Abstract
Background
This study introduces a novel surgical instrument to reduce iatrogenic nerve injuries during procedures such as carpal tunnel and ulnar nerve decompression surgery. These injuries often result from direct damage to surrounding tissues by surgical instruments, whose designs have remained largely unchanged over the past decades. The novel device is a modified surgical forceps that has a deployable surgical scalpel that runs along a groove on the forceps. This design protects important anatomical structures while allowing fast dissection and cutting of fascial layers.
Methods
The process used to develop a novel instrument included computer-aided design (CAD) modeling, 3D printing for prototyping, and the fabrication of an aluminum prototype. Biomechanical testing was performed with the novel device, iris scissors, bandage scissors, and a scalpel on an MTS Static Materials Test System. The peak force to slide-cut, number of cut attempts, and percentage cut on first attempt were compared between the prototype and traditional surgical tools. The materials cut in testing were Ace™ bandage, stockinette, and gauze. Statistical analyses were performed using Welch’s t-tests and Fisher’s exact tests.
Results
Compared to conventional bandage and iris scissors, the novel surgical instrument required significantly less force to cut through an Ace™ bandage, stockinette, and gauze (p < 0.01). The number of cuts required to transect those same materials with the novel device was comparable to that of the scalpel and bandage scissors. Additionally, while there were no differences between the novel device and the other devices for an Ace™ bandage and stockinette, the novel device tended to cut a greater percentage of gauze in one pass than did the iris scissors.
Conclusion
The novel surgical instrument designed in this study required less force compared to conventional scissors, demonstrated cutting efficiency similar to that of a scalpel blade, and had more safety features than either instrument. This study highlights the value of collaboration between biomedical engineering and orthopedic surgery departments on innovation in medical technology, through which new technologies with improved design and functionality demonstrate the potential to reduce iatrogenic injuries.
Keywords: Nerve decompression, Carpal tunnel release, Orthopedic instrument, Forceps, Scalpel
Background
Iatrogenic nerve injuries, which are injuries caused by medical intervention, inflict significant trauma on patients, leading to a range of physical and mental consequences such as sensory loss, paralysis, and pain. Moreover, they result in permanent deficits that encompass functional, psychological, and socioeconomic ramifications [1]. In addition, hospitals and surgeons may experience costs, as iatrogenic nerve injuries account for approximately 3.3% of medical liability proceedings [2].
These iatrogenic injuries are largely due to direct damage from surgical instruments [3]. Despite the risk they carry, the design of instruments used in open surgeries has remained relatively stagnant over the past few decades. Such instruments commonly used in open decompression surgeries include: scalpels, separators, dissection forceps, and surgical scissors [4–6]. Although these instruments are effective at performing decompression procedures, it is clear from the incidence of iatrogenic injuries that there is room for improvement. For instance, while a conventional pair of dissecting scissors has considerable utility, it also harbors significant drawbacks; its cutting edge gradually dulls with each use, increasing the chances of inadvertent damage to surrounding tissue [7]. It is estimated that surgical scissors can be used in up to 40 procedures before needing repair and can be repaired up to 9 times before disposal, with an estimated cost of $1.80 per use [8]. Although surgical scissors are reusable, it should be noted that the sharper the cutting edge, the quicker it will become blunt [6, 7]. Scalpels, on the other hand, are single-use with a reliably sharp edge; however, their use adjacent to vital structures can be dangerous. Plunges and slips can occur and result in damage to surrounding tissue, particularly in less experienced hands such as in academic settings [9].
One common example of a condition requiring surgical intervention, which carries the risk of iatrogenic damage due to these outdated instruments, is carpal tunnel syndrome. Carpal tunnel syndrome is the most common peripheral nerve injury, characterized by numbness or tingling in the wrist due to compression of the median nerve, and affects approximately 3% of all adults in the United States [10]. Intervention often begins with nonsurgical treatment methods, including physical therapy, corticosteroid injections, and wrist bracing [11]; however, 20% of patients do not respond to these treatments [10]. For this subset of patients, surgical intervention may be the next step of treatment, in which an orthopedic surgeon decompresses the median nerve by cutting the fascia constricting the nerve [12]. This surgery has an annual incidence of 400,000-600,000 in the United States alone [13–17]. However, nerve decompression surgery demands exceptional precision due to its complexity, consequently posing a risk of damaging surrounding tissues. In carpal tunnel surgery, there is a 0.49% incidence of iatrogenic nerve injury [18]. Many other surgical procedures may also result in iatrogenic nerve injury, including ulnar nerve decompression, which has similar surgical steps and risk factors [19].
Given the risks associated with the currently used surgical tools, a novel instrument was created that captures the utility of the scissors and the sharpness of the scalpel, while improving their safety and enhancing operative efficiency. The resulting device resembles forceps, such that tissue planes can be spread and separated so tissue can be easily cut under tension, with a channel and central groove along one arm to house and allow forward movement of a modified scalpel handle and blade (Fig. 1). This study describes the design and development of this device, and quantifies the cutting ability as compared to its gold standard counterparts in the laboratory. This force comparison is meant to demonstrate similar cutting ability to current techniques with a design that limits iatrogenic injuries.
Fig. 1.
(A) Fully assembled prototype of the novel device with the modified scalpel slider below. (B) Diagram of the prototype in use, which follows these steps: (1) Insert device beneath fascia of interest. (2) Use forceps to spread tissue until taut. (3) Push scalpel forward, along the groove. (4) Cut all the way through the tissue, repeating steps 1–3 as needed
Methods
Instrument fabrication
The instrument concept was 3D modeled using computer-aided design (CAD) software (SolidWorks® Office Premium 2007 SP3.1, SolidWorks Corporation, Concord, MA, USA). Figure 2 shows a labeled diagram of the novel device in SolidWorks.
Fig. 2.
Exploded view of novel device on SolidWorks
The SolidWorks files (.DXF) were converted into .OMX files with ProtoMAX software. A sheet of aluminum 6061 was thinned (Eisen S-3AII Vertical Mill), and the desired components were outlined (Omax ProtoMAX WaterJet). Finally, the rough cuts were further modified using a mill to allow the component pieces to appropriately mate at their respective junctions. The manufactured components included a right and left tine, a scalpel handle, and scalpel housing. A stainless-steel shoulder screw (McMaster-Carr, 94035A112) was used to unite the tines.
Biomechanical testing
Four instruments were tested, including the novel instrument, a #10 scalpel (Southmedic Inc., Barrie, Ontario, Canada), bandage scissors (Medline Industries, Northfield, IL), and iris scissors (Medline Industries, Northfield, IL) (Fig. 3). Medical supplies of varying elasticity and strength were selected as testing media (Fig. 4), and included gauze (Medline Industries, Northfield, IL), stockinette (Tetra Medical Supply Corp. Niles, IL), and Ace™ bandage (Medline Industries, Northfield, IL).
Fig. 3.
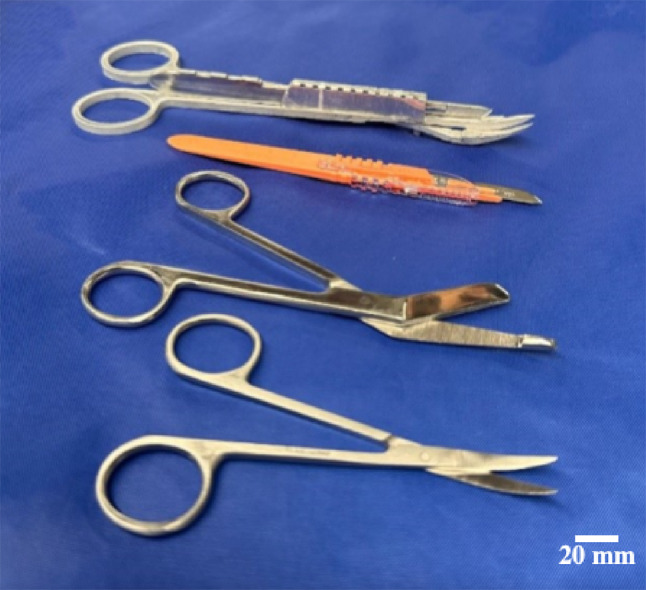
Four instruments used in biomechanical testing to determine the efficacy of the novel device. From top to bottom: novel device, scalpel, bandage scissors, and iris scissors
Fig. 4.
Three materials on which the Force, Number of Cuts, and Percentage Cut tests were conducted. From top to bottom: Ace™ bandage, stockinette, and gauze
Each material was affixed to a uniaxial testing machine (MTS Criterion C43) between two tension grips (MTS, Advantage Screw Action 2000 Grips) connected to a 1 kN load cell (MTS, LPS.103) and preloaded to 19.58 +/- 0.65 N prior to each trial. Figure 5 shows the setup and process of this testing. Three variables were measured: peak force to slide-cut by pushing with an open static tine(s), number of cut attempts to completely section the material, and percentage cut on first attempt. Five trials were performed for each combination of cutting device and material, with a new instrument or blade used for each. The peak force was defined as the difference between the maximum force measured during the trial and the minimum force measured prior to reaching the maximum force. The percentage of cut completion was measured as the length cut by the device divided by the total length of cut necessary to separate the material into two pieces. The values for percentage cut were evaluated with the following ranges: 0–24%, 25–49%, 50–74%, 75–99%, and 100% cut.
Fig. 5.
Iris scissors (A) and the novel device (B) were affixed to a uniaxial testing machine (UTS), and the performance in the Number of Cuts test was evaluated
Statistical analysis
The small sample sizes (5 trials for each device on each material) were considered when selecting applicable statistical tests. Thus, for the Force and Number of Cuts tests, Welch’s t-tests were used to analyze the performance of the novel device compared to the scalpel, iris and bandage scissors. Since the data for the Percentage Cut test were categorical, Fisher’s exact tests were performed using an online statistical calculator [20]. MATLAB (R2022b) was used for the Welch’s t-tests and for graphical presentation.
Results
Force test
The novel device required significantly less force to cut through all materials than both bandage scissors and iris scissors (p < 0.01, for each combination). Compared to the scalpel for cutting stockinette, the novel device required significantly less force (p < 0.05), but not compared to the scalpel for cutting gauze and the Ace™ bandage. For all three materials, the scalpel also required significantly less force than did the iris and bandage scissors (p < 0.01), but there was no statistically significant difference between the bandage and iris scissors. The results are shown in Fig. 6.
Fig. 6.
Peak force measured when cutting through an (A) Ace™ bandage, (B) stockinette, and (C) gauze with each of the cutting devices. * p < 0.05, ** p < 0.01
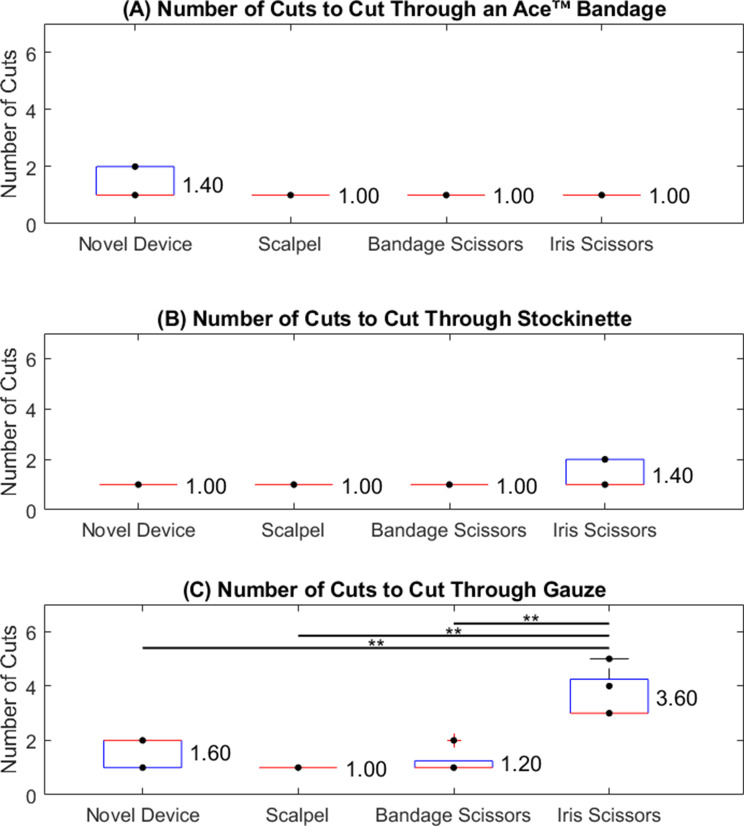
Number of cuts test
The #10 scalpel blade required the fewest cuts, consistently transecting each material by applying only one stroke. The bandage scissors averaged 1.0 cut for the stockinette and Ace™ bandage, and 1.2 cuts for the gauze. The novel instrument averaged 1.0 cut for the stockinette, 1.4 for the Ace™ bandage, and 1.6 for the gauze. The iris scissors required 1.4 cuts for the stockinette and 1 cut for the Ace™ bandage. For gauze, 3.6 attempts were required for the iris scissors, which was significantly greater than the cuts using the other instruments (p < 0.01). Figure 7 shows the results of these tests.
Fig. 7.
Number of Cuts test with each cutting device on an (A) Ace™ Bandage, (B) stockinette, and (C) gauze. **p < 0.01
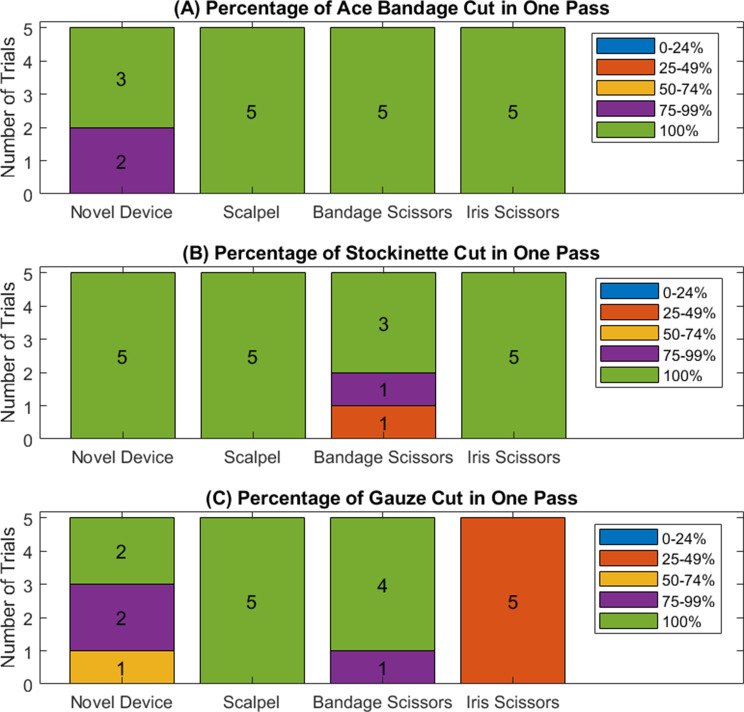
Percentage cut test
The scalpel, bandage scissors, and iris scissors were able to cut through the Ace™ bandage in one pass for every trial. The novel device cut through the Ace™ bandage in one pass, except for two instances where it cut through 75–99% of the Ace™ bandage in one pass. The scalpel, bandage scissors, and novel device cut through the stockinette in one pass for every trial. The iris scissors cut through the stockinette in one pass except for two instances, where the first pass cut through 25–50% and 75–99% of the stockinette one time each. The scalpel cut through the gauze in one pass every time. When using the bandage scissors, the gauze was cut in one pass every time except for one instance where the first cut was 75–99%. The novel device cut through the gauze completely in two instances, 75–99% in another two instances, and 50–75% in one instance. The iris scissors cut through 25–50% of the gauze for all trials. The only statistically significant difference between devices on each material was when comparing iris scissors to each of the other devices (novel device, scalpel, and bandage scissors) when used on gauze (p < 0.01 for each comparison). Figure 8 is a graphical representation of the results of this test.
Fig. 8.
Percentage of material cut by each cutting device for an (A) Ace™ bandage, (B) stockinette, and (C) gauze
Discussion
Iatrogenic injuries to peripheral neurovascular structures continue to occur while surgery is performed on the appendicular skeleton. Although surgeons are familiar with the current instrumentation and its technical use, there are few built-in safeguards available to prevent injury. The primary aim of this study was to clearly delineate the fabrication process from idea conception to a fully functional surgical instrument and evaluate its cutting ability against established benchmarks in the field. The creation of the novel surgical device was driven by the imperative to enhance the safety profile of existing open surgical instruments, while ensuring an intuitive design that seamlessly integrates with modern techniques. In this proof-of-concept biomechanical study, a method was created for collaboratively designing, iterating, and testing a new surgical instrument. The functional performance of the described novel device matches that of a scalpel blade when assessing peak cutting force with a design that limits iatrogenic injury. Furthermore, significantly less force is needed to transect the Ace™ bandage, stockinette, or gauze in comparison to both iris and bandage scissors.
The novel device created in this study was designed to serve as a hybrid instrument, functioning similarly to a tonsil for blunt dissection, while also incorporating the capabilities of a scalpel, resulting in a protected, sharp transection. By incorporating a groove into the tine for the blade to follow, the design ensures that only the area of interest is cut in a controlled manner. The groove serves the additional function of allowing the blade to cut tissue without the dulling of the blade’s edge. However, limitations do exist with this novel surgical device. Combining the forceps and scalpel components leads to a larger instrument with decreased maneuverability and flexibility during surgery as compared to free scalpel. The scalpel is constrained by the scalpel slider holder, which limits the orientation to cut only along the modified right tine; thus, it cannot be maneuvered as freely as a traditional scalpel. Therefore, this device is limited to open surgeries, and would not be useful to minimally invasive procedures. It should be noted the spreading-related motion would not be impacted by the increased height.
The functionality metrics of the novel surgical instrument, scalpel blade, bandage scissors and iris scissors were compared using common medical-grade textiles. The peak force, percentage of surface cut in one attempt, and number of cut attempts to completely transect an Ace™ bandage, stockinette and gauze were analyzed.
The peak force was evaluated to simulate the effort of a surgeon performing a sliding cut, where a lower force can lead to better control and decreased risk of plunging into unintended structures. Comparing peak force, the scalpel and novel instrument performed similarly, and both required significantly less force than either the bandage or iris scissors. A scalpel is unlikely to be used for a sliding cut anywhere other than on skin due to its limited ability to control cutting depth, thereby placing deeper structures at risk of injury. Alternatively, the novel instrument provides a fixed and controlled cut superficial to its tine making the slide cut an ideal application for its use. The larger exerted forces observed in the bandage and iris scissors may be spuriously elevated due to the quality of the disposable instruments used in this study compared to surgical grade instruments. Disposable instruments were selected to ensure a new and consistent manufactured edge for each variable tested and to allow for reproducibility. The novel surgical instrument provides a consistently sharp cut, requiring low force at a fixed and protected depth making it ideal for settings where slide cutting techniques are deployed. Furthermore, the scalpel blade on the novel instrument can be replaced for each operation.
The percentage cut was calculated by measuring the sectioned length after one attempt and was intended to reflect the working length of the instrument. Subsequently, the total number of required attempts was recorded to quantify the overall effectiveness of the instruments under the different material properties of the Ace™ bandage, stockinette and gauze. The scalpel blade cut each material’s length in one pass across 15 trials and showed its efficiency and reliability in settings where safety can be controlled externally. Similarly, the bandage scissors cut the gauze and stockinette in a single attempt and had just one instance in which the Ace™ bandage was < 100% transected in one pass. This may be due to the bandage scissors being larger and more rigid than the other instruments, allowing for highly reliable cutting function but conversely less practicality in surgical settings. The iris scissors required the greatest number of attempts and cut the least amount of the intended length across all trials compared to the other instruments; this may be a result of their narrow tines and fine tips, which are best utilized for meticulous dissection through a small window. The novel surgical device performed inferiorly to the bandage scissors but was superior to the iris scissors in terms of percentage cut and total number of attempts. The narrow tines of the novel device allow for use in settings that require fine dissection in which bandage scissors cannot operate and with improved cutting ability compared to the iris scissors.
Conclusion
This novel instrument requires less force to dissect material than do iris scissors and bandage scissors, while maintaining a cutting efficacy similar to that of a traditional scalpel. Furthermore, its design qualities allow the user to dissect tissue while safely avoiding any critical blood vessels or nerve bundles. This tool is the product of collaboration between biomedical engineers and orthopedic surgeons, resulting in a novel orthopedic instrument designed to reduce iatrogenic injuries during procedures requiring a layer-by-layer dissection approach.
Acknowledgements
The resources obtained to conduct this study were provided by the Biomedical Engineering Department at the College of Engineering, University of Wisconsin - Madison and University of Wisconsin Hospitals and Clinics. The authors would like to thank the UW BME design program, medical student Kat Tan, and BME students Madeline Johnson, Maggie Anderson, Alexis Block, Noah Hamrin, Jason Hahn, and Emma Flemmer for their initial design ideas for the project.
Author contributions
KM and JP were responsible for supervising the study. ZS, PW, MP, LF, LD, SC, SG, and KM contributed to the prototyping and testing of the novel device. MP and LF analyzed and interpreted data, using software to create figures and perform statistical tests. All authors drafted the article and approved the final manuscript.
Funding
The authors received no specific funding for this study.
Data availability
The datasets from this study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have filed for a provisional patent (TISSUE MANIPULATION AND CUTTING DEVICE AND RELATED METHODS, U.S. Patent Application No.: 63/607,999) for the novel device mentioned in the manuscript, under the University of Wisconsin-Madison and in conjunction with the Wisconsin Alumni Research Foundation.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bage T, Power DM. Iatrogenic peripheral nerve injury: a guide to management for the orthopaedic limb surgeon. EFORT Open Rev. 2021;6(8). [DOI] [PMC free article] [PubMed]
- 2.Antoniadis G, Kretschmer T, Pedro MT, König RW, Heinen C, Richter HP. Iatrogenic nerve injuries: prevalence, diagnosis and treatment. Deutsches Ärzteblatt Int. 2014. [DOI] [PMC free article] [PubMed]
- 3.Rasulić L, Savić A, Vitošević F et al. Iatrogenic peripheral nerve injuries—Surgical Treatment and Outcome: 10 years’ experience. World Neurosur. 2017;103. [DOI] [PubMed]
- 4.Chammas M, Boretto J, Burmann LM, Ramos RM, Neto FS, Silva JB. Carpal tunnel syndrome – Part II (treatment). Revis Bras Ortop. 2014;49(5). [DOI] [PMC free article] [PubMed]
- 5.Barnett SA, Shah SA, Ahmad RI. Endoscopic proximal median nerve decompression: an alternative treatment for Pronator Syndrome. J Hand Surg Global Online. 2021;3(4). [DOI] [PMC free article] [PubMed]
- 6.Siegmeth AW, Hopkinson-Woolley JA. Standard Open decompression in carpal tunnel syndrome compared with a modified open technique preserving the superficial skin nerves: a prospective Randomized Study. J Hand Surg. 2006;31(9). [DOI] [PubMed]
- 7.Cordero I. Sharpening and tightening surgical scissors. Community Eye Health. 2011;24(76). [PMC free article] [PubMed]
- 8.Rizan C, Brophy T, Lillywhite R, Reed M, Bhutta MF. Life cycle assessment and life cycle cost of repairing surgical scissors. Int J Life Cycle Ass. 2022;27(6).
- 9.Reddy, Narra G. Preventable errors: never events. J Evol Med Dent Sci. 2014;5(29).
- 10.Viera AJ. Management of carpal tunnel syndrome. Am Fam Physician. 2003;68(2). [PubMed]
- 11.Carlson H, Colbert A, Frydl J, Arnall E, Elliot M, Carlson N. Current options for nonsurgical management of carpal tunnel syndrome. Int J Clin Rheumatol. 2010;5(1). [DOI] [PMC free article] [PubMed]
- 12.Aslani HR, Alizadeh K, Eajazi A et al. Comparison of carpal tunnel release with three different techniques. Clin Neurol Neurosurg. 2012;114(7). [DOI] [PubMed]
- 13.Padua L, Cuccagna C, Giovannini S, Coraci D, Pelosi L, Loreti C et al. Carpal tunnel syndrome: updated evidence and new questions. Lancet Neurol. 2023;22(3). [DOI] [PubMed]
- 14.Cranford CS, Ho JY, Kalainov DM, Hartigan BJ. Carpal Tunnel Syndrome. J Am Acad Orthop Surg. 2007;15(9). [DOI] [PubMed]
- 15.Schmid AB, Fundaun J, Tampin B. Entrapment neuropathies: a contemporary approach to pathophysiology, clinical assessment, and management. International Association for the Study of Pain. 2020;5(4). [DOI] [PMC free article] [PubMed]
- 16.Fajardo M, Kim SH, Szabo RM. Incidence of Carpal Tunnel Release: Trends and Implications Within the United States Ambulatory Care Setting. J Hand Surg. 2012;37(8). [DOI] [PubMed]
- 17.Shin EK. Endoscopic Versus Open Carpal tunnel release. Curr Rev Musculoskelet Med. 2019;12(4). [DOI] [PMC free article] [PubMed]
- 18.Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of Endoscopic and Open Carpal Tunnel Release. The Journal of Arthroscopic & Related Surgery. 2006;22(9). [DOI] [PubMed]
- 19.Shulman B, Bekisz J, Lopez C, Maliha S, Mahure S, Hacquebord J. The Association between Concomitant Ulnar Nerve Compression at the elbow and carpal tunnel syndrome. American Association for Hand Surgery. 2020;15(3). [DOI] [PMC free article] [PubMed]
- 20.Vasavada N. Fisher’s Test for Exact Count Data. 2016. https://astatsa.com/FisherTest/Result/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets from this study are available from the corresponding author upon request.