INTRODUCTION
Glaucoma is estimated to affect approximately 80 million people and is the leading cause of irreversible blindness worldwide.1 The disease is characterized by a progressive optic neuropathy that, if left untreated, may lead to visual dysfunction and disability. Estimation of rates of structural and functional deterioration over time are essential in guiding management.2 Although many patients with glaucoma progress slowly over time and may be treated conservatively, others may show rapid deterioration with substantial risk for becoming disabled during their lifetimes, requiring aggressive treatment to slow down disease progression.3
Most investigations of rates of glaucoma progression have focused on relatively small populations from clinical trials and cohort studies.4–6 These studies tend to have quite restrictive inclusion and exclusion criteria with rigid follow-up schemes that lead to higher rates of adherence to treatment. Therefore, they may not represent how the disease behaves in a real-world setting. Although rates of visual field loss have been recently reported in large clinical populations,7–9 these studies have not included an assessment of structural changes over time. Many patients may show signs of deterioration to the optic nerve and retinal nerve fiber layer (RNFL) without concomitant visual field changes on standard automated perimetry (SAP).10 These structural changes can be captured by imaging technologies, such as spectral-domain optical coherence tomography (SD OCT) and have been shown to be predictive of future visual field loss and quality of life outcomes.11
The widespread incorporation of electronic health records (EHR) and computerized testing in clinical practice has precipitated a rapid growth in the availability of data from patients receiving routine clinical care. Unlike clinical trial data, EHR data reflect ordinary clinical care and can be used to yield valuable information about how a particular disease impacts a real-world clinical population. Analyses of these data may better represent “real” individuals, and reduce issues of random selection and bias, giving better estimates of expected disease trajectories under regular care.12, 13
In the present work, we evaluated rates of structural and functional change with SD OCT and SAP from a large EHR database of patients with glaucoma and suspected of having the disease who were receiving routine clinical care. We were particularly interested in comparing the proportions of eyes that could be categorized as having fast rates of change under the different modalities of testing, and their relationship with disease severity. Such knowledge may help guide the use of these tests to detect individuals who are at risk for visual disability and may need more aggressive management.
METHODS
This was a retrospective cohort study of patients from the Duke Glaucoma Registry (DGR), a database of electronic medical records developed by the Vision, Imaging and Performance (VIP) Laboratory. The database consisted of adults at least 18 years of age with glaucoma or glaucoma suspect diagnoses who were evaluated at the Duke Eye Center or its satellite clinics between 2009 and 2019. The Duke University Institutional Review Board approved this study with a waiver of informed consent due to the retrospective nature of this work. All methods adhered to the tenets of the Declaration of Helsinki for research involving human subjects and were conducted in accordance with regulations of the Health Insurance Portability and Accountability Act.
The database contained clinical information from baseline and follow-up visits, including patient diagnostic and procedure codes, medical history, stereoscopic optic disc photographs, and the results of all Spectralis SD OCT (Heidelberg Engineering, GmbH, Dossenheim, Germany) scans and SAP acquired with the Humphrey Field Analyzer (HFA, versions II and III; Carl Zeiss Meditec, Inc., Dublin, CA) during the study period.
Participant selection
Patients were included in the study if they had glaucoma or suspicion of glaucoma based on International Classification of Diseases (ICD) codes at baseline. Subjects were also required to have at least 2 good-quality SD OCT scans over a minimum follow-up period of 6 months and at least 2 reliable SAP tests over the same study period. Tests performed after any diagnosis of uveitis, retinal detachment, retinal or malignant choroidal tumors, non-glaucomatous disorders of the optic nerve and visual pathways, exudative, atrophic and late-stage dry age-related macular degeneration, amblyopia and venous or arterial retinal occlusion according to ICD codes were excluded. In addition, tests performed after treatment with panretinal photocoagulation, according to Current Procedural Terminology (CPT) codes, were also excluded. ICD and CPT codes used for inclusion and exclusion in the study are further described in Supplemental Table 1 (available at AJO.com).
SD OCT and SAP testing
RNFL thickness measurements were obtained from a 12-degree (for single circle scans) or a 3.45mm-diameter peripapillary circle scan (for scans from the Glaucoma Mode Premium Edition) acquired using the Spectralis SD OCT, as described in detail previously.14 Tests were acquired using the latest available software version at the time of the scan and exported using the latest available version at the time of the analysis (software version 6.8). For each scan, the global average RNFL thickness was calculated as the average of thicknesses of all points from the 360 degrees around the optic nerve head. This parameter was used to assess rates of change in RNFL thickness over time.
The device’s eye-tracking capability was used during image acquisition to adjust for eye movements. All scans that had a quality score lower than 15 were excluded from this analysis. Furthermore, since manual review of all tests was impractical, scans that had average global RNFL thickness measurements with implausible values were excluded (i.e., lower than 20 and greater than 150 μm). Those cutoffs represent measurements above the higher range of reported RNFL thickness for normal controls and below the lower range for glaucoma subjects15–17 and may indicate the presence of acquisition or segmentation errors in the presence of otherwise good quality scores.18 From the total of 47,571 eligible circle scans (i.e. after exclusions for ICD, CPT codes, and insufficient longitudinal data), 2,072 (4.4%) tests were excluded due to low quality scores and 556 (1.2%) were further excluded due to implausible average RNFL thickness values. When more than one good-quality test was available for the same date, the mean global RNFL thickness of all tests from that date was used in the analyses.
Visual field analysis included 24–2 and 30–2 Swedish Interactive Threshold Algorithm (SITA) tests with size III white stimulus. Since SAP was already widely used in clinical care when SD OCT was introduced, all SAP tests acquired more than 6 months before the first SD OCT test or more than 6 months after the last SD OCT test were excluded from the study. This ensured that eyes were evaluated in a corresponding time period for both tests. Visual fields were excluded if they had >33% fixation losses or >15% false-positives. From a total of 28,079 eligible visual fields, 4,993 (17.2%) tests had high fixation losses and 1,934 (6.7%) had high false-positives.
SAP mean deviation (MD) was the parameter used to assess rates of change in visual fields over time.
After excluding those eyes with a minimum follow-up of less than 6 months for both tests, the final sample then consisted of 29,548 SD OCT tests acquired over 27,182 SD OCT visits and 19,812 SAP tests.
Data Analyses
Rates of change were estimated using longitudinal linear mixed models for each parameter (i.e. SD OCT global peripapillary RNFL thickness or SAP MD) over time in each diagnostic group. This standard technique has been described in detail elsewhere.19 In brief, mixed models take into account the natural correlation of such data over time, as well as the fact that each patient may contribute with two eyes for the analyses. Differences in rates of change among eyes and subjects are taken into account by introducing random slopes and random intercepts. Best linear unbiased prediction (BLUP) was used to estimate individual slopes of change for each eye. These estimates are more precise than those obtained by ordinary least squares linear regression,20, 21 notably in the presence of a small number of tests over time as can occur with some eyes. As the number of tests increases, BLUP estimates become essentially identical to those obtained from ordinary least squares regression (OLS).
Individual slopes were then classified into groups according to pre-established cutoffs for each parameter. For global RNFL thickness, the cut-offs were: slow, if change was slower than −1.0 μm/year; moderate, if between −1.0 and −2.0 μm/year; and fast, if change was faster than −2.0 μm/year. For SAP MD, cut-offs were: slow, if change was slower than −0.5 dB/year; moderate if between −0.5 and −1.0 dB/year; and fast if faster than −1.0 dB/year. These cutoffs were based on modifications of previous definitions7, 8 to account for the differences in the dynamic range of SAP and SD OCT. Weighted Cohen’s kappa and confusion matrix plot were used to asses agreement between the classification of progression from both metrics.
Eyes were also classified into 4 groups of disease severity, according to the baseline visual field test. A ‘normal’ visual field was defined based on MD and pattern standard deviation within 95% confidence limits and glaucoma hemifield test results within normal limits. Abnormal SAP tests were classified into ‘mild’, ‘moderate’ or ‘severe’ visual field loss, using the Hodapp-Parrish-Anderson (HPA) classification criteria.22 This widely used classification applies cut-offs based on SAP MD and number and location of abnormal points in the pattern deviation plot to define the severity of visual field loss.
Generalized estimating equations (GEE) with robust sandwich variance estimator23 were used in logistic regression statistical analyses comparing the odds of detecting change by each test for each baseline diagnosis, while adjusting for potential correlations between both eyes from the same individual. In addition, we investigated the relationship between rates of change (i.e., proportions of eyes in each category) and the baseline visual field severity category.
The baseline characteristics and demographics were drawn from the date when the first valid SD OCT test for each eye was performed. All statistical analyses were completed in Stata (version 16, StataCorp LP, College Station, TX) within the Protected Analytics Computing Environment (PACE), a highly protected virtual network space developed by Duke University for analysis of identifiable protected health information.
RESULTS
A total of 29,548 SD OCT tests acquired over 27,182 SD OCT visits and 19,812 SAP tests from 6,138 eyes of 3,669 patients were included in the study. 1,997 (54.4%) of subjects were female and 2,243 (61.1%) had self-identified as white or Caucasian. Average age of subjects at baseline was 63.2 ± 12.9 years. Eyes had a mean ± standard deviation (SD) follow-up time of 4.4 ± 2.1 (range: 0.5 to 9.6) years.
According to ICD codes from the baseline visit, 2,649 (43.2%), 2,107 (34.3%), and 1,382 (22.5%) eyes were classified as glaucoma suspects (GS), primary open-angle glaucoma (POAG) and “other glaucoma”, respectively. Supplemental Table 1 (available at AJO.com) reports the specific ICD codes for each diagnostic group. Table 1 summarizes the demographic and clinical characteristics of the whole sample and subgroups. MD at baseline was similar between POAG and other glaucoma diagnoses (P = 0.234), and both were statistically significantly lower than GS (both P < 0.001). Mean baseline RNFL thickness was also different among groups (P < 0.001, all group comparisons), with GS presenting the thickest RNFL at baseline. There were large variations within baseline characteristics (Supplemental Figure 1, available at AJO.com). Of note, 1,703 (27.7%) eyes had a normal visual field at baseline, and 3,052 (49.7%) eyes were classified as mild, 722 (11.8%) as moderate and 661 (10.8%) as severe visual field loss according to the HPA classification.
Table 1.
Demographics and clinical characteristics at baseline of the subjects included in the study.
| Characteristic | Overall | Diagnosis at baseline | ||
|---|---|---|---|---|
| GS | POAG | Other | ||
| Subject-specific | ||||
| Number of patients, n (%) | 3,669 (100.0) | 1,548 (42.2) | 1,271 (34.6) | 866 (23.6) |
| Age (years), Mean ± SD |
63.2 ± 12.9 |
61.0 ± 12.7 |
66.0 ± 11.4 |
62.9 ± 14.6 |
| Sex, female (%) | 1,997 (54.4) | 882 (57.0) | 650 (51.1) | 472 (54.5) |
| Race, (%) White or Caucasian Black or African American Other |
2,243 (61.1) 1,091 (29.7) 335 (9.1) |
941 (60.8) 472 (30.5) 135 (8.7) |
775 (61.0) 393 (30.9) 103 (8.1) |
540 (61.5) 228 (26.3) 98 (11.3) |
| Eye-specific | ||||
| Number of eyes, n (%) | 6,138 (100.0) | 2,649 (43.2) | 2,107 (34.3) | 1,382 (22.5) |
| Follow-up (years), Mean ± SD |
4.4 ± 2.1 |
4.4 ± 2.0 |
4.6 ± 2.2 |
4.3 ± 1.9 |
| SD OCT | ||||
| Number of visits, n (%) | 27,182 (100.0) | 11,228 (41.3) | 9,829 (36.2) | 6,125 (22.5) |
| Number of visits per eye, Mean ± SD [range] |
4.4 ± 2.0 [2–15] |
4.2 ± 1.7 [2–14] |
4.7 ± 2.2 [2–15] |
4.4 ± 2.1 [2–14] |
| Baseline mean RNFL thickness (μm), Mean ± SD Median (IQR) |
79.6 ± 16.7 81.0 (69.0; 92.0) |
87.0 ± 13.3 88.0 (79.0; 96.0) |
72.8 ± 15.9 73.0 (62.0; 83.0) |
75.6 ± 18.0 76.0 (62.0; 88.0) |
| Baseline mean SD OCT quality, Mean ± SD Median (IQR) |
23.8 ± 4.2 24.0 (21.0; 27.0) |
24.2 ± 4.2 24.0 (21.0; 27.0) |
23.5 ± 4.1 24.0 (21.0; 26.0) |
23.7 ± 4.1 24.0 (21.0; 26.0) |
| SAP | ||||
| Number of tests, n (%) | 19,812 (100.0) | 7,898 (39.9) | 7,436 (37.5) | 4,478 (22.6) |
| Number of tests per eye, n (%) Mean ± SD [range] |
3.2 ± 1.6 [2–15] |
3.0 ± 1.3 [2–9] |
3.5 ± 1.8 [2–15] |
3.2 ±1.6 [2–13] |
| Baseline SAP MD (dB), Mean ± SD Median (IQR) |
−3.78 ± 5.29 −2.07 (−4.79; −0.58) |
−2.16 ± 3.59 −1.35 (−2.92; −0.16) |
−5.02 ± 5.85 −2.95 (−6.86; −1.14) |
−4.99 ± 6.20 −2.81 (−6.75; −0.95) |
| Baseline SAP PSD (dB), Mean ± SD Median (IQR) |
3.67 ± 3.28 2.10 (1.59; 4.38) |
2.52 ± 2.18 1.79 (1.46; 2.42) |
4.70 ± 3.77 2.76 (1.84; 7.08) |
4.29 ± 3.56 2.47 (1.68; 6.33) |
| Baseline VFI (%), Mean ± SD Median (IQR) |
91 ± 15 97 (92; 99) |
96 ± 9 98 (96; 99) |
88 ± 17 95 (85; 98) |
88 ± 18 96 (87; 99) |
GS = glaucoma suspect; IQR = interquartile range; MD = mean deviation; POAG = primary open-angle glaucoma; RNFL = retinal nerve fiber layer; SAP = Standard Automated Perimetry; SD OCT = Spectral-Domain Optical Coherence; SD = standard deviation
The mean rate of change for global RNFL thickness in the overall population was −0.73 ± 0.80 μm/year (median −0.67, IQR: −1.03 to −0.35 μm/year). GS eyes had the slowest mean rate of change (−0.64 ± 0.72 μm/year). Eyes diagnosed with POAG had a mean rate of change of −0.76 ± 0.71 μm/year, while eyes classified as “other glaucoma” had the fastest mean rate of change (−0.89 ± 1.03 μm/year) among the diagnostic groups (P < 0.05, all group comparisons). For rates of MD change over time, GS had significantly slower rates of change than the other diagnostic groups (both P < 0.001), but POAG and other glaucoma types had similar rates of MD loss over time (P = 0.206). Table 2 summarizes the rates of change in structure and function for each of the diagnostic groups.
Table 2.
Rates of Change for Spectral-Domain Optical Coherence Tomography (SD OCT) Retinal Nerve Fiber Layer (RNFL) Thickness and Standard Automated Perimetry (SAP) Mean Deviation (MD) According to Glaucoma Diagnosis at Baseline.
| Diagnosis | n = 6,138 eyes of 3,669 subjects | ||||||
|---|---|---|---|---|---|---|---|
| mean | SD | median | IQR | p5 | p15 | ||
| Rates of SD OCT RNFL thickness change (μm/year) | |||||||
| GS | −0.64 | 0.72 | −0.59 | −0.90 | −0.28 | −1.69 | −1.14 |
| POAG | −0.76 | 0.71 | −0.73 | −1.04 | −0.45 | −1.91 | −1.29 |
| Other | −0.89 | 1.03 | −0.79 | −1.24 | −0.39 | −2.61 | −1.59 |
| Overall | −0.73 | 0.80 | −0.67 | −1.03 | −0.35 | −1.96 | −1.30 |
| Rates of SAP MD change (dB/year) | |||||||
| GS | 0.01 | 0.27 | 0.04 | −0.06 | 0.13 | −0.39 | −0.15 |
| POAG | −0.20 | 0.39 | −0.15 | −0.32 | 0.00 | −0.78 | −0.47 |
| Other | −0.14 | 0.42 | −0.10 | −0.31 | 0.07 | −0.77 | −0.48 |
| Overall | −0.09 | 0.36 | −0.04 | −0.22 | 0.09 | −0.67 | −0.36 |
GS = glaucoma suspect; IQR = interquartile range; MD = mean deviation; p5 = 5th percentile; p15 = 15th percentile; POAG = primary open-angle glaucoma; RNFL = retinal nerve fiber layer; SAP = Standard Automated Perimetry; SD OCT = Spectral-Domain Optical Coherence; SD = standard deviation
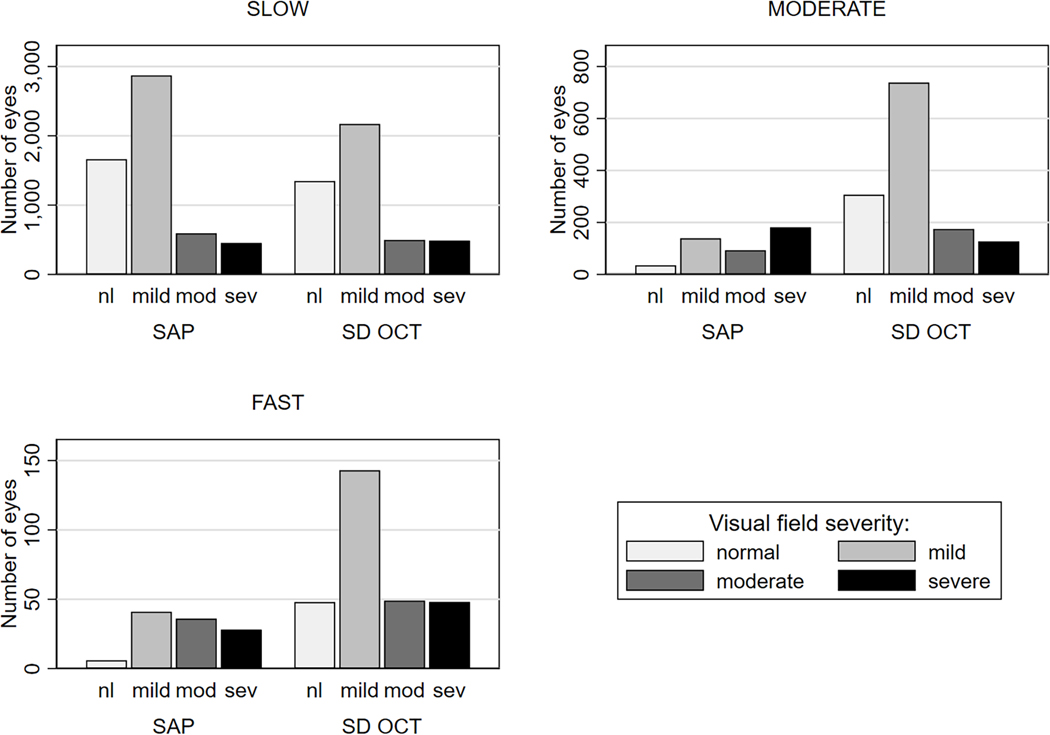
The number of eyes from the sample classified as slow, moderate, and fast progressors according to the previously defined cut-offs is illustrated in Figure 1. Although the majority of eyes were classified as slow progressors, there were important differences according to diagnoses and test. Table 3 breaks down the proportions of individual eyes for each rate of progression, according to the baseline diagnosis and SAP severity. For POAG eyes, 597 (28.3%) eyes were classified with at least moderate rate of change by SD OCT versus 284 (13.5%) eyes by SAP, and the odds of detecting an eye progressing at a moderate or faster rate of change versus slow rate of change was 2.5 times higher for SD OCT than for SAP (P < 0.001, Supplemental Table 2, available at AJO.com). Even greater differences were seen for the other diagnostic groups, with corresponding proportions for the GS group of 20.1% vs. 3.1% (P < 0.001); and 36.7% vs. 14.0% (P < 0.001) for the “other glaucoma” group. Overall, SD OCT also detected a greater proportion of fast progressors in all diagnostic groups compared to SAP (P < 0.001).
Figure 1.
Frequency of Eyes Classified as Slow, Moderate, and Fast Progressors According to Rates of Change of Spectral-Domain Optical Coherence Tomography (SD OCT) Retinal Nerve Fiber Layer (RNFL) Thickness and Standard Automated Perimetry (SAP) Mean Deviation (MD). Eyes Are Separated in Levels of Visual Field Severity at Baseline. nl = normal visual field; mod = moderate; sev = severe.
Table 3.
Classification of Progression According to Rates of Change in Spectral-Domain Optical Coherence Tomography (SD OCT) Retinal Nerve Fiber Layer (RNFL) Thickness and Standard Automated Perimetry (SAP) Mean Deviation (MD) for Each Diagnosis and Level of Visual Field Severity at Baseline.
| Progression Rate Classificationa,b | Test | By Diagnosis | By SAP Severity | Overall (6,138 eyes) | |||||
|---|---|---|---|---|---|---|---|---|---|
| GS (2,649 eyes) | POAG (2,107 eyes) | Other (1,382 eyes) | Normal (1,703 eyes) | Mild (3,052 eyes) | Moderate (722 eyes) | Severe (661 eyes) | |||
| By individual groups | |||||||||
| Slow, n (%) | SD OCT | 2,118 (79.9) | 1,510 (71.7) | 875 (63.3) | 1,348 (79.2) | 2,171 (71.1) | 498 (69.0) | 486 (73.5) | 4,503 (73.4) |
| SAP | 2,567 (96.9) | 1,823 (86.5) | 1,189 (86.0) | 1,662 (97.5) | 2,872 (94.1) | 593 (82.1) | 452 (68.4) | 5,579 (90.9) | |
| Moderate, n (%) | SD OCT | 452 (17.1) | 510 (24.2) | 385 (27.9) | 307 (18.0) | 738 (24.2) | 175 (24.2) | 127 (19.2) | 1,347 (21.9) |
| SAP | 62 (2.3) | 228 (10.8) | 158 (11.4) | 35 (2.1) | 139 (4.6) | 93 (12.9) | 181 (27.4) | 448 (7.3) | |
| Fast, n (%) | SD OCT | 79 (3.0) | 87 (4.1) | 122 (8.8) | 48 (2.8) | 143 (4.7) | 49 (6.8) | 48 (7.3) | 288 (4.7) |
| SAP | 20 (0.8) | 56 (2.7) | 35 (2.6) | 6 (0.4) | 41 (1.3) | 36 (5.0) | 28 (4.2) | 111 (1.8) | |
| By cumulative groups | |||||||||
| Moderate and fast, n (%) | SD OCT | 531 (20.1) | 597 (28.3) | 507 (36.7) | 355 (20.8) | 881 (28.9) | 224 (31.0) | 175 (26.5) | 1,635 (26.6) |
| SAP | 82 (3.1) | 284 (13.5) | 193 (14.0) | 41 (2.5) | 180 (5.9) | 129 (17.9) | 209 (31.6) | 559 (9.1) | |
Rates of SD OCT global RNFL thickness change: slow if slower −1.0 μm/year; moderate, if between −1.0 and −2.0 μm/year; fast, if faster than −2.0 μm/year.
Rates of SAP MD change: slow if slower than −0.5 dB/year; moderate if between −0.5 and −1.0 dB/year; fast if faster than −1.0 dB /year.
GS = glaucoma suspect; MD = mean deviation; POAG = primary open-angle glaucoma; SAP = Standard Automated Perimetry; SD OCT = Spectral-Domain Optical Coherence Tomography; RNFL = retinal nerve fiber layer.
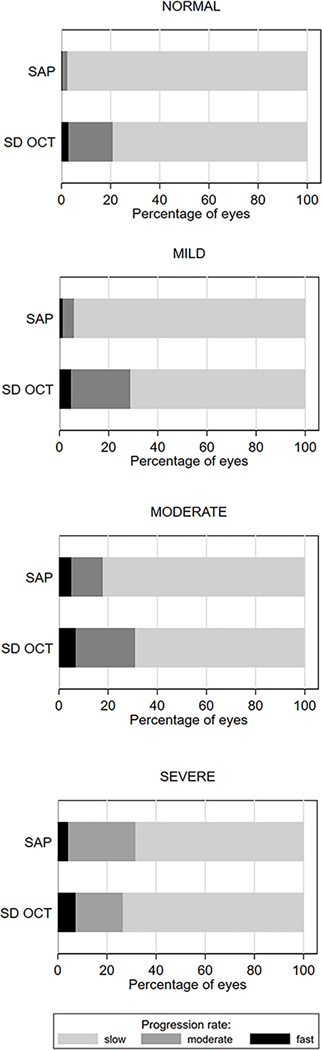
Figure 2 presents the proportion of eyes progressing at each rate of change (i.e. slow, fast, or moderate) within each group of visual field severity at baseline. A significantly larger proportion of eyes were detected as progressing at least at a moderate rate of change by SD OCT than by SAP for eyes with a normal visual field test at baseline (20.8% vs. 2.5%; P < 0.001), and also for a mild (28.9% vs 5.9%; P < 0.001) and moderate visual field defect at baseline (31.0% vs 17.9%; P < 0.001). For eyes with severe visual field loss at baseline, SAP detected more eyes (31.6%) with moderate rates of change or faster than SD OCT (26.5%), but this difference was not statistically significant (P = 0.055).
Figure 2.
Proportion of Eyes Classified as Slow, Moderate, and Fast Progressors by Spectral-Domain Optical Coherence Tomography (SD OCT) Retinal Nerve Fiber Layer (RNFL) Thickness and Standard Automated Perimetry (SAP) Mean Deviation (MD) Slopes of Change. Eyes Are Grouped in Levels of Severity of Visual Field Loss at Baseline.
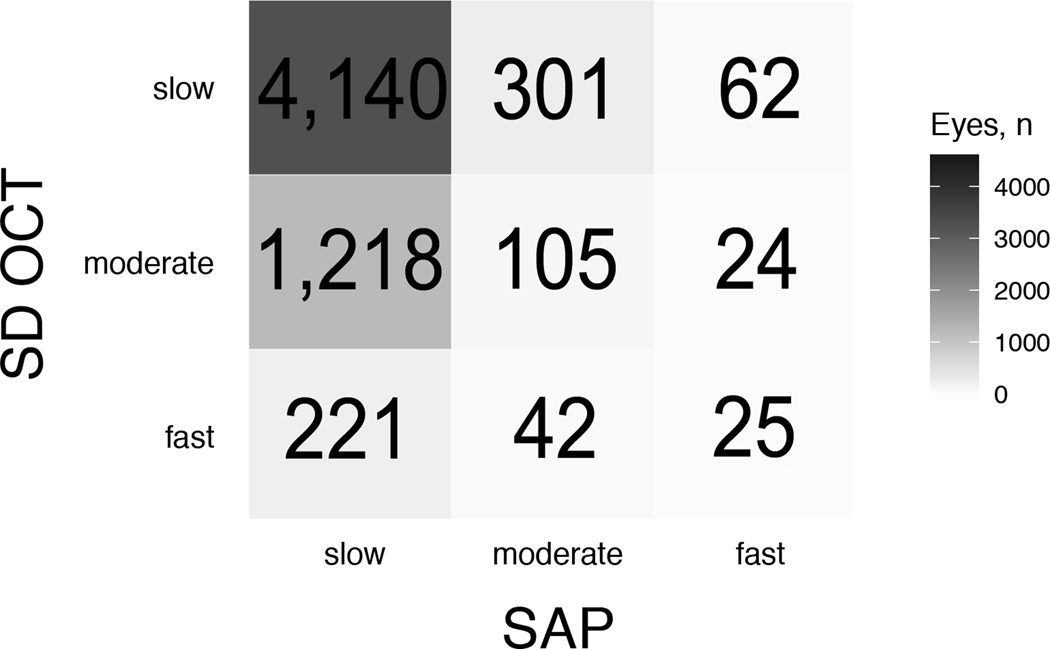
Significant disagreements between SD OCT and SAP for the classification of rates of progression as slow, moderate or fast can be seen in Figure 3. For example, of the 288 eyes categorized as fast progressors by SD OCT, 221 (76.7%) were deemed as slow progressors by SAP. Conversely, of the 111 eyes categorized as fast progressors by SAP, 62 (55.9%) were classified as slow progressors by SD OCT. Of the total number of 6,138 eyes, SD OCT and SAP agreed in 4,270 (weighted Cohen’s kappa = 0.059; P < 0.001).
Figure 3.
Confusion Matrix for the Number of Eyes Classified as Slow, Moderate, and Fast Progressors by Spectral-Domain Optical Coherence Tomography (SD OCT) and Standard Automated Perimetry (SAP).
DISCUSSION
In this large study of EHR data, we assessed the rates of change in structure and function in a population with glaucoma or glaucoma suspect diagnoses followed at a tertiary care center. Although most patients under routine clinical care had relatively slow rates of progression, many eyes had rates of change that could potentially incur significant risk of visual disability if sustained over time. The assessment of rates of change showed striking disagreements between SD OCT and SAP, indicating a clear need for monitoring both structure and function at all stages of glaucoma.
We present the largest analysis of longitudinal SD OCT and SAP results to date, with over 6,100 eligible eyes undergoing routine clinical care over a follow-up period that extended up to 9.6 years. Approximately 40% of the eyes had at least 5 years of follow-up (Supplemental Figure 1, available at AJO.com). The average rate of RNFL thickness change in the whole population was −0.73 μm/year, a number that is considerably faster than previous reports of age-related loss in RNFL thickness. Using a different SD OCT device, two previous longitudinal studies estimated average rates of global RNFL thinning from aging to be approximately −0.50 μm/year.24, 25 Estimates for Spectralis OCT have generally been even lower.16, 26 Although rates of RNFL thickness change have been reported in previous studies for glaucoma and suspect populations, they were limited to relatively small cohort studies or clinical trial data.27–30 Due to patient selection and strict protocol adherence, such estimates may not directly reflect common disease trajectories seen in clinical practice. Furthermore, as shown in our study, a large clinical population is needed to be able to estimate more precisely the subgroups of patients progressing at different categories of rates of change throughout the range of disease severity.
For functional change, the average rate of SAP MD was −0.09 ± 0.36 dB/year, which is similar to the mean rate of −0.15 dB/year reported by Chauhan and colleagues7 in a population of 2,324 eyes. However, more importantly than reporting the average rates of change was the analysis of the proportions of eyes progressing at each category of change. Such analyses revealed very interesting findings, since a significant proportion of eyes had rates of change that could be classified as at least moderate over time. For SD OCT, 1,635 of the 6,138 (26.6%) eyes were considered to be progressing at least at a moderate pace, or with a rate of RNFL loss faster than −1.0 μm/year, which is at least twice the expected age-related loss. For SAP, 559 (9.1%) were classified as at least moderate progressors, with losses greater than −0.5 dB/year. It should be noted that such a linear rate of change would correspond to a 5 dB total loss over a 10-year course of the disease, which might be enough to produce disability, notably if occurring in the better eye of a patient or in the presence of an already damaged field at baseline.31 The proportions of eyes progressing at a fast rate of change were 7.3% and 4.2% for SD OCT and SAP, respectively. These are also substantial, considering the devastating impact that such rate of change may have on patient outcomes.
The analyses of rates of progression according to disease severity revealed several important findings. In line with previous studies, SD OCT detected a much larger number of eyes with mild visual field defect that were progressing at faster rates compared to SAP.10, 32, 33 If an eye started follow-up with a normal visual field, the odds of detecting a progression at a moderate or faster rate of change was almost 11 times higher for SD OCT than for SAP (P < 0.001). However, perhaps the most remarkable finding was the proportion of eyes with severe disease at baseline that were identified with moderate or fast progression by SD OCT. These eyes had an average MD of −15.54 ± 6.47 dB (median −14.88 dB, IQR −12.18 to −19.32 dB) at baseline and approximately 26.5% of them were classified as progressing at a moderate or fast pace by SD OCT; a number lower, but not significantly different, from the 31.6% eyes detected as progressing at a moderate or fast rate by SAP in this group (P = 0.055). It should be noted, however, that these were not necessarily the same eyes, as Figure 3 illustrates. As a matter of fact, most eyes classified as fast by SD OCT would have been classified as slow by SAP and vice-versa. This result demonstrates that both structural and functional tests should be used to monitor glaucoma throughout the whole disease continuum and that SD OCT still has a very important place in monitoring eyes with advanced glaucoma.
Although our study highlights the importance of SD OCT even in eyes with advanced glaucoma, previous reports in the literature have suggested that SD OCT RNFL would become relatively ineffective to detect change in advanced stages due to a floor effect.10, 32, 34 The mean baseline RNFL thickness for eyes classified as severe by HPA criteria was 59.3 ± 15.9 μm (median: 57.0 μm; IQR: 48.0 to 69.0 μm). Therefore, when one takes into account the range of potential floor levels presented in the work of Bowd et al17 (38.0 ± 4.2 μm), it is clear that there would still be a very significant amount of RNFL to be tracked in these patients, which may explain why SD OCT still can be useful in these situations.
The cutoffs used to classify rates as slow, moderate, and fast were arbitrary and different levels could potentially be used. They were based on an a priori assessment of the dynamic ranges of the instruments, as well as previous reports in the literature.7, 8 For example, an eye progressing at a rate of −1.0 dB/year, would lose 10 dB in 10 years, an amount enough to cause functional disability31, 35 and corresponding to about 1/3 of the dynamic range of the HFA (i.e. range of 30 units). Similarly, a rate of −2.0 μm/year of RNFL loss would cause a 20 μm loss over the same period, which is also approximately 1/3 of the dynamic range reported for SD OCT (i.e. ranging 60 units).17 These numbers should not be taken as implying a linear correspondence between SAP MD and SD OCT RNFL thickness losses. In fact, previous studies have clearly established a nonlinear relationship between these metrics.28, 36–38
Also, when evaluating whether an individual’s rate of loss is clinically relevant, clinicians should take into account factors such as age, life expectancy, and the severity of functional or structural baseline damage in both eyes. Clinically important rates of glaucoma progression are the ones that put a patient at risk of future functional impairment or reduction of vision-related quality of life during his/her lifetime.39 Of note, the large proportion of eyes with severe disease that were progressing at a moderate or faster rate of change in this study helps explain why blindness from glaucoma is still a relatively common phenomenon, even for subjects under clinical care.40–42
While the size of our study is a major strength, working with EHR data has inherent limitations. Retrospective data carries an intrinsic risk of inaccurately coded or missing data. For example, the majority of subjects diagnosed with “other glaucoma” were coded with an unspecified classification of glaucoma rather than a specific etiology. Coding for POAG or glaucoma suspect was done by the attending physicians, without following a prespecified guideline other than general coding guidelines, and application of these guidelines may differ from physician to physician. However, the analysis of clinical data from SAP and SD OCT in the diagnostic categories was very consistent. Of note, we only used the ICD code at the time of the first reliable SD OCT to establish the diagnostic category, but some patients changed diagnoses over time. This could reflect disease progression or mis-categorizations at the initial visit, especially if the first visit was not with a glaucoma specialist. However, we elected to use the baseline diagnosis as a way to predict the expected behavior of the disease for a given patient at the initial clinical encounter. Since patients in this study received care in a tertiary referral center, the follow-up was quite variable, and some patients may have been referred only for an intervention rather than for continued follow-up. This justifies our more inclusive criteria, allowing patients with a minimum of 2 tests over follow-up. To generate more precise estimates, we used BLUPs, which takes into account the results obtained by evaluating the whole sample of eyes, giving less weight to estimates obtained from eyes with few measurement occasions and/or large intra-individual variability (that is, more “noise”).43 In eyes with large numbers of measurements over time, BLUP and OLS estimates give fundamentally similar results. Nevertheless, a large proportion of eyes had at least 5 years of follow-up, the timeframe that is relevant for most clinical decision making in glaucoma. Finally, we did not assess whether changes in cataract or visual acuity over time impacted test performance and these may be significant confounders.44, 45 Future studies should incorporate analysis of other risk factors in an attempt to predict which eyes are at risk for exhibiting fast progression over time.
In conclusion, although average rates of change in structure and function in glaucoma may be considered relatively slow, a significant proportion of patients show rates of change that can potentially result in vision-related disability if sustained over time. Our results indicate that both structural and functional tests should be used to monitor the disease, regardless of the amount of baseline damage, and that SD OCT still has an important role in detecting fast progressors in eyes with advanced glaucoma.
Supplementary Material
Supplemental Figure 1. Distribution of the Eyes Included According to Baseline (A) Age, (B) Global Retinal Nerve Fiber Layer (RNFL) Thickness and (C) Mean Deviation (MD), (D) Follow-up Time, and (E) Number of Spectral-Domain Optical Coherence Tomography (SD OCT) and (F) Standard Automated Perimetry (SAP) Tests Per Eye.
ACKNOWLEDGEMENTS
a. Funding/Support:
Supported in part by the National Institutes of Health/National Eye Institute grant EY029885 (FAM). The funding organizations had no role in the design or conduct of this research.
Footnotes
DISCLOSURE
b. Disclosures: A.A.J.: none. A.C.T.: none. E.B.M.: none. C.N.U: none. T.E.: none. S.I.B.: none. H.C.T.: none. S.A.: Aerie Pharmaceuticals (C), Camras Vision (C), Regenex Bio (C), Noveome Biotherapeutics (C). F.A.M.: Aeri Pharmaceuticals (C); Allergan (C, F), Annexon (C); Biogen (C); Carl Zeiss Meditec (C, F), Galimedix (C); Google Inc. (F); Heidelberg Engineering (F), IDx (C); nGoggle Inc. (P), Novartis (F); Stealth Biotherapeutics (C); Reichert (C, F)
The coauthors have seen and agree with each of the changes made to the manuscript in this revision and to the way his or her name is listed.
Supplemental Material available at AJO.com
REFERENCES
- 1.Mariotti SP. Global Data on Vision Impairments 2010. Bull World Health Organ. Switzerland: World Health Organization, 2012:1–14. [Google Scholar]
- 2.Caprioli J. The importance of rates in glaucoma. Am J Ophthalmol 2008;145(2):191–2. [DOI] [PubMed] [Google Scholar]
- 3.European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition - Chapter 3: Treatment principles and options Supported by the EGS Foundation: Part 1: Foreword; Introduction; Glossary; Chapter 3 Treatment principles and options. Br J Ophthalmol 2017;101(6):130–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration.The AGIS Investigators. Am J Ophthalmol 2000;130(4):429–40. [DOI] [PubMed] [Google Scholar]
- 5.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007;114(11):1965–72. [DOI] [PubMed] [Google Scholar]
- 6.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120(6):714–20; discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 7.Chauhan BC, Malik R, Shuba LM, Rafuse PE, Nicolela MT, Artes PH. Rates of glaucomatous visual field change in a large clinical population. Invest Ophthalmol Vis Sci 2014;55(7):4135–43. [DOI] [PubMed] [Google Scholar]
- 8.Heijl A, Buchholz P, Norrgren G, Bengtsson B. Rates of visual field progression in clinical glaucoma care. Acta Ophthalmol 2013;91(5):406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JH, Rabiolo A, Morales E, et al. Risk Factors for Fast Visual Field Progression in Glaucoma. Am J Ophthalmol 2019;207:268–278. [DOI] [PubMed] [Google Scholar]
- 10.Abe RY, Diniz-Filho A, Zangwill LM, et al. The Relative Odds of Progressing by Structural and Functional Tests in Glaucoma. Invest Ophthalmol Vis Sci 2016;57(9):OCT421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medeiros FA, Gracitelli CP, Boer ER, Weinreb RN, Zangwill LM, Rosen PN. Longitudinal changes in quality of life and rates of progressive visual field loss in glaucoma patients. Ophthalmology 2015;122(2):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krumholz HM. Big data and new knowledge in medicine: the thinking, training, and tools needed for a learning health system. Health Aff (Millwood) 2014;33(7):1163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Research Council. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. Washington, DC: The National Academies Press, 2011. [PubMed] [Google Scholar]
- 14.Leite MT, Rao HL, Zangwill LM, Weinreb RN, Medeiros FA. Comparison of the diagnostic accuracies of the Spectralis, Cirrus, and RTVue optical coherence tomography devices in glaucoma. Ophthalmology 2011;118(7):1334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varma R, Bazzaz S, Lai M. Optical tomography-measured retinal nerve fiber layer thickness in normal latinos. Invest Ophthalmol Vis Sci 2003;44(8):3369–73. [DOI] [PubMed] [Google Scholar]
- 16.Patel NB, Lim M, Gajjar A, Evans KB, Harwerth RS. Age-associated changes in the retinal nerve fiber layer and optic nerve head. Invest Ophthalmol Vis Sci 2014;55(8):5134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowd C, Zangwill LM, Weinreb RN, Medeiros FA, Belghith A. Estimating Optical Coherence Tomography Structural Measurement Floors to Improve Detection of Progression in Advanced Glaucoma. Am J Ophthalmol 2017;175:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asrani S, Essaid L, Alder BD, Santiago-Turla C. Artifacts in spectral-domain optical coherence tomography measurements in glaucoma. JAMA Ophthalmol 2014;132(4):396–402. [DOI] [PubMed] [Google Scholar]
- 19.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38(4):963–74. [PubMed] [Google Scholar]
- 20.Medeiros FA, Zangwill LM, Weinreb RN. Improved prediction of rates of visual field loss in glaucoma using empirical Bayes estimates of slopes of change. J Glaucoma 2012;21(3):147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medeiros FA, Zangwill LM, Mansouri K, Lisboa R, Tafreshi A, Weinreb RN. Incorporating risk factors to improve the assessment of rates of glaucomatous progression. Invest Ophthalmol Vis Sci 2012;53(4):2199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodapp E, Parrish RK, Anderson DR. Clinical decisions in glaucoma. St Louis: Mosby, 1993. [Google Scholar]
- 23.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42(1):121–30. [PubMed] [Google Scholar]
- 24.Wu Z, Saunders LJ, Zangwill LM, Daga FB, Crowston JG, Medeiros FA. Impact of Normal Aging and Progression Definitions on the Specificity of Detecting Retinal Nerve Fiber Layer Thinning. Am J Ophthalmol 2017;181:106–113. [DOI] [PubMed] [Google Scholar]
- 25.Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a prospective analysis of age-related loss. Ophthalmology 2012;119(4):731–7. [DOI] [PubMed] [Google Scholar]
- 26.Vianna JR, Danthurebandara VM, Sharpe GP, et al. Importance of Normal Aging in Estimating the Rate of Glaucomatous Neuroretinal Rim and Retinal Nerve Fiber Layer Loss. Ophthalmology 2015;122(12):2392–8. [DOI] [PubMed] [Google Scholar]
- 27.Hammel N, Belghith A, Weinreb RN, Medeiros FA, Mendoza N, Zangwill LM. Comparing the Rates of Retinal Nerve Fiber Layer and Ganglion Cell-Inner Plexiform Layer Loss in Healthy Eyes and in Glaucoma Eyes. Am J Ophthalmol 2017;178(38–50. [DOI] [PubMed] [Google Scholar]
- 28.Liu T, Tatham AJ, Gracitelli CP, Zangwill LM, Weinreb RN, Medeiros FA. Rates of Retinal Nerve Fiber Layer Loss in Contralateral Eyes of Glaucoma Patients with Unilateral Progression by Conventional Methods. Ophthalmology 2015;122(11):2243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee WJ, Kim YK, Park KH, Jeoung JW. Trend-based Analysis of Ganglion Cell-Inner Plexiform Layer Thickness Changes on Optical Coherence Tomography in Glaucoma Progression. Ophthalmology 2017;124(9):1383–1391. [DOI] [PubMed] [Google Scholar]
- 30.Shin JW, Sung KR, Lee GC, Durbin MK, Cheng D. Ganglion Cell-Inner Plexiform Layer Change Detected by Optical Coherence Tomography Indicates Progression in Advanced Glaucoma. Ophthalmology 2017;124(10):1466–1474. [DOI] [PubMed] [Google Scholar]
- 31.Abe RY, Diniz-Filho A, Costa VP, Wu Z, Medeiros FA. Predicting Vision-Related Disability in Glaucoma. Ophthalmology 2018;125(1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Dastiridou A, Francis BA, et al. Comparison of Glaucoma Progression Detection by Optical Coherence Tomography and Visual Field. Am J Ophthalmol 2017;184:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung CK-s, Cheung CYL, Weinreb RN, et al. Evaluation of Retinal Nerve Fiber Layer Progression in Glaucoma: A Study on Optical Coherence Tomography Guided Progression Analysis. Investigative Ophthalmology & Visual Science 2010;51(1):217–222. [DOI] [PubMed] [Google Scholar]
- 34.Lavinsky F, Wu M, Schuman JS, et al. Can Macula and Optic Nerve Head Parameters Detect Glaucoma Progression in Eyes with Advanced Circumpapillary Retinal Nerve Fiber Layer Damage? Ophthalmology 2018;125(12):1907–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jammal AA, Ogata NG, Daga FB, Abe RY, Costa VP, Medeiros FA. What Is the Amount of Visual Field Loss Associated With Disability in Glaucoma? Am J Ophthalmol 2019;197:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medeiros FA, Zangwill LM, Bowd C, Mansouri K, Weinreb RN. The structure and function relationship in glaucoma: implications for detection of progression and measurement of rates of change. Invest Ophthalmol Vis Sci 2012;53(11):6939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung CK, Liu S, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma a prospective analysis with neuroretinal rim and visual field progression. Ophthalmology 2011;118(8):1551–7. [DOI] [PubMed] [Google Scholar]
- 38.Miglior S, Zeyen T, Pfeiffer N, et al. Results of the European Glaucoma Prevention Study. Ophthalmology 2005;112(3):366–75. [DOI] [PubMed] [Google Scholar]
- 39.Saunders LJ, Medeiros FA, Weinreb RN, Zangwill LM. What rates of glaucoma progression are clinically significant? Expert Rev Ophthalmol 2016;11(3):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant WM, Burke JF, Jr. Why do some people go blind from glaucoma? Ophthalmology 1982;89(9):991–8. [DOI] [PubMed] [Google Scholar]
- 41.Oliver JE, Hattenhauer MG, Herman D, et al. Blindness and glaucoma: a comparison of patients progressing to blindness from glaucoma with patients maintaining vision. Am J Ophthalmol 2002;133(6):764–72. [DOI] [PubMed] [Google Scholar]
- 42.Rossetti L, Digiuni M, Montesano G, et al. Blindness and Glaucoma: A Multicenter Data Review from 7 Academic Eye Clinics. PLoS One 2015;10(8):e0136632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson GK. That BLUP is a Good Thing: The Estimation of Random Effects. Statistical Science 1991;6(1):15–32. [Google Scholar]
- 44.Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R, Group CS. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology 2011;118(9):1766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Advanced Glaucoma Intervention Study. 2. Visual field test scoring and reliability. Ophthalmology 1994;101(8):1445–55. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Distribution of the Eyes Included According to Baseline (A) Age, (B) Global Retinal Nerve Fiber Layer (RNFL) Thickness and (C) Mean Deviation (MD), (D) Follow-up Time, and (E) Number of Spectral-Domain Optical Coherence Tomography (SD OCT) and (F) Standard Automated Perimetry (SAP) Tests Per Eye.