Abstract
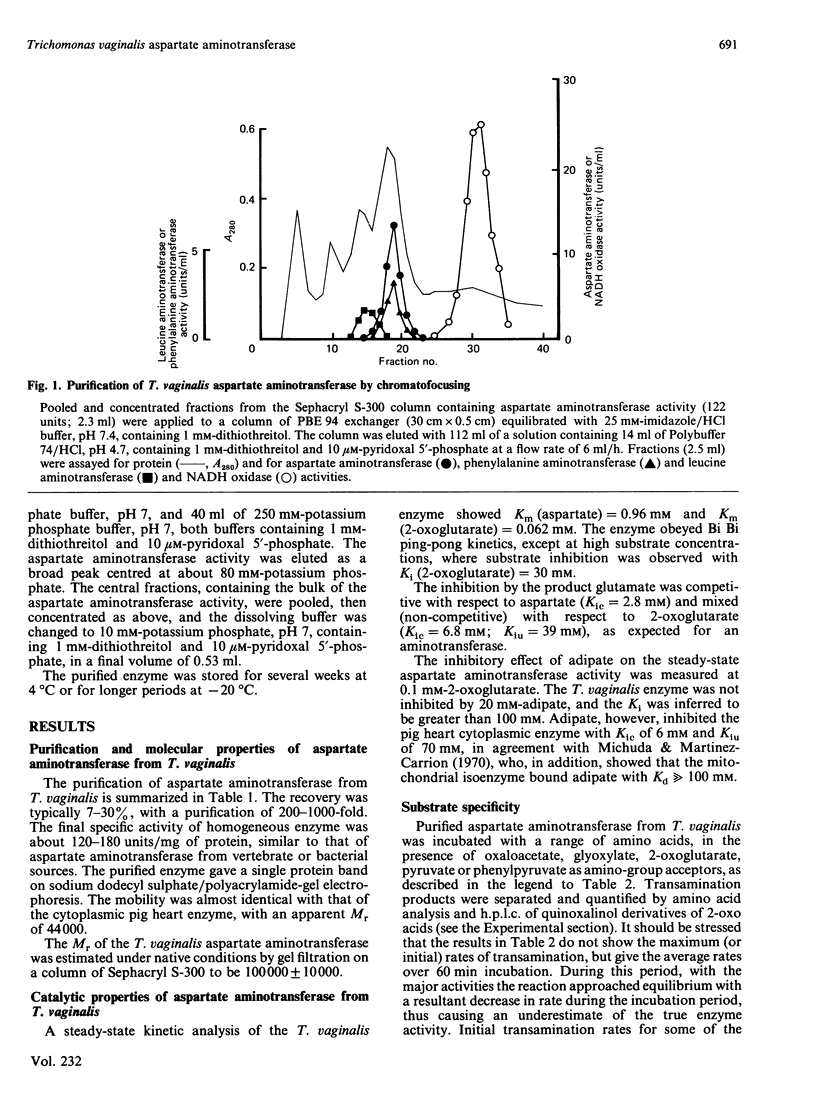
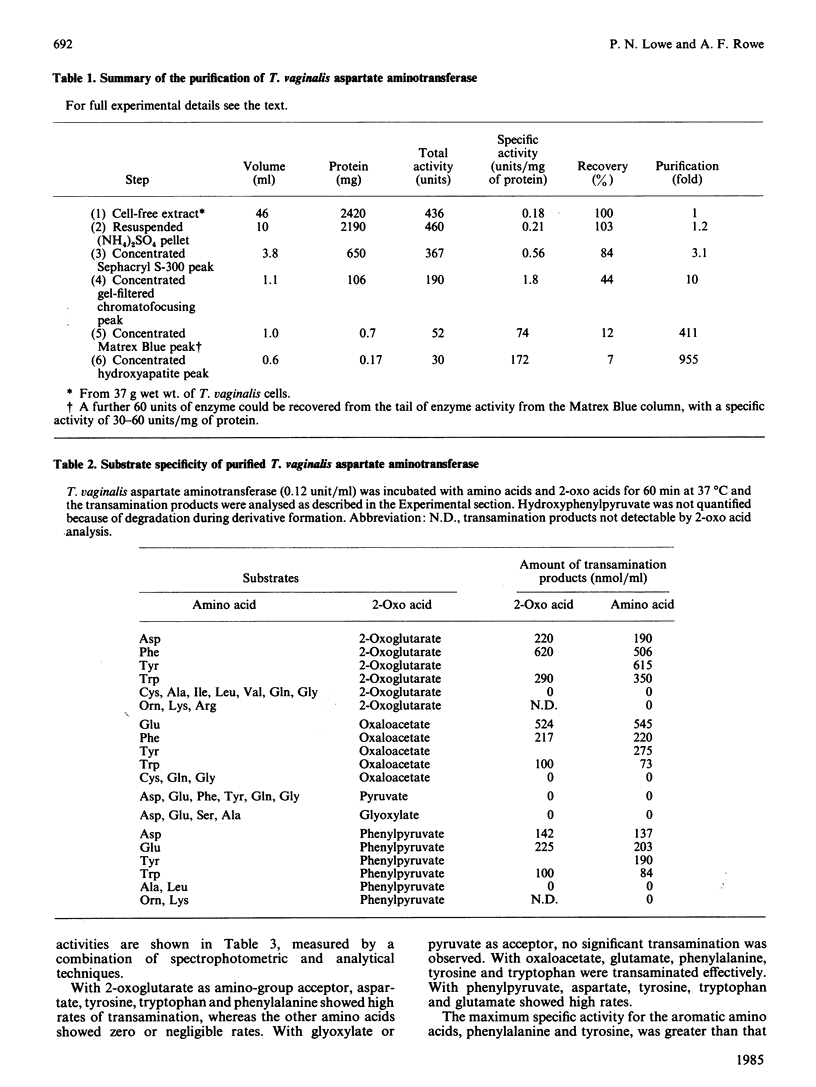
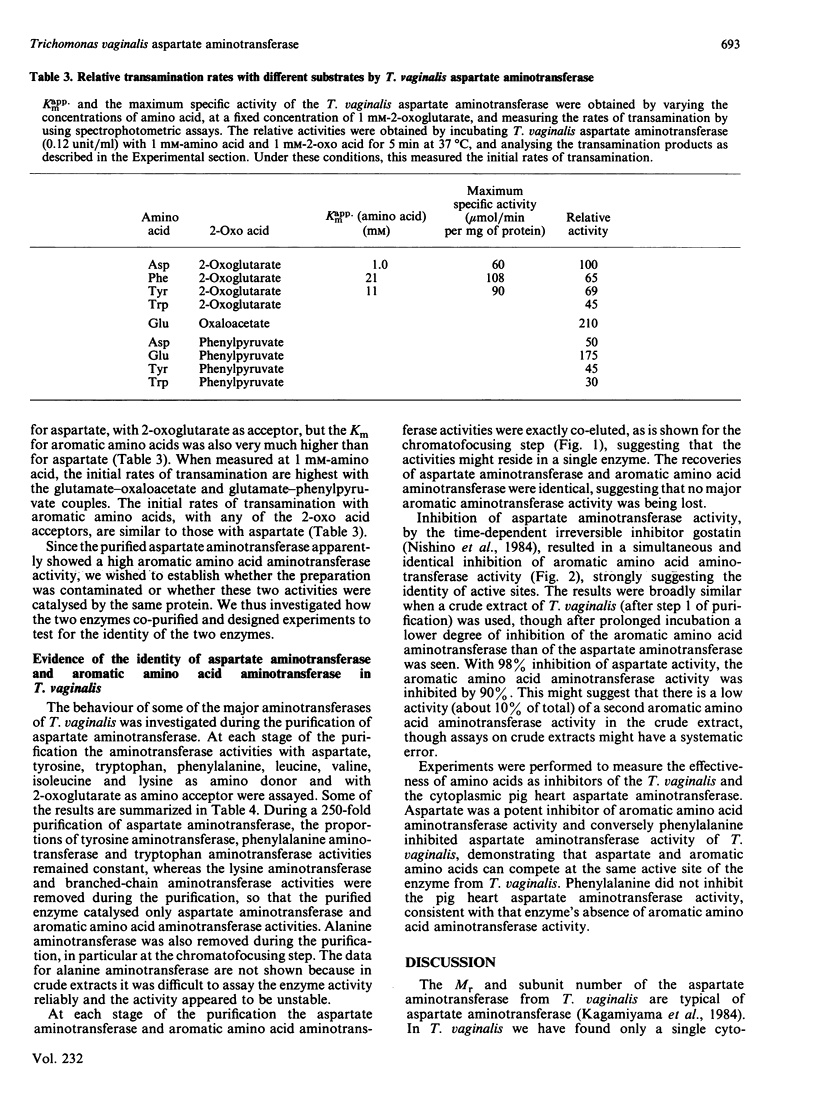
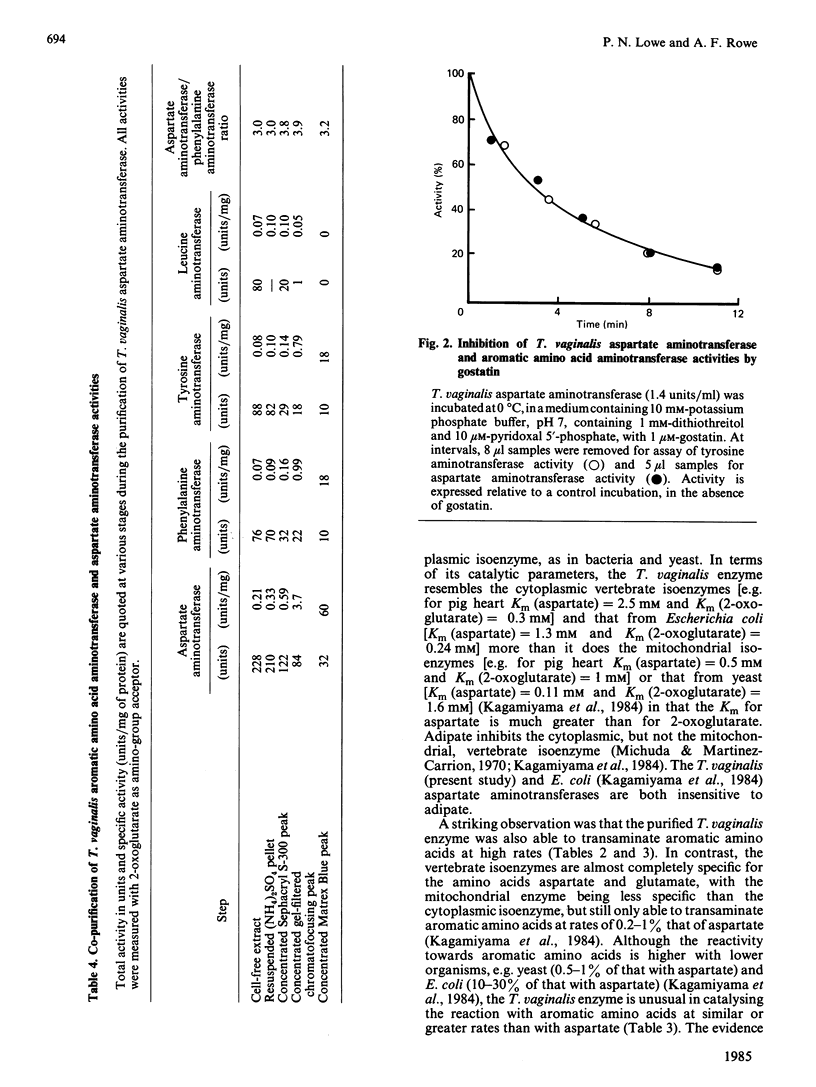
Aspartate: 2-oxoglutarate aminotransferase from the anaerobic protozoon Trichomonas vaginalis was purified to homogeneity and characterized. It is a dimeric protein of overall Mr approx. 100000. Only a single isoenzyme was found in T. vaginalis. The overall molecular and catalytic properties have features in common with both the vertebrate cytoplasmic and mitochondrial isoenzymes. The purified aspartate aminotransferase from T. vaginalis showed very high rates of activity with aromatic amino acids as donors and 2-oxoglutarate as acceptor. This broad-spectrum activity was restricted to aromatic amino acids and aromatic 2-oxo acids, and no significant activity was seen with other common amino acids, other than with the substrates and products of the aspartate: 2-oxoglutarate aminotransferase reaction. Co-purification and co-inhibition, by the irreversible inhibitor gostatin, of the aromatic amino acid aminotransferase and aspartate aminotransferase activities, in conjunction with competitive substrate experiments, strongly suggest that a single enzyme is responsible for both activities. Such high rates of aromatic amino acid aminotransferase activity have not been reported before in eukaryotic aspartate aminotransferase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cazzulo J. J. Protein and amino acid catabolism in Trypanosoma cruzi. Comp Biochem Physiol B. 1984;79(3):309–320. doi: 10.1016/0305-0491(84)90381-x. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Tsuchiya H., Todoriki H., Naruse H. High-performance liquid chromatographic determination of alpha-keto acids in human urine and plasma. Anal Biochem. 1982 May 1;122(1):173–179. doi: 10.1016/0003-2697(82)90267-6. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Calhoun D. H. Intracellular roles of microbial aminotransferases: overlap enzymes across different biochemical pathways. Crit Rev Microbiol. 1981;8(3):229–266. doi: 10.3109/10408418109085080. [DOI] [PubMed] [Google Scholar]
- Kagamiyama H., Kondo K., Yagi T. Aspartate aminotransferase from E. coli--a comparative study among E. coli, and yeast enzymes and pig heart isozymes. Prog Clin Biol Res. 1984;144B:293–302. [PubMed] [Google Scholar]
- Koike K., Koike M. Fluorescent analysis of alpha-keto acids in serum and urine by high-performance liquid chromatography. Anal Biochem. 1984 Sep;141(2):481–487. doi: 10.1016/0003-2697(84)90074-5. [DOI] [PubMed] [Google Scholar]
- Kondo K., Wakabayashi S., Yagi T., Kagamiyama H. The complete amino acid sequence of aspartate aminotransferase from Escherichia coli: sequence comparison with pig isoenzymes. Biochem Biophys Res Commun. 1984 Jul 18;122(1):62–67. doi: 10.1016/0006-291x(84)90439-x. [DOI] [PubMed] [Google Scholar]
- Le Blancq S. M., Lanham S. M. Aspartate aminotransferase in Leishmania is a broad-spectrum transaminase. Trans R Soc Trop Med Hyg. 1984;78(3):373–375. doi: 10.1016/0035-9203(84)90125-1. [DOI] [PubMed] [Google Scholar]
- Liao J. C., Hoffman N. E., Barboriak J. J., Roth D. A. High-performance liquid chromatography of pyruvic and alpha-ketoglutaric acids and its application to urine samples. Clin Chem. 1977 May;23(5):802–805. [PubMed] [Google Scholar]
- Linstead D. New defined and semi-defined media for cultivation of the flagellate Trichomonas vaginalis. Parasitology. 1981 Aug;83(Pt 1):125–137. doi: 10.1017/s0031182000050101. [DOI] [PubMed] [Google Scholar]
- Martini F., Angelaccio S., Barra D., Doonan S., Bossa F. Primary structure of aspartate aminotransferase from horse heart and comparison with that of other homotopic and heterotopic isoenzymes. Comp Biochem Physiol B. 1983;76(3):483–487. doi: 10.1016/0305-0491(83)90280-8. [DOI] [PubMed] [Google Scholar]
- Mavrides C., Orr W. Multispecific aspartate and aromatic amino acid aminotransferases in Escherichia coli. J Biol Chem. 1975 Jun 10;250(11):4128–4133. [PubMed] [Google Scholar]
- Michuda C. M., Martinez-Carrion M. The isozymes of glutamate-aspartate transaminase. Mechanism of inhibition of dicarboxylic acids. J Biol Chem. 1970 Jan 25;245(2):262–269. [PubMed] [Google Scholar]
- Morino Y., Tanase S., Watanabe T., Kagamiyama H., Wada H. Large-scale preparation of cytosolic and mitochondrial asparatate aminotransferases from pig heart. J Biochem. 1977 Sep;82(3):847–852. doi: 10.1093/oxfordjournals.jbchem.a131760. [DOI] [PubMed] [Google Scholar]
- Nishino T., Murao S., Wada H. Mechanism of inactivation of pyridoxal phosphate-linked aspartate transaminase by gostatin. J Biochem. 1984 May;95(5):1283–1288. doi: 10.1093/oxfordjournals.jbchem.a134733. [DOI] [PubMed] [Google Scholar]
- Ohisalo J. J., Andersson B. M., Viljanen A. A., Andersson S. M. Is there a brain tyrosine aminotransferase? Biochem J. 1982 May 15;204(2):621–622. doi: 10.1042/bj2040621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T., Kagamiyama H., Nozaki M. Aspartate: 2-oxoglutarate aminotransferase from bakers' yeast: crystallization and characterization. J Biochem. 1982 Jul;92(1):35–43. doi: 10.1093/oxfordjournals.jbchem.a133929. [DOI] [PubMed] [Google Scholar]


