Abstract
Background
A wide variety of diseases mimic inflammatory bowel disease (IBD). This study aimed to reduce the misdiagnosis among children with colonic ulcers.
Methods
Eighty-six pediatric patients with colonic ulcers detected by colonoscopy were enrolled in the retrospective study. Children were divided into different groups according to the final diagnosis. The clinical characteristics, laboratory examinations, endoscopic findings, and histopathological results were compared.
Results
IBD (n = 37) was just responsible for 43% of patients with colonic ulceration. Other diagnosis included autoimmune diseases (n = 9), infectious enteritis (n = 13), gastrointestinal allergy (n = 8), and other diseases (n = 19). Comparing IBD and non-IBD groups, children with IBD had a higher frequency of symptoms like weight loss/failure to thrive (P < 0.001), perianal lesions (P = 0.001), and oral ulcers (P = 0.022), and higher expression levels of platelet (P = 0.006), neutrophil-to-lymphocyte ratio (NLR) (P = 0.001), erythrocyte sedimentation rate (P < 0.001), C-reactive protein (P < 0.001), Immunoglobulin G (P = 0.012), Interleukin-1β (P = 0.003), Interleukin-6 (P = 0.024) and TNF-α (P = 0.026), and a wider ulcer distribution in the lower gastrointestinal tract (LGIT) (P < 0.001). Expression levels of hemoglobin (P < 0.001) and albumin (P = 0.001) were lower in IBD patients. Multivariate analysis showed hemoglobin, NLR, Score of ulceration in LGIT, and pseudopolyps contributing to the diagnosis of pediatric IBD with colonic ulcers.
Conclusions
We displayed potential indicators to help diagnose pediatric IBD differentiating from other disorders with colonic ulcers more prudently.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-024-05174-3.
Keywords: Colonoscopy, Ulcer, Colon, Diagnosis, Inflammatory bowel disease
Significance
What is known
• Many diseases can mimic IBD and reducing the misdiagnosis of IBD children is essential for the treatment and prognosis. Inflammatory factors like erythrocyte sedimentation rate, and fecal calprotectin have clinical value in diagnosing IBD.
What is new
• In our study, we investigated that IBD was just responsible for 43% of patients with colonic ulceration.
• Some potential indicators (like hemoglobin, neutrophil-to-lymphocyte ratio and ulcer distribution character) may contribute to the diagnosis of pediatric IBD with colonic ulcers.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-024-05174-3.
Introduction
Colonic ulcerations are a kind of localized defect of mucosal surface tissue found in colon and rectum [1]. The etiology of colonic ulceration is multiple, including inflammatory bowel disease (IBD), infectious colitis, allergic colitis, et al. [2–5]. A differential diagnosis is required for further treatment and prognosis evaluation [6]. It is challenging, and misdiagnosis is not rare in clinical practice. Many diseases (including infection, drug-induced disease, vascular disorders, diverticular disease, diversion proctocolitis, and immune disorders) can mimic IBD in clinical and pathological features [7]. Inflammatory factors like erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and fecal calprotectin (FCP) have been found for potential clinical diagnosis value with IBD but still need higher specificity [8, 9]. In most cases, acute infectious colitis is easily identified by clinical symptoms with no requirement for colonoscopy. But the patients with negative findings in pathogen tests, who present gastrointestinal bleeding, respond poorly to treatment, or have previous chronic gastrointestinal disorders, may undergo colonoscopic examination and confront the complex differential diagnosis [10]. Gastrointestinal-involved vasculitis, such as Immunoglobulin A (IgA) vasculitis and systemic lupus erythematosus (SLE), could be very similar to IBD in the symptoms, endoscopic findings, and histological assessment. European Crohn’s and Colitis Organisation (ECCO) recommended some features to help in the differential diagnosis between primary vasculitis and IBD [7]. The imaging, serological, fecal, and molecular tests could assist differentiation.
The manifestation of IBD in the pediatric population is more complicated and less specific than in adults for a higher ratio of genetic factors involved. IBD is diagnosed based on a comprehensive analysis of clinical manifestations, endoscopic examination, histopathological examination, and needed to distinguish from diseases like intestinal infectious diseases, and intestinal lesions of autoimmune diseases. Therefore, it is prone to be overdiagnosed in patients with colonic ulceration [1, 2, 11]. Pediatricians should also pay attention to gastrointestinal allergic diseases, which are more prevalent in children and increasing [12]. The data focused on the pediatric population is limited. To avoid diagnostic pitfalls, we observed the colonoscopy findings of ulcer features and combined them with the clinical manifestations and biochemical indexes of patients for the further recognition of related diseases, especially between IBD and non-IBD diseases. We summarized the differential manifestations of patients in the study and hoped to provide an alert for the misdiagnosis of children with colonic ulceration.
Materials and methods
Patients and study design
From December 2014 to June 2022, 86 pediatric patients (aged from 1 month to 15 years, and male: female = 42: 44) were analyzed in the study from Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. Inpatients with colonic ulceration discovered by colonoscopy for the first time were eligible for the inclusion criteria. The patients with ulcers isolated in rectum were excluded. All children had relatively complete medical history records.
Demographic parameters, clinical characteristics, and laboratory examinations were collected from the medical record retrieval system. Demographic parameters included age and gender. Clinical characteristics harbored weight loss/failure to thrive, abdominal pain, diarrhea, constipation, hematochezia, fever, perianal lesions, rash, oral ulcer, and joint pain. The colonoscopy findings were analyzed retrospectively in combination with histopathological results. We evaluated all the results of colonoscopies based on a complete series of pictures (at least 3 figures in every part: the terminal ileum, ileocecal part, ascending colon, transverse colon, descending colon, sigmoid colon, and rectum) and the written results. Most patients have complete video recordings. Furthermore, we divided the lower gastrointestinal tract (LGIT) into terminal ileum, ileocecal part, ascending colon, transverse colon, descending colon, sigmoid colon, and rectum; every part with ulceration meant one score, and then we accumulated total score. The etiology was determined by clinical manifestation, examination, and long-term follow-up (one year at least). The IBD was diagnosed after excluding infectious, allergic, immunologic and other diseases. When genetic mutation associated with the primary immune deficiencies was detected, we diagnosed those patients as monogenic IBD-like disorders but not ulcerative colitis (UC) or Crohn’s disease (CD) in this study [7]. For children with unclear pathogens who have experienced acute gastroenteritis, were given empirical medication; infectious enteritis is considered if there is no symptom recurrence during follow-up (within 3–6 months). Nonspecific colonic ulcer was diagnosed in the patients who ruled out other diseases and recovered well without any treatment during the follow-up.
Statistical analysis
All data were analyzed using SPSS 26.0 (SPSS, Inc., IBM, Chicago, Illinois, USA). The measurement data were expressed using a median with an interquartile range (IQR). The enumeration data were presented by frequency or proportions. Mann–Whitney non-parametric test, Pearson’s chi-squared test or Fisher’s exact test were used for the comparison between different groups. A P value < 0.05 was defined as the statistical significance. Then, Backward: likelihood ratio test was used to select the most useful predictive features from the remaining features. Receiver operating characteristic (ROC) curve analysis was used to further identify these features with clinical significance.
Results
The diagnosis of children finding colonic ulcer
Eighty-six pediatric patients finding colonic ulcers were enrolled in the research. Etiological analysis of patients was shown in Table 1. Of note, the most common diagnosis was IBD (37/86), including UC (8/86, 9.3%), CD (22/86, 25.6%), and monogenic IBD-like disorders (7/86, 8.1%) with the mutation of IL10RA, EP300 mutation, PIK3CD mutation, Wiskott-Aldrich Syndrome, and immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, respectively. Otherwise, there were autoimmune diseases (6 cases of IgA vasculitis (7.0%), 2 cases of other vasculitis (2.3%), and 1 case of SLE (1.2%), respectively), and gastrointestinal allergy (2 cases of food protein-induced enterocolitis syndrome (FPIES) (2.3%), 3 cases of eosinophilic gastroenteritis (3.5%), and 3 cases of other gastrointestinal allergy diseases (3.5%), respectively). A high ratio of children was also diagnosed with infectious enteritis (13/86, 15.1%). The etiology harbored Mycobacterium tuberculosis, Salmonellae, Campylobacter jejuni, and opportunistic pathogens (Clostridium difficile, Candida albicans, and Klebsiella pneumoniae), associated with antibiotic use. Other causes of colonic ulcers included nonspecific colonic ulcers (14/86, 16.3%), neonatal necrotizing enterocolitis (NEC) (1, 1.2%), Hirschsprung’s disease (1, 1.2%), Langerhans cell histiocytosis (LCH) (1, 1.2%), digestive perianastomotic ulcerations (1, 1.2%), and anal fistula (1, 1.2%).
Table 1.
Etiological analysis of patients with colonic ulceration
| Etiology | Number (n = 86) n (%) |
|---|---|
| IBD ( n = 37) | |
| *UC | 8 (9.3%) |
| *CD | 22 (25.6%) |
| Monogenic IBD-like disorders | 7 (8.1%) |
| Non-IBD (n = 49) | |
| Autoimmune diseases | |
| Vasculitis | |
| IgA vasculitis (Henoch-Schönlein purpura) | 6 (7.0%) |
| Other vasculitis | 2 (2.3%) |
| SLE | 1 (1.2%) |
| Infectious enteritis | 13 (15.1%) |
| Mycobacterium tuberculosis | 2 (2.3%) |
| Salmonellae | 3 (3.5%) |
| Campylobacter jejuni | 1 (1.2%) |
| Clostridium difficile | 1 (1.2%) |
| Candida albicans | 1 (1.2%) |
| Klebsiella pneumoniae | 1 (1.2%) |
| Unknown | 4 (4.7%) |
| Gastrointestinal allergy | |
| FPIES | 2 (2.3%) |
| Eosinophilic gastroenteritis | 3 (3.5%) |
| Other gastrointestinal allergy | 3 (3.5%) |
| Others | |
| Nonspecific colonic ulcer | 14 (16.3%) |
| NEC | 1 (1.2%) |
| Hirschsprung’s disease | 1 (1.2%) |
| LCH | 1 (1.2%) |
| Digestive perianastomotic ulcerations | 1 (1.2%) |
| Anal Fistula | 1 (1.2%) |
IBD = Inflammatory bowel disease, *UC = Ulcerative colitis with no monogenic abnormality, *CD = Crohn’s disease with no monogenic abnormality, SLE = Systemic lupus erythematosus, FPIES = Food protein-induced enterocolitis syndrome, NEC = Necrotizing enterocolitis, LCH = Langerhans cell histiocytosis
Comparison of clinical characteristics in patients with colonic ulceration
Children were divided into IBD (n = 37) and non-IBD (n = 49) groups for further exploring the potential specific indicators of IBD. Statistical analysis displayed no significant difference between IBD and non-IBD groups in age and gender. The frequency of weight loss/failure to thrive (P < 0.001), perianal lesions (P = 0.001), and oral ulcer (P = 0.022) was significantly higher in IBD group than in non-IBD group (Table 2). The frequency of fever is higher in IBD group (18, 48.6%) than in non-IBD group (14, 28.6%), even though there was no significant difference between two groups (P = 0.057).
Table 2.
Comparison of characteristics of IBD and non-IBD patients
| Events | IBD (n = 37), n (%) |
Non-IBD (n = 49), n (%) |
P value | |
|---|---|---|---|---|
| Age, y, | 0.115 | |||
| Age < 2 y | 8 (21.6%) | 11 (22.4%) | ||
| 2 y ≤ age < 6 y | 3 (8.1%) | 9 (18.4%) | ||
| 6 y ≤ age < 10 y | 8 (21.6%) | 16 (32.7%) | ||
| Age ≥ 10 y | 18 (48.6%) | 13 (26.5%) | ||
| Gender (Male/Female) |
16/21 (43.2%/56.8%) |
26/23 (53.1%/46.9%) |
0.367 | |
| Abnormal findings | ||||
| Weight loss/failure to thrive | 16 (43.2%) | 6 (12.2%) | < 0.001*** | |
| Abdominal pain | 17 (45.9%) | 33 (67.3%) | 0.046* | |
| Diarrhea | 19 (51.4%) | 20 (40.8%) | 0.331 | |
| Constipation | 4 (10.8%) | 6 (12.2%) | 1.000 | |
| Hematochezia | 15 (40.5%) | 25 (51.0%) | 0.335 | |
| Fever | 18 (48.6%) | 14 (28.6%) | 0.057 | |
| †Perianal lesions | 13 (35.1%) | 3 (6.1%) | 0.001** | |
| Rash | 7 (18.9%) | 8(16.3%) | 0.754 | |
| Oral ulcer | 7 (18.9%) | 1 (2.0%) | 0.022* | |
| Joint pain | 4 (10.8%) | 2 (4.1%) | 0.432 | |
IBD = Inflammatory bowel disease
†Perianal lesions = skin tag, perianal abscess, anal fistula, or anal fissure
*P < 0.05, **P < 0.01, ***P < 0.001
Then, we further divided children into IBD (UC or CD with no monogenic abnormality), monogenic IBD-like disorders and non-IBD groups (Table S1 – S2). Statistical analysis showed that the age of children with monogenic IBD-like disorders is significantly younger than children in IBD or non-IBD groups (P < 0.001).
Comparison of biochemical indexes in patients with colonic ulceration
The biochemical indexes were then analyzed in different groups (Table 3, Table S3 – S4). The levels of hemoglobin (P < 0.001) and albumin (P = 0.001) were lower in IBD group than in non-IBD group, while the levels of platelet (P = 0.006), neutrophil-to-lymphocyte ratio (NLR) (P = 0.001), ESR (P < 0.001), CRP (P < 0.001), Immunoglobulin G (IgG) (P = 0.012), and Interleukin-1β (IL-1β) (P = 0.003), IL-6 (P = 0.024) and TNF-α (P = 0.026) were significantly higher in IBD group than in non-IBD group. In addition, the level of FCP was > 1500 ug/g in 22 cases (78.6%) of IBD children and in 14 cases (58.3%) of non-IBD children (P = 0.065) (Table 3). NLR, ESR, CRP and TNF-α showed higher level in CD group than in gastrointestinal allergy group (Table S4).
Table 3.
Comparison of biochemical indexes of IBD and non-IBD patients
| Events | IBD (n = 37) |
Non-IBD (n = 49) |
P value |
|---|---|---|---|
|
Hemoglobin, g/dL, median (IQR) |
109.00, (87.50–121.00) n = 37 |
127.00, (111.50–132.50) n = 49 |
< 0.001*** |
|
Albumin, mg/dL median, (IQR) |
36.60, (31.45–41.25) n = 37 |
42.60, (37.20–45.55) n = 49 |
0.001** |
|
Platelet, 10^9/L median, (IQR) |
397, (310–521) n = 37 |
302, (258–391) n = 49 |
0.006** |
|
D-Dimer median, (IQR) |
0.55, (0.32–0.86) n = 29 |
0.315, (0.23–0.827) n = 42 |
0.124 |
|
NLR median, (IQR) |
2.49, (1.80–4.42) n = 37 |
1.41, (0.96–2.70) n = 49 |
0.001** |
|
AST, U/L median, (IQR) |
29.00, (19.85–40.50) n = 37 |
34.00, (25.35–42.15) n = 49 |
0.081 |
|
ALT, U/L median, (IQR) |
16.00, (10.00–21.00) n = 37 |
19.00, (11.00–26.00) n = 49 |
0.230 |
|
ESR, mm/h, median, (IQR) |
36.50, (20.50–64.25) n = 36 |
16.00, (2.50–26.00) n = 33 |
< 0.001*** |
| CRP, mg/L | < 0.001*** | ||
| < 8, n (%) | 10 (27.0%) | 33 (67.3%) | |
| ≥ 8, n (%) | 27 (73.0%) | 16 (32.7%) | |
|
Positive fecal transferrin, n (%) |
16 (84.2%) | 27 (90.0%) | 0.877 |
|
Positive fecal occult blood, n (%) |
21 (60.0%) | 28 (62.2%) | 0.840 |
| FCP, ug/g | 0.065 | ||
| ≤ 50, n (%) | 1 (3.6%) | 2 (8.3%) | |
| 50 < FCP ≤ 500, n (%) | 1 (3.6%) | 6 (25%) | |
| 500 < FCP ≤ 1000, n (%) | 2 (7.1%) | 2 (8.3%) | |
| 1000 < FCP ≤ 1500, n (%) | 2 (7.1%) | 0 (0.0%) | |
| > 1500, n (%) | 22 (78.6%) | 14 (58.3%) | |
| IgA, g/L | 0.279 | ||
| < 0.7, n (%) | 5 (17.9%) | 7 (26.9%) | |
| 0.7 ≤ IgA < 4, n (%) | 20 (71.4%) | 18 (69.2%) | |
| ≥ 4, n (%) | 3 (10.7%) | 1 (3.8%) | |
| IgG, g/L | 0.012* | ||
| < 7, n (%) | 2 (7.1%) | 7 (26.9%) | |
| 7 ≤ IgG < 16, n (%) | 20 (71.4%) | 18 (69.2%) | |
| ≥ 16, n (%) | 6 (21.4%) | 1 (3.8%) | |
| IL-8, pg/mL | 0.921 | ||
| < 62, n (%) | 10 (43.5%) | 9 (45.0%) | |
| ≥ 62, n (%) | 13 (56.5%) | 11 (55.0%) | |
| IL-1β, pg/mL | 0.003** | ||
| < 5, n (%) | 2 (8.7%) | 10 (50.0%) | |
| ≥ 5, n (%) | 21 (91.3%) | 10 (50.0% | |
| IL-6, pg/mL | 0.024* | ||
| < 5.9, n (%) | 4 (17.4%) | 10 (50.0%) | |
| ≥ 5.9, n (%) | 19 (82.6%) | 10 (50.0%) | |
| IL-10, pg/mL | 0.988 | ||
| < 9.1, n (%) | 15 (65.2%) | 13 (65.0%) | |
| ≥ 9.1, n (%) | 8 (34.8%) | 7 (35.0%) | |
| TNF-α, pg/mL | 0.026* | ||
| < 8.1, n (%) | 0 (0.0%) | 4 (20.0%) | |
| ≥ 8.1, n (%) | 23 (100.0%) | 16 (80.0%) | |
| IL-2R, pg/mL | 0.080 | ||
| < 710, n (%) | 3 (12.5%) | 7 (35.0%) | |
| ≥ 710, n (%) | 21 (87.5%) | 13 (65.0%) | |
| 25-(OH)-VitD, nmol/L | 0.142 | ||
| ≤ 50, n (%) | 16 (80.0%) | 6 (54.5%) | |
| > 50, n (%) | 4 (20.0%) | 5 (45.5%) |
IBD = Inflammatory bowel disease, NLR = neutrophil-to-lymphocyte ratio, AST = Alanine aminotransferase, ALT = Alanine aminotransferase, ESR = Erythrocyte sedimentation rate, CRP = C-reactive protein, FCP = Fecal calprotectin, IgA = Immunoglobulin A, IgG = Immunoglobulin G, IL-8 = Interleukin-8, IL-1β = Interleukin-1β, IL-6 = Interleukin-6, IL-10 = Interleukin-10, TNF-α = tumor necrosis factor-α, IL-2R = Interleukin-2 receptor, 25-(OH)-VitD = 25-hydroxyvitamin D. *P < 0.05, **P < 0.01, ***P < 0.001
The comparison of endoscopic characteristics in patients with colonic ulceration
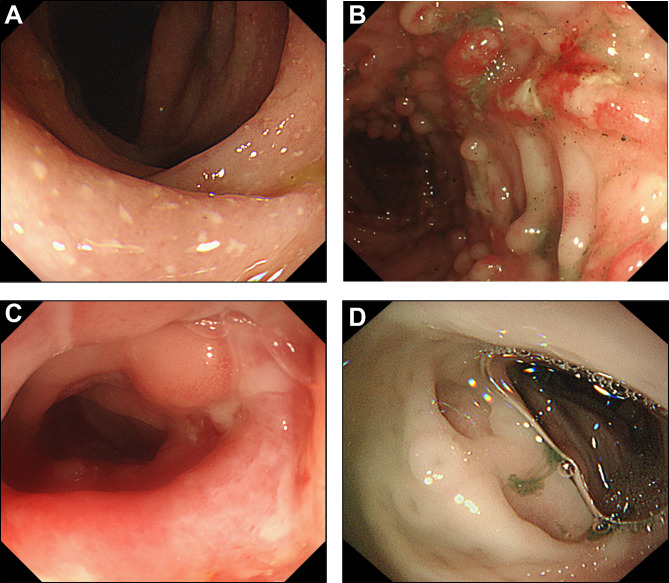
Next, we collected the endoscopic figures of patients with different diagnoses (UC, CD, monogenic IBD-like disorders and non-IBD, respectively) in Fig. 1, and sorted out the endoscopic information of patients (Table 4, Table S5 – S6). The existence of upper gastrointestinal ulceration presented no significant difference in IBD and non-IBD groups. Colonoscopy revealed that ulcers could be found in any segment of the colon, and a wider ulcer distribution range in LGIT was found in IBD group than in non-IBD group (P < 0.001), especially between CD and infectious enteritis groups. Besides, Table 4 displayed that cobblestone appearance, deep ulcer (mainly in CD group as Table S6), and pseudopolyps were mainly showed in IBD group.
Fig. 1.
Endoscopic figures of patients with different diagnosis in colon. (A) UC: multiple continuous shallow ulcers with white coating on the surface. (B) CD: Cobblestone appearance with ulceration. (C) monogenic IBD-like disorder: Ulcerative lesions covered with pus coating, and accompanied by scattered inflammatory polypoid hyperplasia around the ulcer. (D) Allergy: clear multiple ulcers
Table 4.
Comparison of endoscopic characteristics in IBD and non-IBD patients
| Events | IBD (n = 37) |
Non-IBD (n = 49) |
P value |
|---|---|---|---|
| Ulcer distribution | |||
| Upper gastrointestinal ulceration, n (%) | 9 (24.3%) | 4(10.3%) | 0.104 |
|
*Score of ulceration in LGIT, median (IQR) |
5 (2–6) |
2 (1–2) |
< 0.001*** |
| Abnormal findings | |||
| Deep ulcer, n (%) | 16 (43.2%) | 5 (10.2%) | < 0.001*** |
| Pseudopolyps, n (%) | 10 (27.0%) | 2 (4.1%) | 0.004** |
| Bleeding changes, n (%) | 7 (18.9%) | 10 (20.4%) | 1.000 |
| Erythema, n (%) | 1 (2.7%) | 3 (6.1%) | 0.631 |
| Cobblestone appearance, n (%) | 4 (10.2%) | 0 (0.0%) | 0.031* |
| Narrow, n (%) | 3 (8.1%) | 2 (4.1%) | 0.648 |
| Distortion of ileocecal valve, n (%) | 3 (8.1%) | 0 (0.0%) | 0.076 |
IBD = Inflammatory bowel disease, * Score of ulceration in LGIT: We divided the lower gastrointestinal tract (LGIT) into terminal ileum, ileocecal part, ascending colon, transverse colon, descending colon, sigmoid colon and rectum; every part with ulceration meant one score and we accumulated total score. *P < 0.05, **P < 0.01, ***P < 0.001
The comparison of histopathological results in patients with colonic ulceration
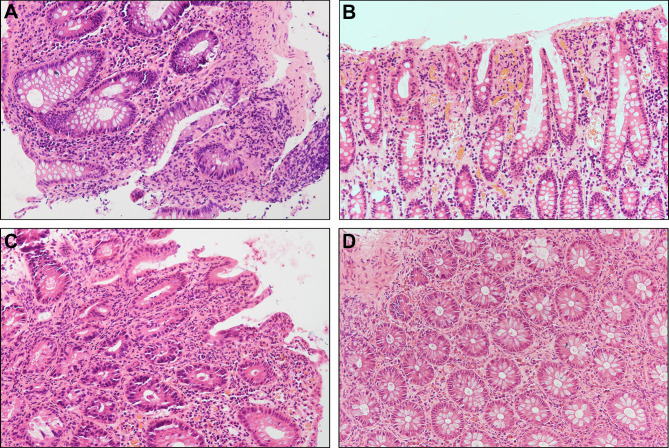
The histopathological presentations were including inflammatory cell infiltration, cryptitis, crypt abscess, granulation tissues hyperplasia, and granuloma (Fig. 2). We found IBD patients presented more pathological changes, including crypt abscess (P = 0.013), granulation tissues hyperplasia (P < 0.001), and granuloma (P = 0.016), compared to non-IBD patients (Table 5). When comparing IBD, monogenic IBD-like disorders and non-IBD groups (Table S7), only the characteristic of granulation tissues hyperplasia had statistical differences between children with monogenic IBD-like disorders (3, 42.9%) and non-IBD groups (1, 2.1%). Table S8 further displayed that CD group had significant ratio of granulation tissues hyperplasia. Meanwhile, there was no significant difference about the number of Eosinophilic granulocytes between patients with IBD and non-IBD groups (Table S9 - S10).
Fig. 2.
Representative images of H&E staining for colon tissues of patients with different diagnosis. (A) UC: formation of crypt abscess with partial gland deformation (200×). (B) CD: acute and chronic inflammatory cell aggregation (200×). (C) monogenic IBD-like disorder: infiltration of plasma cells and neutrophils, occasional crypt abscess (200×). (D) The figure of the child with allergy: numerous eosinophils (200×)
Table 5.
Comparison of histopathological results in IBD and non-IBD patients
| Events | IBD (n = 37) |
Non-IBD (n = 48) |
P value |
|---|---|---|---|
| Cryptitis, n (%) | 26 (70.3%) | 31 (64.6%) | 0.580 |
| Crypt abscess, n (%) | 13 (35.1%) | 6 (12.5%) | 0.013* |
| Granulation tissues hyperplasia, n (%) | 13 (35.1%) | 1 (2.1%) | < 0.001*** |
| Granuloma, n (%) | 9 (24.3%) | 2 (4.2%) | 0.016* |
IBD = Inflammatory bowel disease. *P < 0.05, ***P < 0.001
Multivariate logistic regression analysis of potential indicators of IBD with colonic ulceration
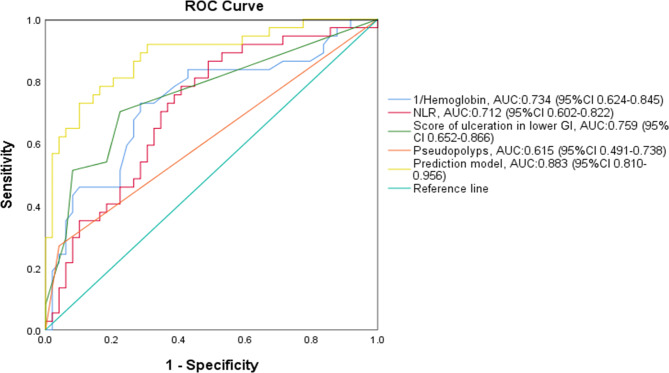
When we finished the analysis of the clinical characteristics, laboratory examinations, histopathological results, and colonoscopy results compared between the IBD and non-IBD groups, multivariate analysis on the data was made for a prediction rule for colonic ulcers in pediatric patients (Table 6). We found the hemoglobin (OR = 0.955, P = 0.002), NLR (OR = 1.368, P = 0.014), Score of ulceration in LGIT (OR = 1.866, P < 0.001), and pseudopolyps (OR = 12.605, P = 0.014) contributing to the diagnosis of pediatric IBD with colonic ulcers. Then ROC curve analysis was used in these variables (Fig. 3). Our findings showed the area under the curve (AUC) > 0.5 in NLR (0.712), Score of ulceration in LGIT (0.759), and pseudopolyps (0.615), and AUC < 0.5 in hemoglobin (0.266). Besides, we found combined diagnosis of these variables had larger AUC (0.883).
Table 6.
Multivariate logistic regression analysis of potential indicators of IBD
| Events | Regression coefficient | OR | 95%CI | P value |
|---|---|---|---|---|
| Hemoglobin | -0.046 | 0.955 | 0.928–0.983 | 0.002** |
| NLR | 0.314 | 1.368 | 1.066–1.757 | 0.014* |
| *Score of ulceration in LGIT | 0.624 | 1.866 | 1.338–2.601 | < 0.001*** |
| Pseudopolyps | 2.534 | 12.605 | 1.685–94.294 | 0.014* |
OR = Odds ratio, CI = Confidence interval, NLR = neutrophil-to-lymphocyte ratio
*Score of ulceration in LGIT: We divided the lower gastrointestinal tract (LGIT) into terminal ileum, ileocecal part, ascending colon, transverse colon, descending colon, sigmoid colon and rectum; every part with ulceration meant one score and we accumulated total score
*P < 0.05, **P < 0.01, ***P < 0.001
Fig. 3.
Receiver operating characteristic (ROC) curve analysis. The figure showed the ROC of hemoglobin (P < 0.001, the cutoff value = 118 with sensitivity of 73.0% and specificity of 71.4%), NLR (P = 0.001, the cutoff value = 1.69 with sensitivity of 78.4% and specificity of 59.2%), Score of ulceration in LGIT (P < 0.001, the cutoff value = 2.50 with sensitivity of 70.3% and specificity of 77.6%), and pseudopolyps (P = 0.070)
Discussion
With the development of endoscopic technology, colonic ulcers were found in children with different diagnosis. Due to the particular stage of growth and development in children and different disease spectrum from adults, it is essential to do early differential diagnosis and provide precise treatment. Our study displayed some potential indicators for the help of the diagnosis of pediatric IBD differentiating from other disorders with colonic ulcers, though we need larger sample size to confirm the findings.
In the total of 86 pediatric patients found with ulcers by colonoscopy, the most common diagnosis was IBD. Early-onset IBD patients (< 10 years), especially very early-onset IBD (VEO-IBD) patients (< 6 years), present a closer relationship with genetic mutations [13]. We defined VEO-IBD patients as monogenic IBD-like disorders when they have a monogenic mutation according to ECCO [7]. In our study, 3 cases were with the mutation of IL10RA, which was of high frequency in monogenic IBD-like disorders, and the conventional therapy of IBD has poor efficacy, even with haematopoietic stem cell transplantation [14, 15]. Four cases were also with genetic abnormality, including EP300 mutation, PIK3CD mutation, Wiskott-Aldrich Syndrome, and IPEX syndrome, respectively. These children need early gene detection to confirm the diagnosis for the severe disease progression [16]. Autoimmune diseases had 9 cases, mainly including IgA vasculitis (6/86). IgA vasculitis is one of the most frequent vasculitides in childhood and had similar gastrointestinal manifestations (abdominal pain, hematochezia) with IBD, which needs care for the prognosis [17]. A total of 8 cases of gastrointestinal allergy were found in our study, and the disease occurs frequently in children. Nambu et al. have found the most common diagnoses except for IBD in children who performed colonoscopies, were eosinophilic gastrointestinal disorders, which need food avoidance [18]. Our findings showed that the levels of NLR, ESR, CRP, and TNF-α may distinguish CD and gastrointestinal allergy. Furthermore, a high proportion of children had intestinal ulcers for infectious enteritis (13/86), which may alert us to strictly control the indications of colonoscopy examination. The level of ESR was also higher in CD group than infectious enteritis group.
We mainly focused on comparing IBD with other diseases and the differences may help to increase the differential diagnosis of disease profile. The results displayed that the age of IBD group was mainly ≥ 10 years (60.0%), while the age of children with monogenic IBD-like disorders was mainly < 2 years (85.7%), which was consistent with previous research [19]. Clinical symptoms of children with colonic ulcers, weight loss/failure to thrive, perianal lesions, and oral ulcers had a higher frequency in IBD children than in non-IBD children. These abnormal findings fulfilled the criteria of clinical features in IBD [20, 21]. Our findings showed less abdominal pain in IBD children. The cause indicates that the mild pain was easily neglected due to other serious complaints in this retrospective study. And abdominal pain is a subjective feeling while children may lack correct expression.
Laboratory examinations presented lower hemoglobin and albumin expression levels in the IBD group than in the non-IBD group. These were typically elevated in active disease while mild IBD could show normal results in routine blood tests [21]. Sabery et al. found anemia as a predictor for IBD was 83% sensitivity, and albumin is also a sensitive maker when combined with inflammatory indexes like CRP or ESR [8, 9]. The intestinal ulcer lesions may contribute to the condition that IBD children are more susceptible to anemia than other diseases. Our multivariate analysis also identified lower hemoglobin with sensitivity of 73.0% and specificity of 71.4%. Platelet aggregation at the colonic mucosae was common in colitis and IBD [22], and our findings of the high level of platelet and D-Dimer in IBD children (age ≥ 6 y) indicated the IBD children had an increased risk for thrombus development than children with other colitis (Table S3). As a marker of reflecting systemic inflammation, NLR has been found to be significantly increased at diagnosis and active disease of IBD children compared with healthy subjects [23–25]. Our results further found that NLR was also considerably higher in IBD group than in non-IBD group, even though all patients had colonic ulceration. When NLR reaching the value of 1.69 (sensitivity of 78.4% and specificity of 59.2%) in a child with colonic ulcerations, we may need to consider IBD.
The expression levels of ESR and CRP were still significantly higher in IBD group. These markers were relatively sensitive and specific to distinguish IBD (especially CD) from other diseases in children with ulcers in colon. Besides, FCP is a kind of protein from neutrophils, considered as a noninvasive and valuable marker of intestinal inflammation [26, 27]. We found that the expression levels of FCP between two groups did not show statistical differences (P = 0.065). FCP in both IBD (78.6%) and non-IBD patients (58.3%) was mainly > 1500 ug/g. The reason indicates that young children have a higher baseline value of FCP compared with normal adults [27]. In addition, the research object in our study were all children with colonic ulceration, whose intestinal inflammation was relatively severe. Herein, FCP may not be sensitive to distinguishing IBD among children with colonic ulcerations.
Otherwise, IgG was also at a higher level in IBD group. IBD is a disorder closely related to immune reaction. IgG was predicted to be associated with IBD severity and response to gut microbiota [28]. Pro-inflammatory factors including IL-1β, and TNF-α, were increasing the expression level in IBD group. The finding indicates that IL-1β may be a potential specific marker of IBD in pediatric patients. The upstream mechanisms and signal pathways of IL-1β may be interesting perspectives of pathogeny which identify IBD from colonic ulcerative diseases, like mediating NLRP3 activation which regulates the generation and maturation of IL-1β [29–31]. In addition, anti-TNF-α drugs have been widely used in treating children with IBD [32]. Anti- IL-1β may also be an effective therapeutic target in IBD patients in the future.
Colonoscopy revealed statistical differences in ulcer distribution between IBD and non-IBD groups. The discrepancy in ulcer distribution range may help to distinguish IBD and non-IBD with colonic ulceration, especially between CD and infectious enteritis. ROC curve analysis indicated that when the ulcer affects more than 2.5 parts (≥ 3 parts in fact) of the intestinal segments, there is a possibility of diagnosis as IBD with sensitivity of 70.3% and specificity of 77.6%. This still needs to be confirmed by increasing the sample size. Deep ulcer (mainly in CD), and pseudopolyps were also more found in IBD group. Otherwise, cobblestone appearance was only found in IBD group, which fulfilled the criteria of endoscopic characteristics in CD [20]. Histopathological results play essential roles in the different diagnoses of intestinal disorders [21]. The characteristics of mucosal tissue with hematoxylin & eosin staining included cryptitis, crypt abscess, granulation tissues hyperplasia, granuloma, lymphocyte proliferation, and eosinophil infiltration. The histological phenotype is hardly distinguished for the overlapping in morphological aspects between IBD and non-IBD patients [10]. In children with colonic ulcers, we found the significant differences in the change of crypt abscess, granulation tissues hyperplasia, and granuloma between IBD and non-IBD groups. Granuloma is a kind of local change of chronic inflammation, which could be observed typically in CD [21]. We did not see significant eosinophil infiltration in IBD (even monogenic IBD-like disorders) or Infectious enteritis groups. Characteristic pathological changes still need further exploration in other intestinal disorders.
We still have some limitations in the research. In our IBD group, there were mainly CD children (n = 22). The sample size of UC is relatively small and still needs to be expanded. A larger sample size is necessary to further verify our findings and reduce bias in data analysis.
Conclusion
In conclusion, the etiologies caused by colonic ulcers are complex and diverse, and primary diseases are IBD, infectious enteritis, autoimmune diseases, and gastrointestinal allergy. Hemoglobin, albumin, NLR, ESR, and CRP could be considered essential reference indicators for CD in children with colonic ulcers. Platelet, IgG, IL-1β, IL-6, and TNF-α may be potential indicators. The sensitivity and specificity still need a large sample size to verify these indexes. Joint monitoring of hemoglobin, NLR, ulcer distribution range, and pseudopolyps may have clinical significance in identifying IBD from other disease when children with colonic ulcers. The differences found in clinical characteristics, laboratory examinations, histopathological results and endoscopic findings of IBD and non-IBD groups in the study are hoped to help further understand intestinal diseases and improve the diagnosis in children with colonic ulcers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Alanine aminotransferase
- AUC
Area under the curve
- CD
Crohn’s disease
- CI
Confidence interval
- CRP
C-reactive protein
- ECCO
European Crohn’s and Colitis Organisation
- ESR
Erythrocyte sedimentation rate
- FCP
Fecal calprotectin
- FPIES
Food protein-induced enterocolitis
- LGIT
Lower gastrointestinal tract
- IBD
Inflammatory bowel disease
- Ig
Immunoglobulin
- IL
Interleukin
- IPEX syndrome
Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome
- IQR
Interquartile range
- LCH
Langerhans cell histiocytosis
- NEC
Neonatal necrotizing enterocolitis
- NLR
Neutrophil-to-lymphocyte ratio
- NLRP3
NOD-like Receptor Family Pyrin Domain Containing 3
- OR
Odds ratio
- ROC
Receiver operating characteristic
- SLE
Systemic lupus erythematosus
- TNF-α
Tumor necrosis factor-α
- UC
Ulcerative colitis
- VEO-IBD
Very early-onset IBD
- 25-(OH)-VitD
25-hydroxyvitamin D
Author contributions
W. Y. and Y. W. developed the study design. Y. Y. and Y. T. collected the data and wrote the manuscript. Y. C., H. F., and Q. W. analyzed the data. W. Y. and Y. W. also reviewed and modified the manuscript. All of the authors approved the manuscript to be published.
Funding
This study was supported by National Natural Science Foundation of China (81701486), and Foundation of Science and Technology Commission of Shanghai Municipality (16ZR1428400).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
In accordance with the Declaration of Helsinki, the study was approved by the Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (XHEC-D-2022-250). Written informed consent was obtained from the parents of all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yaying You and Yijing Tao contributed equally to this work.
Contributor Information
Ying Wang, Email: wangying_ssmu@126.com.
Weihui Yan, Email: yanweihui@xinhuamed.com.cn.
References
- 1.Edden Y, Shih SS, Wexner SD. Solitary rectal ulcer syndrome and stercoral ulcers. Gastroenterol Clin North Am. 2009;38:541–5. [DOI] [PubMed] [Google Scholar]
- 2.Cai J, Li F, Zhou W, Luo HS. Ileocecal ulcer in central China: case series. Dig Dis Sci. 2007;52:3169–73. [DOI] [PubMed] [Google Scholar]
- 3.Lee YJ, Yang SK, Byeon JS, Myung SJ, Chang HS, Hong SS, Kim KJ, Lee GH, Jung HY, Hong WS, Kim JH, Min YI, Chang SJ, Yu CS. Analysis of colonoscopic findings in the differential diagnosis between intestinal tuberculosis and Crohn’s disease. Endoscopy. 2006;38:592–7. [DOI] [PubMed] [Google Scholar]
- 4.Yazici Y, Hatemi G, Bodaghi B, Cheon JH, Suzuki N, Ambrose N, Yazici H. Behcet syndrome. Nat Rev Dis Primers. 2021;7:67. [DOI] [PubMed] [Google Scholar]
- 5.Turner D, Levine A, Escher JC, Griffiths AM, Russell RK, Dignass A, Dias JA, et al. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. J Pediatr Gastroenterol Nutr. 2012;55:340–61. [DOI] [PubMed] [Google Scholar]
- 6.Kaenkumchorn T, Wahbeh G. Ulcerative colitis: making the diagnosis. Gastroenterol Clin North Am. 2020;49:655–69. [DOI] [PubMed] [Google Scholar]
- 7.Feakins R, Torres J, Borralho-Nunes P, Burisch J, Cúrdia Gonçalves T, De Ridder L, Driessen A, Lobatón T, Menchén L, Mookhoek A, Noor N, Svrcek M, Villanacci V, Zidar N, Tripathi M. ECCO topical review on clinicopathological spectrum and differential diagnosis of inflammatory bowel disease. J Crohns Colitis. 2022;16:343–68. [DOI] [PubMed] [Google Scholar]
- 8.Sabery N, Bass D. Use of serologic markers as a screening tool in inflammatory bowel disease compared with elevated erythrocyte sedimentation rate and anemia. Pediatrics. 2007;119:e193–199. [DOI] [PubMed] [Google Scholar]
- 9.Holtman GA, Lisman-van Leeuwen Y, Reitsma JB, Berger MY. Noninvasive tests for inflammatory bowel disease: a meta-analysis. Pediatrics. 2016;137. [DOI] [PubMed]
- 10.Reggiani Bonetti L, Leoncini G, Daperno M, Principi MB, Baronchelli C, Manenti S, Caprioli F, Armuzzi A, Caputo A, Parente P, Cadei M, Villanacci V. Histopathology of non-IBD colitis practical recommendations from pathologists of IG-IBD group. Dig Liver Dis. 2021;53:950–7. [DOI] [PubMed] [Google Scholar]
- 11.Cai L, Chen Y, Xiao SY. Clinicopathologic features of chronic intestinal schistosomiasis and its distinction from Crohn disease. Am J Surg Pathol. 2021;45:430–8. [DOI] [PubMed] [Google Scholar]
- 12.Sicherer SH, Warren CM, Dant C, Gupta RS, Nadeau KC. Food allergy from infancy through adulthood. J Allergy Clin Immunol Pract. 2020;8:1854–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uhlig HH, Schwerd T, Koletzko S, Shah N, Kammermeier J, Elkadri A, Ouahed J, Wilson DC, Travis SP, Turner D, Klein C, Snapper SB, Muise AM, Group CiIS, Neopics. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014;147:990–1007 e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almana Y, Mohammed R. Current concepts in pediatric inflammatory bowel disease; IL10/IL10R colitis as a model disease. Int J Pediatr Adolesc Med. 2019;6:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye Z, Qian L, Hu W, Miao S, Wang Y, Lu J, Zhou Y, Lu X, Zhang Y, Zheng C, Sun H, Tang W, Tang Z, Sun S, Dong K, Qian X, Zhai X, Huang Y. Clinical outcome of infantile-onset inflammatory bowel disease in 102 patients with interleukin-10 signalling deficiency. Aliment Pharmacol Ther. 2022. [DOI] [PubMed]
- 16.Ouahed J, Spencer E, Kotlarz D, Shouval DS, Kowalik M, Peng K, Field M, Grushkin-Lerner L, Pai SY, Bousvaros A, Cho J, Argmann C, Schadt E, McGovern DPB, Mokry M, Nieuwenhuis E, Clevers H, Powrie F, Uhlig H, Klein C, Muise A, Dubinsky M, Snapper SB. Very early onset inflammatory bowel disease: a clinical approach with a focus on the role of genetics and underlying immune deficiencies. Inflamm Bowel Dis. 2020;26:820–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozen S, Sag E. Childhood vasculitis. Rheumatology (Oxford). 2020;59:iii95–100. [DOI] [PubMed] [Google Scholar]
- 18.Nambu R, Hagiwara SI, Kakuta F, Hara T, Shimizu H, Abukawa D, Iwama I, Kagimoto S, Arai K. Current role of colonoscopy in infants and young children: a multicenter study. BMC Gastroenterol. 2019;19:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuenzig ME, Fung SG, Marderfeld L, Mak JWY, Kaplan GG, Ng SC, Wilson DC, Cameron F, Henderson P, Kotze PG, Bhatti J, Fang V, Gerber S, Guay E, Kotteduwa Jayawarden S, Kadota L, Maldonado DF, Osei JA, Sandarage R, Stanton A, Wan M, Benchimol EI. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: systematic review. Gastroenterology. 2022;162:1147–e11591144. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira SB, Monteiro IM. Diagnosis and management of inflammatory bowel disease in children. BMJ. 2017;357:j2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, Kolho KL, Veres G, Russell RK, Paerregaard A, Buderus S, Greer ML, Dias JA, Veereman-Wauters G, Lionetti P, Sladek M, de Martin J, Staiano A, Ruemmele FM, Wilson DC. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795–806. [DOI] [PubMed] [Google Scholar]
- 22.Huang B, Chen Z, Geng L, Wang J, Liang H, Cao Y, Chen H, et al. Mucosal profiling of pediatric-onset colitis and IBD reveals common pathogenics and therapeutic pathways. Cell. 2019;179:1160–e11761124. [DOI] [PubMed] [Google Scholar]
- 23.Şimşek-Onat P, Hizarcioglu-Gulsen H, Ergen YM, Gumus E, Özen H, Demir H, Özen S, Saltık-Temizel İN. Neutrophil-to-lymphocyte ratio: an easy marker for the diagnosis and monitoring of inflammatory bowel disease in children. Dig Dis Sci. 2023;68:233–9. [DOI] [PubMed] [Google Scholar]
- 24.Fu W, Fu H, Ye W, Han Y, Liu X, Zhu S, Li H, Tang R, Wang Q. Peripheral blood neutrophil-to-lymphocyte ratio in inflammatory bowel disease and disease activity: a meta-analysis. Int Immunopharmacol. 2021;101:108235. [DOI] [PubMed] [Google Scholar]
- 25.Zahmatkesh A, Sohouli MH, Hosseini SME, Rohani P. The role of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in the diagnosis and severity of inflammatory bowel disease in children. Eur J Pediatr. 2023;182:4263–70. [DOI] [PubMed] [Google Scholar]
- 26.Ricciuto A, Griffiths AM. Clinical value of fecal calprotectin. Crit Rev Clin Lab Sci. 2019;56:307–20. [DOI] [PubMed] [Google Scholar]
- 27.Lężyk-Ciemniak E, Tworkiewicz M, Wilczyńska D, Szaflarska-Popławska A, Krogulska A. Usefulness of testing for fecal calprotectin in pediatric gastroenterology clinical practice. Med Princ Pract. 2021;30:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rengarajan S, Vivio EE, Parkes M, Peterson DA, Roberson EDO, Newberry RD, Ciorba MA, Hsieh CS. Dynamic immunoglobulin responses to gut bacteria during inflammatory bowel disease. Gut Microbes. 2020;11:405–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aschenbrenner D, Quaranta M, Banerjee S, Ilott N, Jansen J, Steere B, Chen YH, Ho S, Cox K, Arancibia-Cárcamo CV, Coles M, Gaffney E, Travis SP, Denson L, Kugathasan S, Schmitz J, Powrie F, Sansom SN, Uhlig HH. Deconvolution of monocyte responses in inflammatory bowel disease reveals an IL-1 cytokine network that regulates IL-23 in genetic and acquired IL-10 resistance. Gut. 2021;70:1023–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–9. [DOI] [PubMed] [Google Scholar]
- 31.Man SM. Inflammasomes in the gastrointestinal tract: infection, cancer and gut microbiota homeostasis. Nat Rev Gastroenterol Hepatol. 2018;15:721–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peyrin-Biroulet L, Deltenre P, de Suray N, Branche J, Sandborn WJ, Colombel JF. Efficacy and safety of tumor necrosis factor antagonists in Crohn’s disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2008;6:644–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.