional activation of target genes responsible for cell adhesion and migration [10]. In the WNT/Ca2+ pathway, WNT ligands bind to FZD receptors, stimulating heterotrimeric G proteins and subsequently activating phospholipase C (PLC). PLC leads to an increase in intracellular Ca2+ release and a decrease in cyclic guanosine monophosphate (cGMP) levels. This activation results in the activation of two kinases, CaMKII (calcium/calmodulin-dependent protein kinase II) or Caln (calmodulin-dependent phosphatase), as well as protein kinase C, promoting cell migration [11].WNT7B is a WNT ligand that plays different roles in various cancers such as bladder cancer, prostate cancer, pancreatic adenocarcinoma, and colorectal cancer through canonical and non-canonical WNT signaling pathways [12–14]. Increasing evidence suggests that WNT7B is a potential prognostic biomarker and therapeutic target for human cancer [15, 16].However, the clinical significance and underlying mechanisms of action of WNT7B in the development of OSCC remain unclear.
This study aimed to comprehensively investigate the expression of WNT7B in OSCC and its correlation with clinicopathological features. Additionally, we sought to explore the effects and mechanisms of WNT7B in two OSCC cell lines. To our knowledge, this is the first study to investigate the relationship between WNT7B expression and the prognosis of OSCC. By elucidating the role of WNT7B in OSCC, we hope that our research will provide valuable insights into targeted therapy strategies.
Materials and methods
Bioinformatics analysis
The differential expression levels of WNT7B were analyzed in cancer tissue samples obtained from the Cancer Genome Atlas (TCGA) data portal (https://tcga-data.nci.nih.gov/tcga/). Data from patients with head and neck squamous cell carcinoma, including transcriptome profiling and clinical information, were downloaded from TCGA. Fragments per kilobase of transcript per million mapped reads (HTSeq-FPKM) were used to assess mRNA expression patterns. Raw counts (HTSeq counts) were downloaded for differential expression analysis. Additionally, we obtained the OSCC datasets GSE37991 and GSE30784 from the Gene Expression Omnibus (GEO) database, which included tumor and non-tumor pairwise samples as well as tumor and non-tumor samples, respectively. To perform survival analysis, we derived expression and survival data from GSE41613 for OSCC.
Patient specimens and tissue microarrays
This study included 127 patients who underwent surgical resection at the First Affiliated Hospital of Fujian Medical University between 2012 and 2016. Human OSCC tissue microarrays (TMA) were constructed using formalin-fixed paraffin-embedded (FFPE) OSCC tissue blocks obtained from these patients. Non-necrotic cancer areas and normal squamous epithelium were selected and labeled in hematoxylin and eosin H&E sections and corresponding paraffin-embedded tissue blocks. Tissue cylinders with a diameter of 2 mm were cored from the marked areas of the donor block and transferred to the recipient TMA block using a trephine apparatus. The histopathological features of the slides, including stages, tumor differentiation, perineural invasion, and lymph node status, were reviewed by two experienced pathologists according to the American Joint Committee on Cancer (AJCC) 8th edition pathological criteria. Additionally, 10 pairs of OSCC tissues and adjacent normal tissues were obtained from surgical resections at our hospital and quickly frozen in liquid nitrogen and stored at -80 °C. This study was approved by the Institutional Human Experiment and Ethics Committee of the First Affiliated Hospital of Fujian Medical University (FJMU-IACUC 2021 − 0299). All participants have signed an informed consent form. All methods followed by relevant guidelines and regulations, including the Helsinki Declaration.
Animal treatment
A total of 25 5-week-old C57BL mice were purchased from GemPharmatech Co., Ltd. (GuangDong, China) selected as the experimental group. The 4-nitroquinoline 1-oxide (4-NQO) (Sigma, 32-60127, USA) was dissolved in propylene glycol and prepared at a concentration of 200 ppm in drinking water. Five mice were used as controls and received propylene glycol and distilled water without 4-NQO.The euthanasia method followed American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals (2020), tongue tissue samples were collected and stored in FFPE tissues for further experiments. The experimental scheme was approved by the Animal Care and Use Committee of Fujian Medical University in accordance with institutional and Chinese government guidelines for animal experiments.
Immunohistochemistry (IHC)
Immunohistochemistry was performed on FFPE sections cut into 4 μm slide sections from TMA blocks. The primary antibody used was a WNT7B rabbit monoclonal antibody (Abcam,94915) at a dilution of 1:100. The Ventana Benchmark ULTRA automated staining system (Ventana Medical Systems, USA) was used according to the manufacturer’s instructions. Mice tongue tissue and xenografts tumor samples were immobilized, paraffin embedded, sectioned, removed, and H&E staining, antigen extracted according to the manufacturer’s instructions. The primary antibody WNT7B (Abcam,94915) at a dilution of 1:100, Ki-67 (Abcam, ab15580) at a dilution of 1:500 incubated at room temperature for 1 h. After washing PBS three times, incubate the slices with the biotin conjugated second antibody (Maxin) at room temperature for 30 min. After washing three times in PBS, incubate the slices with streptavidin peroxidase (Maxin) at room temperature for 15 min. Then washing three times in PBS, the antibody complex was observed by incubation with diaminobenzidine tetrachloride (DAB) chromogen (Maxin). The scoring was based on the number of positively stained cells in the overall evaluation of all available tumor tissues. The staining intensity was assessed on a scale of 0–3, corresponding to the following categories: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. The frequency of positive cells was determined based on the following criteria: a score of 0 represented less than 5% of positive cells, a score of 1 represented 5–25% of positive cells, a score of 2 represented 26–50% of positive cells, a score of 3 represented 51–75% of positive cells, and a score of 4 represented greater than 75% of positive cells.
ELISA assay
Twenty pairs of blood samples were collected from patients with OSCC and from healthy individuals. Serum was obtained by separating the blood cells through natural sedimentation rate. The WNT7B ELISA Kit (CSB-EL026142MO, P28047) was used according to the manufacturer’s instructions. The expression of WNT7B protein in the samples was determined by calculating it based on the standard curve at a wavelength of 450 nm using an ELISA detector (Pharmacia Biotech, Piscataway, NJ, USA). All participants have signed an informed consent form.
Cell culture and transfection
The human oral cancer cell lines HN6 and HN30, as well as the 293T cell line, were obtained from the American Type Culture Collection. The cells were cultured in Dulbecco’s modified eagle’s medium (DMEM; Gibco, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco). Human oral epithelial cells, H203, were purchased from Procell Life Science & Technology Co., Ltd. (Procell, Wuhan, #CP-H203) and maintained in a complete culture medium for human oral epithelial cells (Procell, Wuhan, #CM-H203). All cell lines were authenticated using short tandem repeat (STR) analysis. The plasmids were designed, synthesized, and validated by sequencing and were then amplified through transformation and expansion. Cell transfection was performed with Lipofectamine 2000 (Invitrogen, Catalog #L11668019), according to the manufacturer’s instructions.
Small interfering RNA (siRNA) transfection
Two different siRNAs and negative control (NC) were purchased from Gene Pharma Corporation. (Gene Pharma, Shanghai, China). RNA interference sequences are listed in Supplementary Table 1. To transfect HN6 and HN30 cells, Lipofectamine RNAiMAX (Invitrogen, Catalog#13778150) was used according to the manufacturer’s instructions.
Cell proliferation assay
Cell proliferation was assessed using Cell Counting Kit-8(CCK8) reagent (Dojindo, Catalog #CK04) according to the manufacturer’s instructions. HN6 and HN30 cells were transfected with small interfering RNAs (siRNAs) or plasmids. The cells were then trypsinized and counted, and 2000 cells were added to each well of a 96-well plate with three replicate wells per condition. The optical density was measured at a wavelength of 450 nm at four time points (1, 2, 3, and 4 days). The colony formation assay further validated cell proliferation. HN30 and HN6 cells overexpressing or knocked down WNT7B were seeded at a density of 2 × 103 cells/well in 6-well plates. The plates were incubated at 37 °C for 10−14 d. After the cells were washed three times with PBS, they were fixed in 4% paraformaldehyde. The colonies were stained with 0.1% crystal violet for 10 min and photographed for further analysis.
Wound healing assay
HN6 and HN30 cells were transfected with small interfering RNAs (siRNAs) or plasmids. The cells were allowed to grow until they formed a confluent monolayer on the plate. A 20 µL pipette tip was used to create scratches on the surface of the cell monolayer. The medium was then replaced with serum-free DMEM. The process of wound healing was observed using a casting microscope (Olympus), and photographs were captured immediately after scratching (0 h) and at the point of complete healing of the scratches.
Transwell assay
Cell migration and invasion abilities were assessed using 24-well plates with transwell chambers, with or without Matrigel (CORNING, 353097). Cells were treated with trypsin and then seeded in each well with 400 µL of serum-free DMEM medium at a density of 4 × 104 cells per well. The lower chamber of the transwell was filled with 800 µL of culture medium containing 10% FBS. Following incubation at 37 °C and 5% CO2 for 48 h, the lower surface of the filter was fixed with 4% paraformaldehyde for 10 min, followed by gentle washing with PBS. Subsequently, the filter was stained with 0.1% crystal violet for 10 min. Migrating cells were observed and photographed and counted.
Quantitative real-time polymerase chain reaction
RNA was extracted from cells and tissues using TRIzol reagent (Invitrogen, Grand Island, NY, USA) for genetic testing. Subsequently, cDNA was synthesized using the Perfect Real Time PrimeScript® RT kit (Takara, Shiga, Japan) and quantified using PCR with the SYBR® Premix Ex TaqTM II (Takara, Shiga, Japan) on a 7300HT real-time PCR system, following the manufacturer’s instructions. The PCR primer pairs are shown in Supplementary Table 2. Additionally, the ΔΔCT method was used to quantify the expression of target genes relative to the control.
Western blot analysis
Total cell protein extracts were prepared using a lysis buffer (Beyotime, China) supplemented with a phosphatase inhibitor (Beyotime, China) and a protease inhibitor (MedChen Express, HY-K0011). Proteins were separated by 8−12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Bio-Rad, 456–1086). The proteins were transferred to a PVDF membrane (Bio-Rad, 1620177). The proteins were probed with specific antibodies in Supplementary Table 3, horseradish peroxidase-conjugated secondary antibodies were used and proteins were visualized using a ChemiDoc XRS + Imager (BIO-RAD, USA). When the expected bands, estimated by the molecular weight ladder and the manufacturer’s instructions of primary antibodies, were separated enough, the blots were cut into 2 or 3 parts prior to incubation with primary antibodies. The original gels and multiple exposure images were shown in Supplementary file.
Co-Immunoprecipitation
The 293T cell lysates were centrifuged at 12,000 rpm for 10 min at 4 °C. Then, 20 µl of protein G agarose beads (MCE, #HY-K0204) were added to the supernatant and incubated at room temperature for 30 min. A total of 40 µl of the supernatant was taken as the input group, while the remaining samples were supplemented with 20 µl of anti-c-MYC magnetic beads (MCE, #HY-K0206) or anti-FLAG magnetic beads (MCE, #HY-K0207) and incubated overnight at 4 °C. Nonspecifically bound proteins were removed by washing with agarose beads. Samples were analyzed by western blotting.
Luciferase reporter assay
The 293T cells were seeded in 48-well plates at a density of 3 × 104 cells per well and subsequently transfected with plasmids WNT7B, TOPflash, and phRL-SV40 Renilla luciferase using Lipofectamine 2000 transfection reagent, following the manufacturer’s instructions. After 48 h, luciferase activity was quantified using the Dual-Glo Luciferase Assay System (Promega).
Lentivirus transduction
The WHT7B short hairpin RNAs (shRNAs) were purchased from TSINGKE (BeiJing, China). The target sequences for shWNT7Bs were as shown in Supplementary Table 4. The WHT7B shRNAs were transfected into the 293T cell lines according to the manufacturer’s protocol. Stable HN6 cell lines were generated by retroviral infection treated with 2 g/mL puromycin for two weeks.
Establishment of xenograft mouse models
5-week-old female BALB/c nude mice were purchased from GemPharmatech Co., Ltd. (GuangDong, China), then randomly divided into three groups. Stable transfected HN6 cells were re-suspended in 0.1 ml serum-free DMEM at a density of 1 × 106 cells and injected into the right axillary of each mouse. Tumor size was measured every four days when the tumor was palpable. The tumor size was adopted by the formula V= (length × width 2)/2. The euthanasia method followed American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals (2020), the tumors were harvested and weighed. Xenografts tumor samples were collected and stored in FFPE tissues for further experiments.The experimental scheme was approved by the Animal Care and Use Committee of Fujian Medical University in accordance with institutional and Chinese government guidelines for animal experiments.
Statistical analysis
Statistical analyses were performed with GraphPad Prism 9.0 or SPSS 19.0. Differences between groups were analyzed using the t-test. One-way ANOVA was used to compare the differences among multiple groups. The correlation between the expression level of WNT7B and the clinical features of OSCC patients was assessed using the χ2-test or Fisher’s exact test. The overall survival (OS) curves were plotted using the Kaplan−Meier method, and the log-rank test was used to compare differences. The following notions were used to indicate significance: n.s. (P > 0.05), *(0.01 < P < 0.05), **(0.001 < P < 0.01), ***(0.0001 < P < 0.001), ****(P < 0.0001).
Results
Upregulation of WNT7B is associated with clinicopathological features and predicts a poor prognosis in OSCC
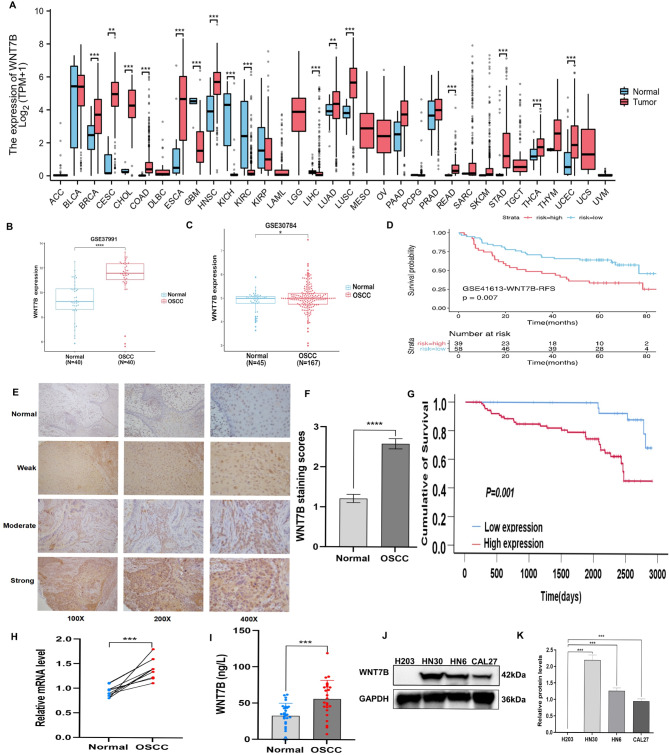
To investigate the expression of WNT7B in various types of cancer, a pan-cancer analysis was conducted using the TCGA database. The analysis revealed a significant overexpression of WNT7B mRNA in tumor tissues compared to adjacent tissues in various types of cancer (Fig. 1A). To further investigate the role of WNT7B in OSCC, its expression in OSCC tissues was significantly higher than normal tissues using the GEO database. WNT7B expression was notably higher in OSCC tissues than in normal tissues (Fig. 1B, C). Kaplan-Meier survival analysis using the Kaplan-Meier Plotter demonstrated a significant correlation between high WNT7B expression and overall survival (OS) in patients with OSCC (Fig. 1D). Immunohistochemical staining of WNT7B was performed in 127 OSCC specimens showed significantly higher expression in OSCC tissues than in normal tissues (Fig. 1E, F). Furthermore, high expression of WNT7B was associated with a poor prognosis in patients with OSCC (Fig. 1G). Our findings revealed a significant increase in WNT7B mRNA expression compared to adjacent normal tissues (Fig. 1H). Furthermore, WNT7B expression was significantly higher in the serum of patients compared to healthy individuals (Fig. 1I). Western blot analysis confirmed the high expression of WNT7B in OSCC cell lines compared to H203 (Fig. 1J, K). These findings provide further evidence supporting WNT7B upregulated in OSCC progression and suggest its clinical significance as a prognostic marker.
Fig. 1.
WNT7B is upregulated in OSCC and indicates worse survival. (A) Pan-cancer analysis of WNT7B expression in cancers. (B, C) TCGA database shows that WNT7B is significantly higher in OSCC compared to normal tissues. (D) TCGA database shows that the high expression of WNT7B correlates with a poor prognosis in OSCC. (E) The representative WNT7B immunohistochemical staining images with different magnifications in OSCC tissues (magnification at ×100, ×200, ×400). (F) The expression of WNT7B is significantly higher in OSCC than in normal tissues examined by IHC. (G) The overall survival rate is significantly different between the high and low expression of WNT7B in patients with OSCC (P = 0.001). (H) qRT-PCR shows that WNT7B mRNA is higher in OSCC than in adjacent tissue. (I) The ELISA assay shows that WNT7B is higher in the serum of patients with OSCC compared to healthy individuals. (J) Western blot analysis shows that WNT7B expression is higher in oral cancer cells than the human normal oral epithelium cells. (K) Quantification of the levels of the WNT7B protein in oral cancer cells and human normal oral epithelium cells. Error bars, mean ± SD; *P < 0.05; **0.001 < P < 0.05; ***P < 0.001; ****P < 0.0001; t-test
Correlations between WNT7B protein expression and clinicopathologic factors
In order to investigate the clinical significance of WNT7B in OSCC, the relationship between the expression of WNT7B and clinicopathologic factors is presented in Table 1. The results indicate that there were no statistically significant differences in WNT7B expression based on gender, age, tumor stage, or degree of differentiation. However, in the high expression WNT7B group, 40 (31.5%) cases exhibited perineural invasion, while 51 (40.2%) cases did not. In contrast, in the low expression WNT7B group, 8 (6.3%) cases showed perineural invasion, while 28 (22.0%) did not. This suggests a statistically significant difference in perineural invasion based on WNT7B expression levels. Furthermore, among the cases with high WNT7B expression, 46 (36.2%) cases demonstrated lymph node metastasis, while 45 (35.4%) cases had no metastasis or lymph node metastasis could not be detected (P < 0.05). This indicates an association between high WNT7B expression and lymph node metastasis and perineural invasion in patients with OSCC.
Table 1.
Correlation between WNT7B expression and OSCC clinicopathological factors
| Factors | Subtypes | Cases | WNT7B Low (%) | WNT7B High (%) | χ2 | P value |
|---|---|---|---|---|---|---|
| Gender | Male | 79 | 24(18.9%) | 55(43.3%) | 0.425 | 0.514 |
| Female | 48 | 12(9.4%) | 36(28.3%) | |||
| Age(years) | < 60 | 83 | 24(18.9%) | 59(46.5%) | 0.038 | 0.845 |
| ≥ 60 | 44 | 12(9.4%) | 32(25.2%) | |||
| Perineural invasion | Present | 48 | 8(6.3%) | 40(31.5%) | 5.183 | 0.023* |
| Absent | 79 | 28(22.0%) | 51(40.2%) | |||
| AJCC stages | I and II | 52 | 11(8.7%) | 41(32.3%) | 3.866 | 0.276 |
| III and IV | 75 | 25(19.7%) | 50(39.4%) | |||
| Differentiation | Well | 60 | 20(15.7%) | 40(31.5%) | 4.097 | 0.129 |
| Moderate | 20 | 2(1.6%) | 18(14.2%) | |||
| Poor | 47 | 14(11.0%) | 33(26.0%) | |||
| Lymph node metastasis | N0 and Nx | 72 | 27(21.3%) | 45(35.4%) | 6.858 | 0.009** |
| N1 and N2 | 55 | 9(7.1%) | 46(36.2%) |
AJCC, American Joint Committee on Cancer; Nx, unknow lymph node status; * P < 0.05, ** P < 0.01
WNT7B participates in the malignant development of oral leukoplakia and carcinoma
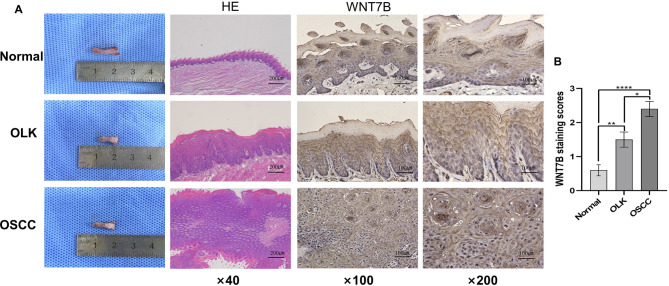
Oral leukoplakia (OLK) is a common oral potential malignant disease. To verify whether WNT7B is involved in the process of OLK developing into OSCC, we constructed an animal model. Macroscopic observations were made to confirm the diagnosis of OLK and OSCC, which was further validated by H&E staining (Fig. 2A). The IHC analysis showed the absence of WNT7B expression in normal tongue tissues, while its expression gradually increased in OLK and OSCC, exhibiting significant statistical differences (Fig. 2B). These findings suggest that WNT7B may plays a pivotal role in the progression of oral leukoplakia to carcinoma.
Fig. 2.
WNT7B participates in the malignant development of oral leukoplakia and carcinoma. (A) Left: Macroscopic observations were made to confirm the diagnosis of normal mucosa, OLK and OSCC, which was further validated by H&E staining. Right: Representative WNT7B immunohistochemical staining images with different magnifications in normal oral mucosa, OLK, and OSCC. (B) The expression of WNT7B is gradually increased in normal oral mucosa, OLK, and OSCC (*P < 0.05, **P < 0.01, ****P < 0.0001)
WNT7B affects the proliferation of OSCC cells
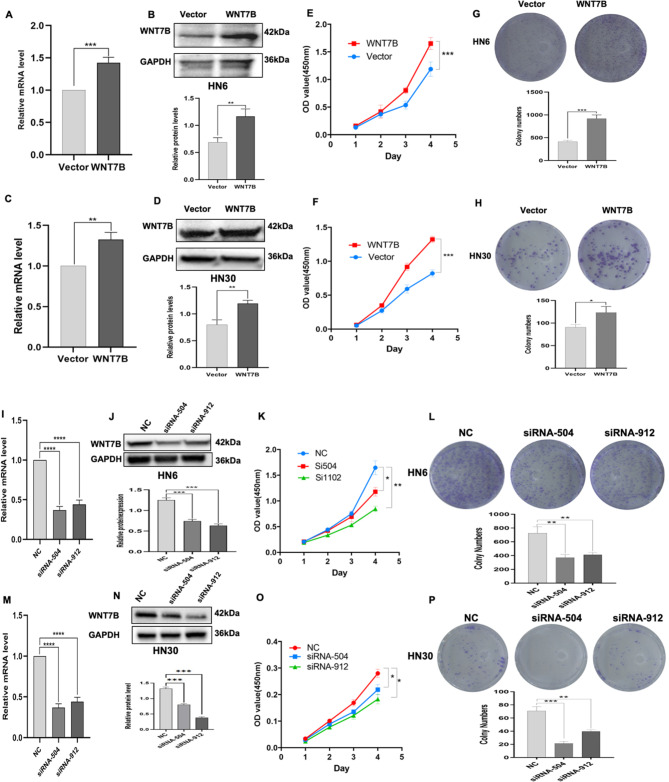
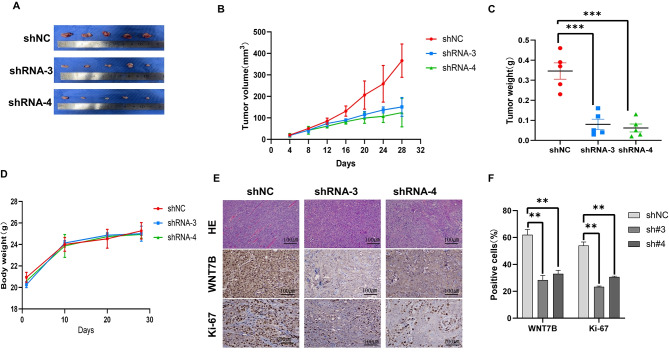
As demonstrated above, the expression of WNT7B is notably elevated in OSCC. To further investigate the impact of WNT7B on cell proliferation ability. Initially, plasmid transfection was performed to enhance the expression of WNT7B in HN6 and HN30 cancer cells. Transfection efficiency was confirmed by the upregulation of WNT7B mRNA (Fig. 3A, C) and protein levels (Fig. 3B, D). The results showed that WNT7B overexpression significantly increased cell viability in HN6 and HN30 cells compared to the vector control group (Fig. 3E, F). Conversely, the effects of silencing WNT7B expression in OSCC cells were also explored by siRNA transfection. Successful downregulation of WNT7B expression was confirmed by qRT-PCR (Fig. 3I, M) and western blotting (Fig. 3J, N), which showed a decrease in WNT7B expression levels. Knockdown of WNT7B expression resulted in reduced viability of OSCC cells compared to negative control (NC) (Fig. 3K, O). Furthermore, WNT7B overexpression improved cells clone formation capacity (Fig. 3G, H), while knockdown of WNT7B reversed this effect (Fig. 3L, P). It can be seen from the above that WNT7B is related to the proliferation ability of OSCC cells. Therefore, we then explored whether the abnormal expression of WNT7B had an impact on the tumorigenesis process in nude mice. After successfully establishing a subcutaneous xenograft model of OSCC cells in nude mice. We found that tumor volume, tumor weight, and tumors in the WNT7B down-regulated group were smaller than those in the control group (Fig. 4A-C). The body weight of nude mice was similar to that of each group (Fig. 4D). In addition, we performed immunohistochemical staining of WNT7B and Ki-67 to assess whether the expression of WNT7B was reduced in xenograft tumor tissues and the effect on proliferation. As shown in (Fig. 4E, F), with the downregulation of WNT7B expression, Ki-67 expression in xenografts also decreased. Based on these findings we found that knocking down WNT7B expression reduces oral squamous cancer cell proliferation in vitro and in vivo. These results further support the role of WNT7B as an oncogene in oral squamous cell carcinoma.
Fig. 3.
WNT7B promotes OSCC cell proliferation. (A-D) qRT-PCR and WB analysis showing the successful overexpression of WNT7B in HN30 and HN6 cell lines. (E, F) CCK8 assay shows a statistically significant difference between OSCC cells with overexpressed WNT7B than controls. (I-N) qRT-PCR and WB analysis shows a successful knockdown of the expression level of WNT7B in HN30 and HN6 cells. (K, O) CCK8 assay shows a statistically significant difference between OSCC cells with knockdown WNT7B and negative control. (G, H) The clone formation ability assay shows that the overexpressed WNT7B increased significantly compared to the control. (L, P) The clone formation ability assay shows knockdown WNT7B is significantly reduced compared to the control. n = 3 independent experiments; error bars, mean ± SD; *P < 0.05, **P < 0.01, ***P < 0.001; ****P < 0.0001, t-test
Fig. 4.
Knockdown of WNT7B inhibits tumor growth of OSCC in vivo. (A) The macroscopic images of tumors in the WNT7B shRNA group were much smaller than those in the negative control group. (B) Tumor volume curves. (C) Weight of the excised tumors. (D) The body weights of each group did not show much difference. (E) Representative images H&E staining and the expression of WNT7B and Ki67 in tumor tissues detected by immunohistochemistry. (F) The expression of WNT7B in the shRNA groups was decreased compared to control, while Ki-67 expression in the shRNA groups was shown to be decreased. ** P < 0.01, *** P < 0.001
WNT7B affects cell migration by inducing the epithelial-mesenchymal transition (EMT)
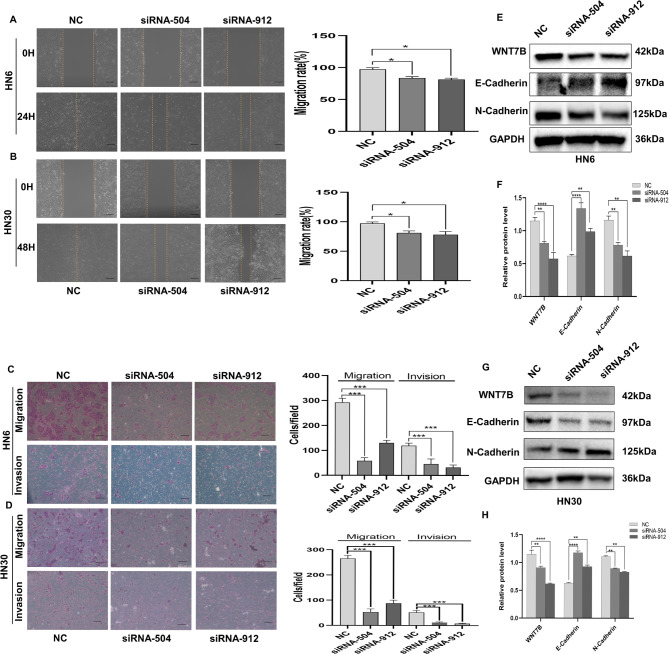
Next, we investigated whether WNT7B affects the motility of OSCC cells. Overexpression of WNT7B in HN6 and HN30 cells significantly accelerated the rate of wound healing compared to that in the control group (Fig. 5A, B). Similarly, migration and invasion assays showed a notable improvement in the migratory and invasive capabilities of cells overexpressing WNT7B (Fig. 5C, D). In contrast, the downregulation of WNT7B expression in OSCC cells yielded opposite results (Fig. 6A-D). Furthermore, we examined the expression of EMT markers in HN30 and HN6 cells. The protein levels of E-cadherin were significantly reduced in cells overexpressing WNT7B compared to those of the vector group, while N-cadherin levels were elevated (Fig. 5E-H). On the contrary, upon WNT7B knockdown in HN30 and HN6 cells, the expression levels of E-cadherin and N-cadherin showed trends opposite to those observed during the overexpression of WNT7B (Fig. 6E-H). These findings suggested a positive correlation between WNT7B expression and the migratory ability of OSCC cells through the induction of EMT.
Fig. 5.
WNT7B promotes the migration and invasion of OSCC cells. (A-D) Wound-healing assay, the migration and invasion assay analyses of changes in migratory capacity as WNT7B is overexpressed in HN6/HN30 cells. Bars, 500 μm. (E-H) Western blot analysis that detected epithelial to mesenchymal transition marker E-cadherin protein levels decreased and the N-cadherin protein increased in HN6 and HN30 cells after WNT7B overexpressed. GAPDH served as a normalization control for WB. n = 3 independent experiments; error bars, mean ± SD; ns, not significant, *P < 0.05, **P < 0.01, ***P < 0.001; t-test
Fig. 6.
WNT7B inhibited epithelial to mesenchymal transition in OSCC cells. (A-D) Wound-healing assay, the migration and invasion assay analyses of changes in migratory capacity as WNT7B was knockdown in HN6/HN30 cells. Bars, 500 μm. (E-H) Western blot analysis detecting epithelial to mesenchymal transition marker E-cadherin protein levels increased and N-cadherin protein levels decreased in HN6/HN30 cells after WNT7B knockdown. GAPDH served as a normalization control for WB. Error bars, mean ± SD; n = 3 independent experiments; error bars, mean ± SD; *P < 0.05, **P < 0.01, ***P < 0.001; t-test
WNT7B may exert its function by binding to FZD7
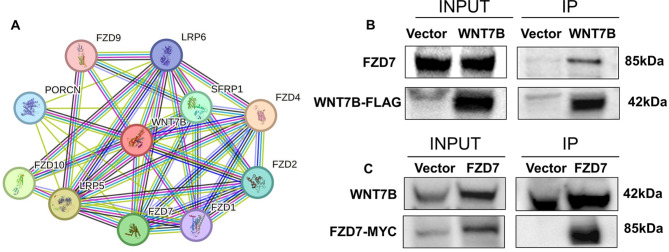
As demonstrated above, WNTs may exert its function by binding to FZD receptors. First, we performed a predictive analysis using the STRING website(https://cn.string-db.org/) to further investigate potential receptors that may interact with WNT7B. This analysis yielded a list of potential receptors that interact with WNT7B (Fig. 7A). Subsequently, we separately overexpressed WNT7B or FZD7 in 293T cells and performed Co-IP experiments (Fig. 7B). Immunoprecipitation was performed using a WNT7B antibody, followed by immunoblotting with an FZD7 antibody to detect the presence of FZD7 bands. In particular, clear FZD7 bands were observed in the IP samples. Similarly, when immunoprecipitation was performed using a WNT7B antibody and immunoblotting was performed using an FZD7 antibody, the striped area ratio of the WNT7B group was significantly higher than that of the control group (Fig. 7C). These findings provide evidence for a direct interaction between FZD7 and WNT7B in OSCC cells. It is demonstrated that the binding of FZD7 and WNT7B is a necessary condition for exerting biological effects in OSCC.
Fig. 7.
WNT7B may interact with FZD7 in OSCC. (A) The protein–protein interaction network of WNT7B is generated by STRING. (B) Overexpressing WNT7B in 293T is immunoprecipitated (IP) with antibody to the FZD7 bands, and clear FZD7 bands are observed in the IP samples. (C) Overexpressing FZD7 in 293T is IP with antibody to the WNT7B bands, the striped area ratio of WNT7B in IP samples increased significantly compared to the control group
WNT7B is correlated with activation of the WNT/β-catenin signaling pathway in OSCC
To determine whether WNT7B activates canonical or non-canonical signaling pathways in OSCC. We observed that WNT7B overexpression resulted in a significant decrease in the levels of phosphorylated β-catenin protein, while total β-catenin protein levels remained unchanged compared to the control group (Fig. 8A-D). This suggests that WNT7B modulates the canonical WNT signaling pathway by influencing the phosphorylation status of β-catenin. In contrast, when WNT7B was silenced using specific siRNAs, there was an increase in phosphorylated β-catenin protein levels, while total β-catenin protein levels remained consistent with the control group (Fig. 8E-H). Furthermore, we performed nuclear cytoplasmic separation experiments to further confirm changes in β-catenin protein expression levels in HN30 and HN6 cells following WNT7B overexpression (Fig. 8I, L). The results revealed that the overexpression of WNT7B significantly increased the levels of β-catenin protein in the nucleus (Fig. 8K, N), indicating the translocation of β-catenin from the cytoplasm to the nucleus. However, the level of β-catenin protein in the cytoplasm remained unchanged compared to the vector control group (Fig. 8J, M). Finally, we used a luciferase reporter assay to further evaluate changes in β-catenin/TCF-mediated transcription activity. The results showed that WNT7B overexpression significantly increased TOP/FOP luciferase activity compared to the control group (Fig. 8O). Based on these findings, it can be inferred that WNT7B activates the WNT/β-catenin signaling pathway, thus promoting cell proliferation and migration capabilities in OSCC.
Fig. 8.
WNT7B activated the WNT/β-catenin signaling pathway in OSCC. (A-D) WB analysis overexpression of WNT7B in HN6/HN30 cells significantly decreased phosphorylated β-catenin protein levels, but no difference in total β-catenin protein levels. (E-H) Knockdown of WNT7B in HN6 and HN30 cells increased phosphorylated β-catenin protein levels, but no difference in total β-catenin protein levels. (I, L) Nuclear cytoplasmic separation assay analysis overexpression of WNT7B in HN6/HN30cells. (J, M) β-catenin protein level in the nucleus was significantly higher than that in the control group. (K, N) There is no significant β-catenin protein level in the cytoplasm. (O) TOP/FOP luciferase reporter assay quantifying the relative overexpression of WNT7B signaling activity in 293T cell, β-catenin/TCF transcriptional activity increased compared to the vector group. Error bars, mean ± SD; n = 3 independent experiments; ns, not significant, *P < 0.05, **P < 0.01, ***P < 0.001; t-test
Discussion
The WNT signaling pathway plays a crucial role in various biological processes. However, its dysfunction is closely associated with cancer occurrence and development [4]. In this study, we performed a bioinformatics analysis and found that upregulated WNT7B expression was associated with OSCC progression and a poor prognosis. Further experimental validation showed that WNT7B was upregulated in OSCC, and its high expression was closely related to lymph node metastasis and perineural invasion, indicating that WNT7B is a significant oncogene in OSCC. Furthermore, high WNT7B expression is associated with a low survival rate in patients with OSCC. We also inferred that WNT7B may be involved in the progression of oral leukoplakia to carcinoma induced by 4-NQO in mice. Our study showed that WNT7B may bind to the FZD7 receptor to promote proliferation, migration, and invasion of OSCC cells by activating the WNT/β-catenin signaling pathway. These findings highlighted the crucial role of WNT7B in OSCC progression and suggested its potential as a valuable prognostic biomarker.
WNTs are secreted glycoproteins with 19 subtypes reported in humans. Increasingly, studies have focused on inhibiting the WNT signaling pathway in tumors using small molecules or monoclonal antibodies [15]. Dysregulation of the WNT/β-catenin signaling pathway was first reported in the occurrence and development of colon cancer [16]. Kayamori et al. [17] found that WNT7A not only significantly overexpressed in tumor cells, but also associated with poor prognosis in patients with OSCC. Huang et al. [18] revealed that WNT7A is upregulated in HNSCC tissues and cells, and it may be an oncogene in OSCC via activating JAK1/STAT3 signaling pathway. In addition, Cierpikowski et al. [19] found that WNT1 and CXCR4 is an independent prognostic factor of patients with OSCC. WNT5A also play pivotal role in the occurrence and development of OSCC [20–22]. All these studies suggest that elucidating the role of WNT signaling pathways in the development and progression of OSCC is important for the treatment of OSCC patients. In this study, we found that the expression of WNT7B increased sequentially during the progression from normal mucosa to leukoplakia and carcinoma using a 4NQO-induced mouse model, suggesting that WNT7B may be involved in the development of oral leukoplakia into cancer. This result is consistent with earlier works [23, 24]. Recent findings underscore the role of the WNT signaling pathway in cell proliferation [25, 26]. For example, the recombinant human WNT7B protein could promote the migration and differentiation of human dental pulp cells by activating WNT/β-catenin and JNK signaling pathways [27]. Our results indicated a significant link between WNT7B expression and tumor cell proliferation in vitro and in vivo. This is consistent with the findings of Liu et al., in which elevated levels of WNT7B promoted HCC cell proliferation [26]. Moreover, increasing evidence suggests that WNT7B promotes the migration of tumor cells and is strongly associated with a poor prognosis [29, 30]. We also found that WNT7B promotes the migration and invasion of OSCC cells. The WNT signaling pathway is closely related to EMT, a key process in cancer progression. E-cadherin and N-cadherin are important molecular makers in EMT [31]. Our results indicate that overexpression of WNT7B decreases the expression level of E-cadherin while increasing the expression level of N-cadherin, while knockdown of WNT7B has the opposite effect. This suggests that WNT7B induces EMT in OSCC cells. Yeo et al. also revealed that myeloid-derived WNT7B plays an essential role in angiogenesis, invasion, and metastasis in breast cancer, therefore, WNT7B is a promising therapeutic target for breast cancer patients [32]. Similarly, Chen et al. demonstrated that Wnt7b promotes proliferation, migration, and invasion by activating the WNT/β-catenin signaling pathway in colorectal carcinoma [33]. Other studies have demonstrated that WNT ligands can elicit various biological effects depending on the receptors with which they interact. Zhang et al. discovered that the interaction between Fzd7/Wnt7b plays a crucial role in stemness and drug resistance in pancreatic cancer cells [34]. In contrast, Sun et al. found that Wnt7B/FZD5 is involved in cell proliferation, DNA damage repair, and stemness in triple-negative breast cancer [35]. In our study, we observed that the binding of WNT7B to FZD7 mediated its biological effects. Furthermore, WNT signaling interacts with other pathways, such as Notch and Sonic Hedgehog, thus contributing to the complexity of cancer pathogenesis [36]. Furthermore, while WNT7B generally acts as an oncogene, promoting tumor progression, its function can vary depending on the specific type of cancer. For example, Na et al. demonstrated that high expression of WNT7B inhibits EMT and stemness and that low expression of WNT7B is associated with a poor prognosis in bladder urothelial carcinoma [12]. Our findings indicate that WNT7B plays an important role in cell proliferation, migration, and EMT in OSCC and may serve as a potential therapeutic target for OSCC.
WNT7B can activate canonical or non-canonical signaling pathways to exert biological effects on cancer [13, 28]. For example, in the androgen-independent growth of castration-resistant prostate cancer cells, WNT7B has been reported to activate protein kinase C (PKC) isoenzyme [13]. Additionally, in pancreatic cancer, WNT7B affects the proliferation of pancreatic progenitor cells through PKC signaling and YY1 [37]. These findings suggest that WNT7B activates non-canonical signaling pathways involving PKC to promote cancer progression in specific contexts. Furthermore, studies have indicated that WNT7B plays an important role in colorectal carcinoma and hepatocellular carcinoma by activating the canonical WNT/β-catenin signaling pathway [14, 38]. Activation of this pathway leads to the accumulation of β-catenin in the cytoplasm and its subsequent transport from the cytoplasm to the nucleus, where it activates the expression of downstream target genes [39]. In our study, overexpression of WNT7B resulted in a decrease in phosphorylated Thr41/Ser45 β-catenin levels and an increase in nuclear β-catenin, indicating activation of WNT/β-catenin signaling. On the contrary, WNT7B knockdown yielded the opposite result. The nuclear-cytoplasmic separation experiment confirmed a significant increase in β-catenin in the nucleus. However, both experiments showed that total β-catenin and cytoplasmic β-catenin protein levels were not statistically significant. This may be attributed to the transient nature of cytoplasmic stability, which is a necessary step preceding nuclear translocation, therefore, the increase in β-catenin may not be as pronounced. Furthermore, the TOP/FOP luciferase reporter assay further confirmed that WNT7B enhanced β-catenin transcriptional activity. Collectively, these findings support the notion that WNT7B activates the canonical WNT signaling pathway in OSCC. However, there are still some limitations in this study that need to be addressed. The precise mechanisms by which WNT7B exerts its biological functions in OSCC remain unclear. Although the study suggests the activation of the WNT/β-catenin signaling pathway as a potential mechanism, more detailed research is needed to uncover the intricacies of this process.
Conclusion
In conclusion, our study provides evidence that WNT7B promotes proliferation, migration, and invasion ability of OSCC cells. As we known, this study is the first to propose a close association between WNT7B and tumor progression, as well as a poor prognosis in OSCC. These results indicate that WNT7B has the potential to serve as a novel prognostic biomarker and target therapy for patients with OSCC.
Data Availability
All data in this study are included in the article and supplementary material files.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
D.Z. and Y.Lu conceived the ideas and designed the experiments. Y.Li, Q.H., T.L., Y.Y. and L.H. performed the experiments. Y.Li, L.H., and T.L. were involved in data analyses and interpretation. D.Z, L.H, and Y.Lu acquired the funding. Y.Li and L.H. wrote the manuscript with the feedback from all authors.
Funding
This research was supported by the National Natural Science Foundation of China (82272868 and 82173180), and the Innovation Foundation of Department of Science and Technology of Fujian (grant number: 2020Y9126), and Fujian Provincial Health Technology Project (Grant number: 2020CXA049), Ningxia Natural Science Foundation Project (grant number: 2023AAC03224).
Data availability
All data in this study are included in the article and supplementary material files.
Declarations
Ethics approval and consent to participants
The study was performed in accordance with the provisions of the Declaration of Helsinki (as revised in Edinburgh 2000) and was approved by the Ethics Committee of the School and Hospital of Stomatology, Fujian Medical University (Approval number: 2020-FMUSS-030). Informed consent was obtained by all participants. All animal procedures were conducted in accordance with the China Animal Welfare Legislation and were approved by the Ethics Committee of Fujian Medical University (Approval Number: IACUC FJMU 2021 − 0299). The study is reported in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Li and Li Huang contributed equally to this work.
Contributor Information
Dali Zheng, Email: dalizheng@fjmu.edu.cn.
Youguang Lu, Email: fjlyg63@fjmu.edu.cn.
References
- 1.Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S, Kerr AR. Oral Cancer Screening: Past, Present, and Future. J Dent Res. 2021;100(12):1313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie H, Ma Y, Li J, et al. WNT7A Promotes EGF-Induced Migration of Oral Squamous Cell Carcinoma Cells by Activating β-Catenin/MMP9-Mediated Signaling. Front Pharmacol. 2020;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36(11):1461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X, Zhang M, Xu F, Jiang S. Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities. Mol Cancer. 2020;19(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Z, Li X, Mao Y, et al. Interferon-dependent SLC14A1 + cancer-associated fibroblasts promote cancer stemness via WNT5A in bladder cancer. Cancer Cell. 2022;40(12):1550–e15657. [DOI] [PubMed] [Google Scholar]
- 7.Cevallos RR, Rodríguez-Martínez G, Gazarian K. Wnt/β-Catenin/TCF Pathway Is a Phase-Dependent Promoter of Colony Formation and Mesendodermal Differentiation During Human Somatic Cell Reprogramming. Stem Cells. 2018;36(5):683–95. [DOI] [PubMed] [Google Scholar]
- 8.Martyn I, Kanno TY, Ruzo A, Siggia ED, Brivanlou AH. Self-organization of a human organizer by combined Wnt and Nodal signalling. Nature. 2018;558(7708):132–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons MJ, Tammela T, Dow LE. WNT as a Driver and Dependency in Cancer. Cancer Discov. 2021;11(10):2413–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol. 2016;99:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Chen Z, Tang Y, Xiao Q. The involvement of noncanonical Wnt signaling in cancers. Biomed Pharmacother. 2021;133:110946. [DOI] [PubMed] [Google Scholar]
- 12.Na L, Wang Z, Bai Y, et al. WNT7B represses epithelial-mesenchymal transition and stem-like properties in bladder urothelial carcinoma. Biochim Biophys Acta Mol Basis Dis. 2022;1868(1):166271. [DOI] [PubMed] [Google Scholar]
- 13.Zheng D, Decker KF, Zhou T, et al. Role of WNT7B-induced noncanonical pathway in advanced prostate cancer. Mol Cancer Res. 2013;11(5):482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang S, Li Q, Liu Y, et al. Activation of WNT7b autocrine eases metastasis of colorectal cancer via epithelial to mesenchymal transition and predicts poor prognosis. BMC Cancer. 2021;21(1):180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nusse R, Clevers H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169(6):985–99. [DOI] [PubMed] [Google Scholar]
- 16.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26. [DOI] [PubMed] [Google Scholar]
- 17.Kayamori K, Katsube KI, Hirai H, Harada H, Ikeda T. Role of Stromal Fibroblast-Induced WNT7A Associated with Cancer Cell Migration Through the AKT/CLDN1 Signaling Axis in Oral Squamous Cell Carcinoma. Lab Invest. 2023;103(10):100228. [DOI] [PubMed] [Google Scholar]
- 18.Huang Q, Xiao Y, Lan T, Lu Y, Huang L, Zheng D. WNT7A promotes tumorigenesis of head and neck squamous cell carcinoma via activating FZD7/JAK1/STAT3 signaling. Int J Oral Sci. 2024;16(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cierpikowski P, Lis-Nawara A, Bar J. Prognostic Value of WNT1, NOTCH1, PDGFRβ, and CXCR4 in Oral Squamous Cell Carcinoma. Anticancer Res. 2023;43(2):591–602. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto T, Kawano S, Matsubara R, et al. Critical roles of Wnt5a-Ror2 signaling in aggressiveness of tongue squamous cell carcinoma and production of matrix metalloproteinase-2 via ∆Np63β-mediated epithelial-mesenchymal transition. Oral Oncol. 2017;69:15–25. [DOI] [PubMed] [Google Scholar]
- 21.Khan W, Haragannavar VC, Rao RS, Prasad K, Sowmya SV, Augustine D, Patil S. P-Cadherin and WNT5A expression in assessment of lymph node metastasis in oral squamous cell carcinoma. Clin Oral Investig. 2022;26(1):259–73. [DOI] [PubMed] [Google Scholar]
- 22.Prgomet Z, Axelsson L, Lindberg P, Andersson T. Migration and invasion of oral squamous carcinoma cells is promoted by WNT5A, a regulator of cancer progression. J Oral Pathol Med. 2015;44(10):776–84. [DOI] [PubMed] [Google Scholar]
- 23.Shiah SG, Hsiao JR, Chang WM, et al. Downregulated miR329 and miR410 promote the proliferation and invasion of oral squamous cell carcinoma by targeting Wnt-7b. Cancer Res. 2014;74(24):7560–72. [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Luo L, Xu C, et al. Tumor specificity of WNT ligands and receptors reveals universal squamous cell carcinoma oncogenes. BMC Cancer. 2022;22(1):790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Zuo X, Sun X, Tian X, Teng Y. Hexokinase 2 Promotes Cell Proliferation and Tumor Formation through the Wnt/β-catenin Pathway-mediated Cyclin D1/c-myc Upregulation in Epithelial Ovarian Cancer. J Cancer. 2022;13(8):2559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dev A Jr, Vachher M, Prasad CP. β-catenin inhibitors in cancer therapeutics: intricacies and way forward. Bioengineered. 2023;14(1):2251696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lv H, Yang J, Wang C, Yu F, Huang D, Ye L. The WNT7B protein promotes the migration and differentiation of human dental pulp cells partly through WNT/beta-catenin and c-Jun N-terminal kinase signalling pathways. Arch Oral Biol. 2018;87:54–61. [DOI] [PubMed] [Google Scholar]
- 28.Liu LJ, Lv Z, Xue X, Xing ZY, Zhu F. Canonical WNT Signaling Activated by WNT7B Contributes to L-HBs-Mediated Sorafenib Resistance in Hepatocellular Carcinoma by Inhibiting Mitophagy. Cancers (Basel). 2022;14(23):5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Liu TY, Peng HT, et al. Up-regulation of Wnt7b rather than Wnt1, Wnt7a, and Wnt9a indicates poor prognosis in breast cancer. Int J Clin Exp Pathol. 2018;11(9):4552–61. [PMC free article] [PubMed] [Google Scholar]
- 30.Arensman MD, Kovochich AN, Kulikauskas RM, et al. WNT7B mediates autocrine Wnt/β-catenin signaling and anchorage-independent growth in pancreatic adenocarcinoma. Oncogene. 2014;33(7):899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murillo-Garzón V, Gorroño-Etxebarria I, Åkerfelt M, et al. Frizzled-8 integrates Wnt-11 and transforming growth factor-β signaling in prostate cancer. Nat Commun. 2018;9(1):1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeo EJ, Cassetta L, Qian BZ, et al. Myeloid WNT7b mediates the angiogenic switch and metastasis in breast cancer. Cancer Res. 2014;74(11):2962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen S, Ding H, Wang K, Guo K. Inhibition of Wnt7b reduces the proliferation, invasion, and migration of colorectal cancer cells. Mol Biol Rep. 2023;50(2):1415–24. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Xu Y, Zhao C. Fzd7/Wnt7b signaling contributes to stemness and chemoresistance in pancreatic cancer. Cancer Med. 2021;10(10):3332–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y, Wang Z, Na L, Dong D, Wang W, Zhao C. FZD5 contributes to TNBC proliferation, DNA damage repair and stemness. Cell Death Dis. 2020;11(12):1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dijksterhuis JP, Baljinnyam B, Stanger K, et al. Systematic mapping of WNT-FZD protein interactions reveals functional selectivity by distinct WNT-FZD pairs. J Biol Chem. 2015;290(11):6789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimura A, Toyoda T, Iwasaki M, Hirama R, Osafune K. Combined Omics Approaches Reveal the Roles of Non-canonical WNT7B Signaling and YY1 in the Proliferation of Human Pancreatic Progenitor Cells. Cell Chem Biol. 2020;27(12):1561–e15727. [DOI] [PubMed] [Google Scholar]
- 38.Tang N, Cai X, Peng L, Liu H, Chen Y. TCP1 regulates Wnt7b/β-catenin pathway through P53 to influence the proliferation and migration of hepatocellular carcinoma cells. Signal Transduct Target Ther. 2020;5(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data in this study are included in the article and supplementary material files.
All data in this study are included in the article and supplementary material files.