Abstract
Abstract
Objectives
This study aimed to examine the relationship between measures of kidney function and impaired lung function in individuals with diabetes and to assess all-cause mortality risk associated with having chronic kidney disease (CKD) and or impaired lung function.
Design
Cross-sectional and retrospective cohort study.
Setting
The National Health and Nutrition Examination Survey 2007–2012.
Participants
A total of 10 809 participants aged over 20 years were included in this study: 9503 with normal spirometry, 951 with preserved ratio impaired spirometry (PRISm) and 355 with variable obstruction (VO).
Exposure and outcome measures
Kidney function measures, including estimated glomerular filtration rate (eGFR) and urinary albumin to creatinine ratio (UACR), were considered exposure variables. PRISm and VO were outcome variables. PRISm was defined as a forced expiratory volume in 1 s (FEV1)<80% predicted and an FEV1/forced vital capacity (FVC) ratio≥0.7, while VO was defined as an FEV1/FVC ratio <0.7 prebronchodilator and ≥0.7 postbronchodilator. In the cross-sectional analysis, multivariate logistic regression models were used to assess the relationship between kidney function measures and spirometry findings. In the retrospective cohort analysis, Cox proportional hazards models were employed to evaluate the impact of having PRISm or VO, combined with CKD, on all-cause mortality.
Results
An increase in UACR was significantly associated with higher odds of PRISm (OR (95% CI)=1.10 (1.01, 1.21), p=0.03). Additionally, eGFR <60 was associated with the odds of variable obstructive lung function (OR (95% CI)=1.72 (1.07, 2.74), p=0.03) compared with eGFR >60. After adjustments, an increase in UACR was associated with higher odds of PRISm in individuals with diabetes (OR (95% CI)=1.21 (1.08, 1.36), p=0.002), and UACR ≥300 mg/g significantly increased odds of having PRISm in idividuals with diabetes (OR (95% CI)=2.34 (1.23, 4.47), p=0.01). During a mean follow-up of 12.3 years, 10 500 deaths occurred. In the diabetic group, compared with normal spirometry without CKD, those with both PRISm and CKD had a significantly increased risk of all-cause mortality (HR (95% CI)=3.46 (1.94, 6.16), p<0.0001).
Conclusion
An elevated UACR and albuminuria were linked to a higher risk of PRISm. Our study emphasises that kidney and lung function are correlated. Further research is necessary to confirm our findings.
Keywords: Diabetic nephropathy & vascular disease; Pulmonary Disease, Chronic Obstructive; Respiratory Function Test
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study used a large nationally representative sample of the US population.
The study explored associations between measures of kidney function and impaired lung function.
This study assessed all cause-mortality risk associated with having both chronic kidney disease and impaired lung function.
The study’s cross-sectional nature meant that causal relationships could not be proven.
The results of this study cannot be generalised to populations outside of the USA.
Introduction
Preserved ratio impaired spirometry (PRISm) is defined as a forced expiratory volume in 1 s (FEV1) less than 80% of predicted value and an FEV1/forced vital capacity (FVC) ratio of 0.7 or greater, with a global prevalence reported between 3% and 20%.1,4 PRISm is a prevalent and unstable condition that can lead to cardiopulmonary diseases15,9 and all-cause mortality in middle-aged to old-aged adults.10 11 Moreover, lung dysfunction is also linked to recurrent hospitalisation12 13 and decreased quality of life.14 There is a lack of conclusive evidence on whether diabetes causes PRISm, but an inverse relationship between type 2 diabetes and pulmonary function is reported,15,18 pointing to the lungs as a potential target for diabetes-related complications, while some suggest that PRISm is associated with the risk of developing diabetes.17 19
Variable obstruction (VO) is characterised by prebronchodilator FEV1/FVC ratio ˂0.7 and a postbronchodilator FEV1/FVC ratio ≥0.7, also termed as ‘bronchodilator responsiveness’. The Global Initiative on Obstructive Lung Disease uses post-BD FEV1/FVC ratio ˂0.7 to define COPD. Recent literature debates this as it does not use the lower limit of normal values of FEV1 and FVC. Buhr et al20 found that VO poses a significant risk of developing COPD in future. Thus, this study used VO—a new pulmonary phenotype—as it reflects the early stage of COPD.
Chronic kidney disease (CKD) is a widespread problem affecting a large portion of the adult population around the world,21 including 13% of adults in the USA22 and 10% of adults in China.23 Research has shown that factors like decreased estimated glomerular filtration rate (eGFR) and albuminuria are linked to a higher risk of death due to heart and blood vessel problems in people with diabetes.24 Apart from restrictive and obstructive lung function, studies analysing associations between PRISm and kidney are not available. A study conducted using data from the National Health and Nutrition Examination Survey (NHANES) found that one in four adults in the USA with CKD also had impaired lung function.25 They also revealed that restrictive lung function is common in people with CKD, and that increased levels of urinary albumin were associated with both restrictive and obstructive lung function.
Diabetes is a prevalent cause of CKD. Recent studies have established a connection between hypoxia and right ventricular dysfunction in CKD, with the latter being potentially linked to impaired lung function.26 The associations between kidney function measures and pulmonary dysfunction, however, have not been extensively investigated, particularly in the diabetes population. Thus, we aimed to examine the relationship between measures of kidney function (eGFR and urinary albumin to creatinine ratio (UACR)) and impaired lung function (PRISm and variable obstructive lung function) in those with diabetes. Furthermore, we sought to determine the all-cause mortality risk associated with CKD and/or various spirometry categories. Our study used a large, nationally representative sample of US adults to provide a comprehensive understanding of the association between kidney and lung function.
Methods
Study design and participants
This was a cross-sectional and retrospective cohort study using the NHANES, a national survey of children and adults in the USA conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention.27 Data were obtained by in-person interviews and medical examinations. The interviews collected self-reported information on demographics, socioeconomic status and health conditions, while the medical examinations included a range of physiological measurements and laboratory tests performed by highly trained medical staff. The complex and multistage probability design of NHANES ensured a representative sample of the US population.28 Moreover, NHANES data have been used in many studies, proving its validity and quality.2529,31 Data used in this study were obtained from three consecutive 2-year survey cycles (2007–2012), for spirometry data were measured in these year cycles only.
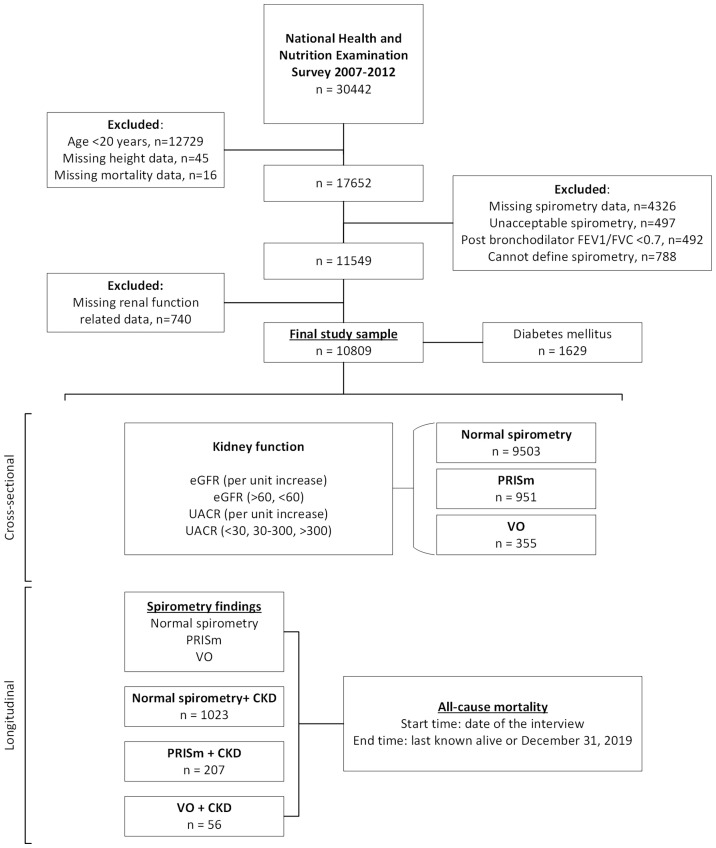
This study included participants aged 20 years or older (n=17 713). We excluded those with missing height data (n=45); missing or unacceptable spirometry data (n=4823); postbronchodilator FEV1/FVC<0.7 (n=492); undefined spirometry (n=788); missing renal function-related data (n=740) and missing mortality data (n=16). Consequently, a total of 10 809 participants were included in the cross-sectional and retrospective cohort analyses (figure 1). In addition, this study complies with the guidelines for reporting cross-sectional studies as specified in the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (online supplemental table 1).32
Figure 1. Study flow chart. National Health and Nutrition Examination Survey 2007–2012. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate (mL/min per 1.73 m2); FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PRISm, preserved ratio impaired spirometry; UACR, urinary albumin to creatinine ratio; VO, variable obstruction.
Assessment of kidney function
The eGFR was calculated using the CKD Epidemiology Collaboration equation.33 The UACR was determined from a spot urine sample and then divided into two categories: less than 30 mg/g and equal to or greater than 30 mg/g. CKD was defined as either an eGFR of less than 60 mL/min/1.73 m2 or a UACR of 30 mg/g or more. The UACR was further categorised into three groups based on the standard cutoffs: normal (less than 30 mg/g), moderately increased albuminuria (30–300 mg/g) and severely increased albuminuria (300 mg/g or more).34 The eGFR was also divided into two categories: less than 60 mL/min/1.73 m2 and greater than 60 mL/min/1.73 m2. We could not use standard eGFR categories (G1–G5) due to insufficient data.34
Assessment of pulmonary function
In the period between 2007 and 2012, the NHANES conducted pulmonary function tests on all adult participants, except for those experiencing chest pain, difficulties with forceful expiration, use of supplemental oxygen, recent surgeries on the eye, chest, or abdomen, recent heart attack, stroke, tuberculosis exposure, coughing up of blood, or a history of a detached retina, collapsed lung, or aneurysm were excluded from the study.35 Ohio 822/827 dry-rolling seal volume spirometers were used to measure pulmonary function during all three survey cycles. NHANES classified the spirometry data based on the quality of data collection, with ‘A’ being the highest quality and exceeding American Thoracic Society standards,36 ‘B’ meeting the standards (ie, adequate technical quality and reproducibility), ‘C’ being potentially usable but not meeting all the standards (ie, some technical issues or lack of reproducibility), ‘D’ being questionable result and ‘F’, invalid. We only used FEV1 and FVC quality grades A, B and C. Quality of the spirometry has been described elsewhere.37
Normal spirometry was defined as an FEV1 ≥80% predicted and an FEV1/FVC ratio ≥0.7. PRISm was defined as a ˂80% predicted and a FEV1/FVC ratio ≥0.7. The presence of variable obstructive lung function was determined by a prebronchodilator FEV1/FVC ratio less than ˂0.7 and a postbronchodilator FEV1/FVC ratio ≥0.7.
Ascertainment of mortality status
The NCHS has linked data from NHANES and other surveys with death certificate records from the National Death Index (NDI) and made available the public-use linked mortality files.38 Mortality status for NHANES participants was ascertained primarily through probabilistic record matching with the NDI through 31 December 2019 using a unique study identifier. More details about the matching methods are available from NCHS.39
Assessment of confounders
In this study, demographic and health information, such as age, sex, race/ethnicity, education, annual household income, smoking status, drinking status and cardiovascular disease (CVD), was self-reported during the household interview. Participants were categorised as current smokers if they had smoked 100 or more cigarettes in their lifetime and reported smoking currently, former smokers if they had smoked 100 or more cigarettes but had quit, and never-smokers if they had not smoked 100 or more cigarettes in their lifetime.40 Body mass index (BMI) was calculated using the participant’s weight in kilograms divided by their height in metres squared. Diabetes was diagnosed using criteria such as the use of insulin or oral hypoglycaemic agents, fasting plasma glucose (FPG) levels of 126 mg/dL or higher, or glycated haemoglobin levels of 6.5% or higher. In a mobile examination centre, three consecutive blood pressure readings were taken. The average of the readings was used in this study. Participants were considered hypertensive if they had a systolic blood pressure of 140 mm Hg or higher and diastolic blood pressure of 90 mm Hg or higher or if they were using antihypertensive medication. The 140/90 mm Hg threshold is based on the International Society of Hypertension guidelines,41 which are commonly applied in clinical practice.
Statistical analysis
In line with the instructions for using NHANES data,27 28 we used the sample weights, clustering and stratification whenever feasible to account for the complex survey design using the ‘survey’ R package (V.4.1-2). This approach allows the final sample to be representative of the US population, despite exclusions. Participant data were downloaded from the NHANES website in November 2022 by the first author.27
We compared baseline characteristics according to spirometry groups by using the analysis of variance for continuous variables and the χ2 test for categorical variables. In the cross-sectional analysis, we employed multivariate logistic regression models to evaluate the association between kidney function indicators—including eGFR (per unit increase) and log-transformed UACR—and spirometry outcomes, specifically PRISm and VO. UACR was log-transformed to address skewness and to meet the assumptions of the logistic regression model. Furthermore, categorical analyses were carried out in three categories of UACR (<30, 30–300 and ≥300 mg/g) and two categories of eGFR (>60 and <60 mL/min per 1.73 m2), where the UACR <30 mg/g and eGFR >60 mL/min per 1.73 m2 categories were used as the reference groups for comparison.
In the retrospective cohort analysis, we defined baseline as the time when participants had their interview. We used person-months, which were counted from baseline to the date of death, loss to follow-up, or 31 December 2019, whichever came first. We used Cox proportional hazards models to calculate the HRs and corresponding 95% CIs for all-cause mortality associated with PRISm and VO. Furthermore, we examined all-cause mortality risk in six groups: (1) normal spirometry without CKD (reference group); (2) normal spirometry with CKD; (3) PRISm without CKD; (4) PRISm with CKD; (5) VO without CKD and (6) VO with CKD. We examined the proportional hazards assumption using Schoenfeld residuals; assumptions were satisfied in all models (global p>0.05).
In all analyses, we adjusted for demographic variables such as age, sex, ethnicity, education and annual household income in model 1. We further adjusted for BMI, hypertension, smoking, CVD and diabetes mellitus (DM) in model 2. In regression models, we checked multicollinearity using vif() function of ‘car’ package (V.3.1-2) in R, where the variance inflation factor was below 2, indicating very low multicollinearity. The statistical analysis was conducted by using R V.4.2.1, and a p<0.05 was established as the threshold for statistical significance in all analyses.
Participants and public involvement
Participants were not involved in the design, conduct, reporting or dissemination plans of our research.
Results
Baseline characteristics
This study included 10 809 participants. Baseline characteristics of the included participants are shown in table 1. The average age of the study participants was 43.43(SE, 0.35) years, and 51.7% of participants were female. Among the included participants, 951 were found to have PRISm and 355 had variable obstructive spirometry (table 1). A noteworthy observation was that the variable obstructive group had a higher mean age (49.6 years) and a higher proportion of male participants (61.9%). The majority of the participants across all spirometry categories had middle/high school education or higher. Additionally, the PRISm group had a lower proportion of individuals earning an annual income of ≥US$65 000 compared with other categories (35.6% vs 64.4%).
Table 1. Baseline characteristics stratified by spirometry categories, NHANES 2007–2012, n=10 809.
| Variable | Entire cohort | Normal | PRISm* | Variable obstruction† |
| n | 10 809 | 9503 | 951 | 355 |
| Age | 43.43 (0.35) | 42.80 (0.35) | 47.87 (0.71) | 49.57 (1.28) |
| Sex | ||||
| Female (%) | 5588 (51.7) | 4966 (52.26) | 487 (52.55) | 135 (38.10) |
| Male (%) | 5221 (48.3) | 4537 (47.74) | 464 (47.45) | 220 (61.90) |
| Ethnicity | ||||
| Mexican American (%) | 1869 (17.29) | 1728 (9.63) | 103 (6.00) | 38 (3.98) |
| Non-Hispanic Black (%) | 2234 (20.67) | 1964 (10.71) | 216 (13.05) | 54(5.71) |
| Non-Hispanic White (%) | 519 (41.81) | 3978 (67.59) | 324 (56.51) | 217 (82.93) |
| Other Hispanic (%) | 231 (11.39) | 1096 (6.06) | 108 (6.44) | 27 (2.70) |
| Others(multiracial) (%) | 956 (8.84) | 737 (6.01) | 200 (18.00) | 19 (4.69) |
| Education | ||||
| College and above (%) | 5800 (53.7) | 5146 (63.21) | 465 (52.79) | 189 (63.10) |
| Middle and high school (%) | 3974 (36.79) | 3447 (31.83) | 392 (41.31) | 135 (34.06) |
| Primary school and less (%) | 1027 (9.51) | 903 (4.96) | 93 (5.90) | 31 (2.85) |
| Annual Household Income | ||||
| <US$65 000 (%) | 6970 (67.17) | 6079 (55.03) | 671 (64.43) | 220 (50.88) |
| ≥US$65 000 (%) | 3406 (32.83) | 3040 (44.97) | 242 (35.57) | 124 (49.12) |
| WCC | 7.10 (0.04) | 7.06 (0.04) | 7.58 (0.11) | 7.20 (0.15) |
| Neutrophils% | 58.25 (0.17) | 58.23 (0.17) | 58.42 (0.45) | 58.53 (0.52) |
| BMI | 28.78 (0.11) | 28.66 (0.12) | 31.39 (0.38) | 26.82 (0.41) |
| Waist circumference | 97.84 (0.31) | 97.39 (0.30) | 104.77 (0.83) | 95.36 (1.12) |
| HOMA_IR | 3.60 (0.09) | 3.50 (0.09) | 5.17 (0.34) | 2.90 (0.25) |
| FPG (mg/dL) | 104.38 (0.67) | 103.44 (0.66) | 115.52 (2.67) | 103.94 (2.18) |
| HbA1c | 5.56 (0.01) | 5.52 (0.01) | 5.99 (0.06) | 5.63 (0.06) |
| CRP | 0.35 (0.01) | 0.34 (0.01) | 0.53 (0.07) | 0.28 (0.03) |
| Diabetes duration | 10.54 (0.52) | 10.39 (0.61) | 11.33 (1.03) | 9.78 (1.19) |
| Pulmonary function | ||||
| FVC (mL) | 4160.42 (15.08) | 4236.20 (14.81) | 3100.05 (36.40) | 4385.11 (69.68) |
| FEV1(mL) | 3313.75 (13.37) | 3403.04 (12.51) | 2395.07 (27.64) | 2968.83 (47.53) |
| FEV1/FVC | 0.80 (0.00) | 0.80 (0.00) | 0.78 (0.00) | 0.68 (0.00) |
| PEF (mL) | 8545.97 (37.18) | 8713.33 (35.71) | 6848.72 (88.19) | 7854.04 (139.94) |
| Postbronchodilator | ||||
| FEV1 | 3299.94 (39.50) | 3614.85 (66.78) | 3077.69 (110.17) | 3184.70 (48.17) |
| FVC | 4421.16 (55.19) | 4673.57 (90.89) | 3980.77 (138.21) | 4346.15 (69.27) |
| FEV1/FVC | 0.75 (0.00) | 0.78 (0.00) | 0.77 (0.01) | 0.73 (0.00) |
| PEF | 8513.87 (101.23) | 8722.72 (173.26) | 8035.92 (344.23) | 8459.33 (126.06) |
| Prebronchodilator | ||||
| FEV1% | 0.98 (0.00) | 1.00 (0.00) | 0.74 (0.00) | 0.86 (0.01) |
| FVC% | 0.99 (0.00) | 1.01 (0.00) | 0.76 (0.00) | 0.99 (0.01) |
| Pre-FEV1/FVC% | 0.99 (0.00) | 0.99 (0.00) | 0.97 (0.00) | 0.86 (0.00) |
| Pre-PEF% | 1.06 (0.00) | 1.08 (0.00) | 0.88 (0.01) | 0.94 (0.01) |
| Difference (post–pre) | ||||
| FEV1 | 0.25 (0.02) | 0.35 (0.01) | −0.79 (0.03) | −0.14 (0.05) |
| FVC | 0.23 (0.01) | 0.30 (0.01) | −0.77 (0.03) | 0.44 (0.07) |
| FEV1/FVC | 0.12 (0.02) | 0.22 (0.02) | −0.17 (0.03) | −1.49 (0.02) |
| PEF | 0.20 (0.02) | 0.27 (0.02) | −0.58 (0.04) | −0.12 (0.06) |
| Kidney function | ||||
| UACR | 21.09 (1.68) | 19.66 (1.89) | 41.86 (6.74) | 15.51 (2.52) |
| eGFR | 98.34 (0.49) | 98.90 (0.50) | 95.61 (0.73) | 90.70 (1.72) |
| UACR (continuous) | 1.98 (0.02) | 1.96 (0.02) | 2.26 (0.06) | 1.94 (0.05) |
| UACR (categorical) | ||||
| <30 mg/g | 9858 (91.2) | 8758 (93.88) | 780 (86.91) | 320 (91.99) |
| ≥300 mg/g | 151 (1.4) | 111 (0.69) | 36 (2.45) | 4 (1.18) |
| 30–300 mg/g | 800 (7.4) | 634 (5.43) | 135 (10.64) | 31 (6.83) |
| Diabetes mellitus | ||||
| No (%) | 9049 (84.74) | 8094 (90.46) | 655 (72.56) | 300 (88.07) |
| Yes (%) | 1629 (15.26) | 1284 (9.54) | 290 (27.44) | 55 (11.93) |
| Chronic kidney disease | ||||
| No (%) | 9523 (88.1) | 8480 (91.44) | 744 (83.12) | 299 (85.24) |
| Yes (%) | 1286 (11.9) | 1023 (8.56) | 207 (16.88) | 56 (14.76) |
| Cardiovascular disease | ||||
| No (%) | 10 156 (93.98) | 9008 (96.00) | 821 (87.88) | 327 (92.45) |
| Yes (%) | 651 (6.02) | 494 (4.00) | 130 (12.12) | 27 (7.55) |
| Smoking | ||||
| Former (%) | 2302 (21.31) | 1981 (21.79) | 217 (22.73) | 104 (28.25) |
| Never (%) | 6273 (58.07) | 5620 (59.53) | 501 (53.69) | 152 (42.02) |
| Current (%) | 2228 (20.62) | 1897 (18.68) | 232 (23.58) | 99 (29.73) |
| Alcohol | ||||
| Drinker (%) | 1588 (15.74) | 1352 (12.94) | 181 (20.11) | 55 (10.79) |
| Never (%) | 1241 (12.3) | 1061 (9.16) | 154 (14.86) | 26 (6.25) |
| Current (%) | 7261 (71.96) | 6456 (77.90) | 551 (65.03) | 254 (82.95) |
| Hypertension | ||||
| No (%) | 7059 (65.31) | 6357 (70.86) | 485 (54.57) | 217 (64.55) |
| Yes (%) | 3750 (34.69) | 3146 (29.14) | 466 (45.43) | 138 (35.45) |
Values are means (SE) or percentages.
PRISm was defined as FEV1<80% predicted and FEV1/FVC≥0.7.
Variable obstruction was defined as prebronchodialator FEV1/FVC<0.7 and postbronchodialator FEV1/FVC≥0.7.
BMIbody mass indexCRPC reactive proteineGFRestimated glomerular filtration rateFEV1forced expiratory volume in 1 sFVCforced vital capacityHbA1chaemoglobin A1cHOME-IRhomeostatic model assessment of insulin resistanceNHANESNational Health and Nutrition Examination SurveyPEFpeak expiratory flowPRISmpreserved ratio impaired spirometryUACRurinary albumin to creatinine ratioWCCwhite cell count
In comparison to those with normal spirometry, individuals with PRISm demonstrated elevated levels of various markers of inflammation and metabolic dysfunction, including higher white cell count, BMI, waist circumference, homeostatic model assessment of insulin resistance, FPG, haemoglobin A1c, C reactive protein and longer duration of DM. This pattern was not observed in the variable obstructive spirometry group. Furthermore, individuals with PRISm were more likely to suffer from comorbidities such as DM, CKD, CVD and hypertension compared with those with normal spirometry. Both PRISm and variable obstructive groups were primarily composed of never-smokers (53.7% and 42.0%, respectively) and current drinkers (65.0% and 83.0%, respectively).
In terms of pulmonary and renal function, individuals with PRISm exhibited lower pulmonary function indices, including FVC, FEV1 and peak expiratory flow, as well as reduced kidney function as indicated by elevated UACR and decreased eGFR. Furthermore, in categorising UACR by clinical thresholds, those with PRISm were more likely to have UACR ≥30 mg/g (13.1% vs 6.1% for normal spirometry and 13.1% vs 8.0% for variable obstructive spirometry, respectively).
Associations between measures of kidney function and spirometry categories
Entire cohort
Compared with normal spirometry, a significant association between an increase in UACR and odds of PRISm (OR 1.10 (95% CI 1.01 to 1.21), p=0.03) was observed. This association remained significant even after full adjustment for potential confounders. In contrast, no significant association was found between a decrease in eGFR and PRISm (table 2).
Table 2. Odds of PRISm and variable obstruction based on eGFR and urinary albumin-creatinine ratio (UACR) in the entire cohort.
| PRISm* | Variable obstruction† | |||||||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| eGFR‡ | 1.00 (1.00,1.01) | 0.30 | 1.00 (1.00,1.01) | 0.28 | 1.00 (0.99,1.00) | 0.31 | 0.99 (0.98,1.00) | 0.11 |
| UACR‡ | 1.24(1.14,1.36) | <0.0001 | 1.10(1.01,1.21) | 0.03 | 0.97 (0.84,1.12) | 0.70 | 0.95 (0.81,1.12) | 0.55 |
| UACR<30 | reference | |||||||
| UACR 30–300 | 1.80(1.42,2.29) | <0.0001 | 1.33 (1.01,1.76) | 0.05 | 1.21 (0.79,1.85) | 0.36 | 1.23 (0.79,1.91) | 0.35 |
| UACR>300 | 3.01(1.68,5.41) | <0.001 | 1.86 (1.01,3.42) | 0.05 | 1.83 (0.47,7.16) | 0.37 | 1.94 (0.47,8.03) | 0.35 |
| eGFR>60 | reference | |||||||
| eGFR<60 | 1.23 (0.85,1.79) | 0.26 | 1.11 (0.78,1.58) | 0.56 | 1.62(1.03,2.56) | 0.04 | 1.72(1.07,2.74) | 0.03 |
Model 1: adjusted by age, sex, ethethnicity, education, annual household income; Model 2: adjusted by model 1+BMI, hypertension, smoking, cardiovascular disease;.PRISm was defined as FEV predicted and FEV/; was defined as pre-bronchodialator FEV/ post-bronchodialator FEV/; As continuous variable
Bold values denote statistical significance (p < 0.05)
PRISm was defined as FEV1<80% predicted and FEV1/FVC≥0.7.
Variable obstruction was defined as prebronchodialator FEV1/FVC<0.7 and postbronchodialator FEV1/FVC≥0.7.
As continuous variable.
BMIbody mass indexeGFRestimated glomerular filtration rateFEV1forced expiratory volume in 1 sFVCforced vital capacityPRISmpreserved ratio impaired spirometry
Both moderately and severely increased albuminuria significantly increased the odds of PRISm (OR 1.80 (95% CI 1.42 to 2.29), p<0.0001 and 3.01 (95% CI 1.68 to 5.41), p<0.001, respectively) after controlling for demographic variables such as age, sex, ethnicity, education and annual household income. No significant association was observed in the variable obstructive group. Additionally, individuals with eGFR <60 mL/min/1.73 m2 had significantly increased odds of VO compared with those with eGFR >60 mL/min/1.73 m2 (OR 1.72 (95% CI 1.07 to 2.74), p=0.03).
Additional analysis (online supplemental table 2) revealed a significant association between CKD and an increased odds of both PRISm and variable obstructive lung function after full adjustment (OR 1.29 (95% CI 1.02 to 1.64), p=0.03 and 1.53 (95% CI 1.08 to 2.16), p=0.02, respectively). Furthermore, individuals with both CKD and DM had increased odds of having PRISm; however, this association lost significance after full adjustment.
Participants with diabetes
Compared with normal spirometry, an increase in UACR was associated with higher odds of PRISm in individuals with diabetes (OR 1.21 (95% CI 1.08 to 1.36), p=0.002) after full adjustment. Similar to the overall cohort, an increase in UACR was not associated with variable obstructive lung function in individuals with diabetes (as shown in table 3).
Table 3. Odds of PRISm and variable obstruction based on eGFR and urinary albumin-creatinine ratio (UACR) among individuals with diabetes.
| PRISm* | Variable obstruction† | |||||||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| eGFR‡ | 1.00 (0.99, 1.01) | 0.59 | 1.00 (0.99, 1.01) | 0.93 | 0.99 (0.96,1.02) | 0.44 | 0.99 (0.96,1.01) | 0.39 |
| UACR‡ | 1.27 (1.13, 1.43) | <0.001 | 1.21 (1.08, 1.36) | 0.002 | 0.94 (0.71,1.24) | 0.66 | 0.90 (0.68,1.18) | 0.43 |
| UACR<30 | Reference | |||||||
| UACR 30–300 | 1.59 (1.12, 2.26) | 0.01 | 1.33 (0.89, 1.99) | 0.16 | 1.08 (0.33,3.52) | 0.89 | 0.99 (0.35,2.81) | 0.99 |
| UACR>300 | 2.78 (1.47, 5.26) | 0.002 | 2.34 (1.23, 4.47) | 0.01 | 1.01 (0.12,8.43) | 0.99 | 1.00 (0.15,6.81) | 1.00 |
| eGFR>60 | Reference | |||||||
| eGFR<60 | 1.07 (0.71, 1.61) | 0.74 | 1.05 (0.69, 1.61) | 0.81 | 2.56 (0.74,8.87) | 0.13 | 2.43 (0.65,9.09) | 0.18 |
Model 1: adjusted by age, sex, ethethnicity, education, annual household income; Model 2: adjusted by model 1+BMI, hypertension, smoking, cardiovascular disease;.PRISm was defined as FEV predicted and FEV/; was defined as pre-bronchodialator FEV/ post-bronchodialator FEV/; As continuous variable
Bold values denote statistical significance (p < 0.05)
PRISm was defined as FEV1<80% predicted and FEV1/FVC≥0.7.
Variable obstruction was defined as prebronchodialator FEV1/FVC<0.7 and postbronchodialator FEV1/FVC≥0.7.
As continuous variable.
BMIbody mass indexeGFRestimated glomerular filtration rateFEV1forced expiratory volume in 1 sFVCforced vital capacityPRISmpreserved ratio impaired spirometry
Compared with normal UACR, those with severely increased albuminuria had significantly higher odds of having PRISm (OR 2.34 (95% CI 1.23 to 4.47), p=0.01). Although a higher risk of VO was observed for individuals with diabetes and eGFR<60 mL/min/1.73 m2, it was statistically insignificant (OR 2.43 (95% CI 0.65 to 9.09), p=0.18).
Mortality risk associated with spirometry categories and CKD
Among 10 809 participants, 634 deaths occurred during a mean follow-up of 9.8 years (105 737.5 person-years). After controlling potential confounding variables, PRISm was associated with elevated risk for all-cause mortality (HR 1.61 (95% CI 1.21 to 2.13), p≤0.001) (table 4). A similar trend was observed in those with diabetes, but it was not statistically significant after full adjustment. However, those with PRISm still had a relatively high risk of mortality (HR 1.47 (95% CI 0.98, 2.21), p=0.06). Unadjusted analysis indicated that the all-cause mortality risk associated with variable obstructive lung function was significantly higher, but the significance diminished after adjustment for confounders.
Table 4. All-cause mortality risk associated with PRISm and variable obstruction among the entire cohort and individuals with diabetes.
| Entire cohort | Diabetes* | |||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Normal function | Reference | Reference | ||
| PRISm† | ||||
| Model 1 | 2.03(1.49, 2.76) | <0.0001 | 1.64(1.10, 2.45) | 0.02 |
| Model 2 | 1.61(1.21, 2.13) | <0.001 | 1.47 (0.98, 2.21) | 0.06 |
| Variable obstruction‡ | ||||
| Model1 | 1.27 (0.73, 2.22) | 0.39 | 0.73 (0.20, 2.67) | 0.63 |
| Model2 | 1.17 (0.68, 2.02) | 0.57 | 0.59 (0.15, 2.26) | 0.44 |
Model 1: adjusted by age, sex, ethnicity, education, annual household income; Model 2: adjusted by model1+BMI, hypertension, smoking, cardiovascular disease, diabetes mellitus.
Bold values denote statistical significance (p < 0.05)
Diabetes was defined based on the use of insulin or oral hypoglycaemic agents, fasting plasma glucose ≥126 mg/dL or glycated haemoglobin ≥6.5%.
PRISm was defined as FEV1<80% predicted and FEV1/FVC≥0.7.
Variable obstruction was defined as prebronchodialator FEV1/FVC<0.7 and postbronchodialator FEV1/FVC≥0.7.
BMIbody mass indexFEV1forced expiratory volume in 1 sFVCforced vital capacityPRISmpreserved ratio impaired spirometry
In the diabetic group, compared with normal spirometry without CKD, individuals with PRISm and CKD had an increased all-cause mortality risk (online supplemental table 3). Although a similar trend was observed for participants with normal lung function and CKD, the fully adjusted HR was relatively higher (HR 3.46 (95% CI 1.94 to 6.16), p<0.0001) for those with PRISm and CKD. When compared with PRISm without CKD, those with CKD and PRISm had a significantly increased risk of all-cause mortality (online supplemental table 4). No significant associations were found between all-cause mortality risk and PRISm without CKD, VO with CKD and VO without CKD.
Discussion
In a representative sample of US adults, we found that elevated UACR was associated with higher odds of PRISm. Furthermore, severely increased albuminuria (UACR ≥300 mg/g) increases the risk of PRISm in individuals with diabetes. We also observed that having PRISm and CKD together was associated with a significantly higher risk of all-cause mortality. To our knowledge, this is the first study to investigate the risk of PRISm associated with measures of kidney function.
Albuminuria25 and CKD42 increase the risk of restrictive lung function, defined by FVC ˂80% predicted and FEV1/FVC ratio ≥0.7. Navaneethan et al also observed that a decrease in eGFR was associated with higher odds of obstructive lung function.25 Atherosclerosis Risk in Communities study with a 25-year follow-up revealed that lower FVC (%predict) and restrictive spirometry increase the risk of end-stage renal disease (ESRD).43 In contrast, lower lung function indices such as FEV1 and FVC correlate with incident CKD, suggesting a potential bidirectional relationship.44 45 Interestingly, Kirkman et al46 used cardiopulmonary exercise test data and found that individuals with CKD had reduced peak oxygen uptake (VO2 max) compared with healthy participants. Furthermore, a study of Japanese patients showed that proteinuria and decreased eGFR were associated with reduced DLCO.47 Most of the evidence studied associations between kidney function and restrictive spirometry. However, our study focused on PRISm, defined by FEV1 ˂80% predicted and FEV1/FVC ratio ≥0.7. We found that an increase in UACR and albuminuria increases the risk of PRISm. Recently, PRISm has been proposed as an early phase of chronic obstructive pulmonary disease (COPD) as PRISm can transition to COPD48; albuminuria was found to reduce lung function and increase incident COPD.49 Overall, our findings, with previous evidence, point out that kidney dysfunction contributes to lung function impairment. However, this association is still an area of research in diabetes despite diabetes being a leading cause of CKD. This study revealed that individuals with diabetes and albuminuria were at a higher risk of restrictive lung disease (OR 8.57 (95% CI 3.4 to 21.9)).50 Moreover, those with diabetes and albuminuria were reported to have lower FVC, FEV1, and higher dyspnoea scores.51 Similarly, we observed a higher risk of PRISm in those with severe albuminuria and diabetes. Albuminuria and eGFR may be predictors of ESRD attributed to diabetes as severely increased albuminuria was linked with an increased risk of ESRD incidence, and incorporating eGFR values with albuminuria improved the prediction of ESRD incidence.52 We found that those with PRISm and CKD were at higher risk of all-cause death compared with those without, emphasising the need to evaluate not only kidney function but also lung function in patients with diabetes.
The mechanisms underlying the interplay between kidney and lung remain to be elucidated. Kidneys are responsible for fluid homoeostasis, acid-base balance and vascular tone. Changes in these might lead to lung dysfunction. Hypoxia has been shown to cause renal vasoconstriction, leading to impaired renal perfusion, as well as increased inflammation and oxidative stress, which can contribute to the development of albuminuria.53 Systemic inflammation, commonly seen in CKD, may cause lung function impairment.54 The relationship between kidney function and lung function is further complicated by factors such as central venous pressure and endothelial dysfunction,26 55 both of which have been linked to impaired renal and lung function.56 57 Metabolic syndrome components have been linked to kidney58 and lung function,59 suggesting a potential mediation. The pathophysiology between these two organs is likely multifactorial and requires further investigation.
More and more people, especially older people, are suffering from multimorbidity, characterised as having two or more chronic diseases simultaneously. We found that diabetic individuals with impaired lung function and CKD are at high risk of all-cause death, suggesting that early monitoring of kidney function in individuals with pulmonary dysfunction could help identify those at risk. Our findings underscore the critical importance of addressing both CKD and lung dysfunction, especially in patients with diabetes. However, further research is needed to clarify and confirm this association, elucidate the underlying mechanisms, identify high-risk populations and develop interventions to prevent or treat lung dysfunction in patients with CKD.
Our study had several strengths; we used a large and nationally representative sample, including participants from NHANES 2007 to 201225 survey cycles with high-quality spirometry data. Additionally, we also analysed associations between measures of kidney function, spirometry types and mortality in individuals with diabetes. Research in this direction mostly uses a fixed ratio of FEV1/FVC ˂0.70 (COPD), which may lead to underdiagnosis of COPD in younger individuals and overdiagnosis in older individuals. Following Buhr et al,20 we included those with VO, also known as bronchodilator responsiveness, defined by prebronchodilator FEV1/FVC ratio ˂0.7 and a postbronchodilator FEV1/FVC ratio ≥0.7. However, present study results should be interpreted with caution as they are subject to a few limitations. Due to the cross-sectional nature of this study, the causality of associations cannot be proven. Additionally, our study only included US population, so our findings may not apply to other populations. Considering the study sample size, participants with diabetes were relatively less. Moreover, the odds of PRISm and mortality risk were not compared with those without diabetes. Therefore, our findings need further validation through additional research with larger samples. Furthermore, some covariates used in the study depended on the self-report by the participant, which may produce biases such as recall and social desirability bias. Unfortunately, we could only use two clinical categories of eGFR (<60 and >60) due to limited data, while eGFR from 60 to 89 may also represent kidney damage. Future studies should use more clinical categories and analyse their impact on the lungs. Although we controlled for potential risk factors, residual confounding cannot be ruled out entirely due to the complex pathophysiology between kidney disease and lung function. For example, in polycystic kidney disease, cysts may develop in the liver, which may hinder the expansion of the thoracic cavity, potentially impacting lung function. Moreover, we could not conduct the planned sensitivity analysis to exclude participants with lung cancer due to high non-response rates. However, we did assess the robustness of our findings by analysing all-cause mortality risk with alternative reference categories (online supplemental table 4). From a statistical point of view, some limitations should also be acknowledged. In logistic regression models, we did not conduct formal assessments of validity, variable selection or goodness of fit. This may influence our findings’ robustness and generalisability, leading to potential biases. However, this method has been used in similar NHANES studies.31 In Cox regression, we only tested the fundamental assumption of proportional hazard (online supplemental table 5 and 6). Violations of other assumptions could lead to biased estimates and affect the validity of findings. Finally, we did not apply multiple testing corrections, which may impact the interpretability of findings. As our analysis was exploratory, we presented uncorrected p values, aligning with our study objectives. Future studies should compare the risk for PRISm and death in individuals with and without diabetes. It would be interesting to see whether pulmonary function indices (FEV1, FVC, etc) are related with eGFR and/or UACR.
Conclusion
Our study found that an increased UACR was associated with a higher risk of PRISm and having CKD and PRISm together increases the risk of all-cause mortality. Our study emphasises the need to evaluate lung function in patients with CKD and highlights the intricate relationship between the two organs. Future large-scale prospective studies are necessary to clarify the associations. The underlying mechanisms are unclear and warrant further research.
supplementary material
Footnotes
Funding: None
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-075955).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Data availability free text: Open access data are available at the NHANES website (http://www.cdc.gov/nchs/nhanes.htm).
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Ethics approval: NHANES was approved by the National Center for Health Statistics Research Ethics Review Board under Continuation of Protocol. The data were obtained from NHANES and all subjects signed the informed consent during the recruitment period.
Contributor Information
Ikramulhaq Patel, Email: ikramulhaq@mail.ccmu.edu.cn.
Hong-Jian Gong, Email: 1161249624@qq.com.
Hui Xu, Email: xh1195361950@163.com.
Yin-He Chai, Email: 1149103351@qq.com.
Yu-Shun Qiao, Email: 574230547@qq.com.
Jin-Yan Zhang, Email: 3055828525@qq.com.
Meng-Ting Zhang, Email: 545300328@qq.com.
Coen D A Stehouwer, Email: cda.stehouwer@gmail.com.
Jianbo Zhou, Email: jbzhou@ccmu.edu.cn.
Data availability statement
The datasets used and/or analysed in the current study are available from the corresponding author on reasonable request.
References
- 1.Wijnant SRA, De Roos E, Kavousi M, et al. Trajectory and mortality of preserved ratio impaired spirometry: the Rotterdam Study. Eur Respir J. 2020;55:1901217. doi: 10.1183/13993003.01217-2019. [DOI] [PubMed] [Google Scholar]
- 2.Wan ES, Castaldi PJ, Cho MH, et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15:89. doi: 10.1186/s12931-014-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mannino DM, McBurnie MA, Tan W, et al. Restricted spirometry in the Burden of Lung Disease Study. Int J Tuberc Lung Dis. 2012;16:1405–11. doi: 10.5588/ijtld.12.0054. [DOI] [PubMed] [Google Scholar]
- 4.Higbee DH, Granell R, Davey Smith G, et al. Prevalence, risk factors, and clinical implications of preserved ratio impaired spirometry: a UK Biobank cohort analysis. Lancet Respir Med. 2022;10:149–57. doi: 10.1016/S2213-2600(21)00369-6. [DOI] [PubMed] [Google Scholar]
- 5.Wan ES, Balte P, Schwartz JE, et al. Association Between Preserved Ratio Impaired Spirometry and Clinical Outcomes in US Adults. JAMA. 2021;326:2287–98. doi: 10.1001/jama.2021.20939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marott JL, Ingebrigtsen TS, Çolak Y, et al. Trajectory of Preserved Ratio Impaired Spirometry: Natural History and Long-Term Prognosis. Am J Respir Crit Care Med. 2021;204:910–20. doi: 10.1164/rccm.202102-0517OC. [DOI] [PubMed] [Google Scholar]
- 7.Sin DD, Wu L, Man SFP. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–9. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal SK, Heiss G, Barr RG, et al. Airflow obstruction, lung function, and risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) study. Eur J Heart Fail. 2012;14:414–22. doi: 10.1093/eurjhf/hfs016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Truelsen T, Prescott E, Lange P, et al. Lung function and risk of fatal and non-fatal stroke. The Copenhagen City Heart Study. Int J Epidemiol. 2001;30:145–51. doi: 10.1093/ije/30.1.145. [DOI] [PubMed] [Google Scholar]
- 10.Schünemann HJ, Dorn J, Grant BJ, et al. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. 2000;118:656–64. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 11.Hole DJ, Watt GC, Davey-Smith G, et al. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313:711–5. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mannino DM, Higuchi K, Yu T-C, et al. Economic Burden of COPD in the Presence of Comorbidities. Chest. 2015;148:138–50. doi: 10.1378/chest.14-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest. 2015;147:989–98. doi: 10.1378/chest.14-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheaton AG, Ford ES, Thompson WW, et al. Pulmonary function, chronic respiratory symptoms, and health-related quality of life among adults in the United States--National Health and Nutrition Examination Survey 2007-2010. BMC Public Health. 2013;13:854. doi: 10.1186/1471-2458-13-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Borst B, Gosker HR, Zeegers MP, et al. Pulmonary function in diabetes: a metaanalysis. Chest. 2010;138:393–406. doi: 10.1378/chest.09-2622. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R-H, Cai Y-H, Shu L-P, et al. Bidirectional relationship between diabetes and pulmonary function: a systematic review and meta-analysis. Diabetes Metab. 2021;47:101186. doi: 10.1016/j.diabet.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Li G, Jankowich MD, Lu Y, et al. Preserved Ratio Impaired Spirometry, Metabolomics, and the Risk of Type 2 Diabetes. J Clin Endocrinol Metab. 2023;108:e769–78. doi: 10.1210/clinem/dgad140. [DOI] [PubMed] [Google Scholar]
- 18.Lecube A, Simó R, Pallayova M, et al. Pulmonary Function and Sleep Breathing: Two New Targets for Type 2 Diabetes Care. Endocr Rev. 2017;38:550–73. doi: 10.1210/er.2017-00173. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Lu Y, Qiao Y, et al. Role of Pulmonary Function in Predicting New-Onset Cardiometabolic Diseases and Cardiometabolic Multimorbidity. Chest. 2022;162:421–32. doi: 10.1016/j.chest.2021.12.663. [DOI] [PubMed] [Google Scholar]
- 20.Buhr RG, Barjaktarevic IZ, Quibrera PM, et al. Reversible Airflow Obstruction Predicts Future Chronic Obstructive Pulmonary Disease Development in the SPIROMICS Cohort: An Observational Cohort Study. Am J Respir Crit Care Med. 2022;206:554–62. doi: 10.1164/rccm.202201-0094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill NR, Fatoba ST, Oke JL, et al. Global Prevalence of Chronic Kidney Disease – A Systematic Review and Meta-Analysis. PLoS ONE. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coresh J, Selvin E, Stevens LA, et al. Prevalence of Chronic Kidney Disease in the United States. JAMA. 2007;298:2038. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. The Lancet. 2012;379:815–22. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 24.Dal Canto E, Elders PJM, Heijden AA, et al. Kidney function measures and cardiovascular outcomes in people with diabetes: the Hoorn Diabetes Care System cohort. Diabetologia. 2022 doi: 10.1007/s00125-022-05826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navaneethan SD, Mandayam S, Arrigain S, et al. Obstructive and Restrictive Lung Function Measures and CKD: National Health and Nutrition Examination Survey (NHANES) 2007-2012. Am J Kidney Dis. 2016;68:414–21. doi: 10.1053/j.ajkd.2016.03.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damman K, van Deursen VM, Navis G, et al. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–8. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC) About the national health and nutrition examination survey. 2024. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm Available.
- 28.National health and nutrition examination survey (NHANES) survey methods and analytic guidelines. 2024. https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx Available.
- 29.Lim HI, Jun SJ, Lee SW. Glomerular hyperfiltration may be a novel risk factor of restrictive spirometry pattern: Analysis of the Korea National Health and Nutrition Examination Survey (KNHANES) 2009-2015. PLoS One. 2019;14:e0223050. doi: 10.1371/journal.pone.0223050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Qian H, Zhong S, et al. The relationship between triglyceride-glucose index and albuminuria in United States adults. Front Endocrinol. 2023;14:1215055. doi: 10.3389/fendo.2023.1215055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu TD, Fawzy A, Brigham E, et al. Association of Triglyceride-Glucose Index and Lung Health: A Population-Based Study. Chest. 2021;160:1026–34. doi: 10.1016/j.chest.2021.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elm E von, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007:806–8. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levey AS, Stevens LA, Schmid CH, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med. 2009;150:604. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens PE, Ahmed SB, Carrero JJ, et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105:S117–314. doi: 10.1016/j.kint.2023.10.018. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention Centers for disease control and prevention spirometry - 1st test & 2nd test bronchodilator studies. 2014
- 36.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 37.National Health and Nutrition Examination Survey 2011-2012 data documentation, codebook, and frequencies. spirometry - pre and post-bronchodilator (SPX_G) 2011. https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/SPX_G.htm Available.
- 38.National Center for Health Statistics Division of Analysis and Epidemiology Continuous NHANES public-use linked mortality files. 2019. https://www.cdc.gov/nchs/data-linkage/mortality-public.htm Available.
- 39.National Center for Health Statistics The linkage of national center for health statistics survey data to the national death index — 2019 linked mortality file (LMF) linkage methodology and analytic considerations. 2022. https://www.cdc.gov/nchs/data-linkage/mortality-methods.htm Available.
- 40.Ford ES, Mannino DM, Wheaton AG, et al. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination surveys from 1988-1994 to 2007-2010. Chest. 2013;143:1395–406. doi: 10.1378/chest.12-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 42.Kim J, Lee C-H, Lee HY, et al. Association between Comorbidities and Preserved Ratio Impaired Spirometry: Using the Korean National Health and Nutrition Examination Survey IV-VI. Respiration. 2022;101:25–33. doi: 10.1159/000517599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sumida K, Kwak L, Grams ME, et al. Lung Function and Incident Kidney Disease: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2017;70:675–85. doi: 10.1053/j.ajkd.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaigham S, Christensson A, Wollmer P, et al. Low lung function and the risk of incident chronic kidney disease in the Malmö Preventive Project cohort. BMC Nephrol. 2020;21:124. doi: 10.1186/s12882-020-01758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim SH, Kim HS, Min HK, et al. Obstructive spirometry pattern and the risk of chronic kidney disease: analysis from the community-based prospective Ansan-Ansung cohort in Korea. BMJ Open. 2021;11:e043432. doi: 10.1136/bmjopen-2020-043432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirkman DL, Muth BJ, Stock JM, et al. Cardiopulmonary exercise testing reveals subclinical abnormalities in chronic kidney disease. Eur J Prev Cardiol. 2018;25:1717–24. doi: 10.1177/2047487318777777. [DOI] [PubMed] [Google Scholar]
- 47.Nakade Y, Toyama T, Furuichi K, et al. Impact of kidney function and urinary protein excretion on pulmonary function in Japanese patients with chronic kidney disease. Clin Exp Nephrol. 2014;18:763–9. doi: 10.1007/s10157-013-0920-7. [DOI] [PubMed] [Google Scholar]
- 48.Lange P, Celli B, Agustí A, et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med. 2015;373:111–22. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 49.Oelsner EC, Balte PP, Grams ME, et al. Albuminuria, Lung Function Decline, and Risk of Incident Chronic Obstructive Pulmonary Disease. The NHLBI Pooled Cohorts Study. Am J Respir Crit Care Med. 2019;199:321–32. doi: 10.1164/rccm.201803-0402OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kopf S, Groener JB, Kender Z, et al. Breathlessness and Restrictive Lung Disease: An Important Diabetes-Related Feature in Patients with Type 2 Diabetes. Respiration. 2018;96:29–40. doi: 10.1159/000488909. [DOI] [PubMed] [Google Scholar]
- 51.Klein OL, Aviles-Santa L, Cai J, et al. Hispanics/Latinos With Type 2 Diabetes Have Functional and Symptomatic Pulmonary Impairment Mirroring Kidney Microangiopathy: Findings From the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Diabetes Care. 2016;39:2051–7. doi: 10.2337/dc16-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berhane AM, Weil EJ, Knowler WC, et al. Albuminuria and estimated glomerular filtration rate as predictors of diabetic end-stage renal disease and death. Clin J Am Soc Nephrol. 2011;6:2444–51. doi: 10.2215/CJN.00580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and markers of inflammation: data from the Third National Health and Nutrition Examination. Am J Med. 2003;114:758–62. doi: 10.1016/s0002-9343(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 54.Mukai H, Ming P, Lindholm B, et al. Restrictive lung disorder is common in patients with kidney failure and associates with protein-energy wasting, inflammation and cardiovascular disease. PLoS One. 2018;13:e0195585. doi: 10.1371/journal.pone.0195585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anand IS. Cardiorenal syndrome: a cardiologist’s perspective of pathophysiology. Clin J Am Soc Nephrol. 2013;8:1800–7. doi: 10.2215/CJN.04090413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stam F, van Guldener C, Schalkwijk CG, et al. Impaired renal function is associated with markers of endothelial dysfunction and increased inflammatory activity. Nephrol Dial Transplant. 2003;18:892–8. doi: 10.1093/ndt/gfg080. [DOI] [PubMed] [Google Scholar]
- 57.Hancox RJ, Thomas L, Williams MJA, et al. Associations between lung and endothelial function in early middle age. Respirology. 2020;25:89–96. doi: 10.1111/resp.13556. [DOI] [PubMed] [Google Scholar]
- 58.Thomas G, Sehgal AR, Kashyap SR, et al. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2011;6:2364–73. doi: 10.2215/CJN.02180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen W-L, Wang C-C, Wu L-W, et al. Relationship between lung function and metabolic syndrome. PLoS One. 2014;9:e108989. doi: 10.1371/journal.pone.0108989. [DOI] [PMC free article] [PubMed] [Google Scholar]