Abstract
Background
In patients with chronic obstructive pulmonary disease (COPD), the clinical use of the minute ventilation-carbon dioxide production (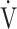 E-
E-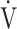 CO2) slope has been reported as a measure of exercise efficiency, but the oxygen uptake efficiency slope (OUES), i.e., the slope of oxygen uptake (
CO2) slope has been reported as a measure of exercise efficiency, but the oxygen uptake efficiency slope (OUES), i.e., the slope of oxygen uptake (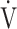 O2) versus the logarithmically transformed
O2) versus the logarithmically transformed 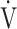 E, has rarely been reported.
E, has rarely been reported.
Methods
We hypothesized that the 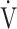 E-
E-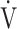 CO2 slope is more useful than OUES in clinical use for the pathophysiological evaluation of COPD. Then, we investigated the cardiopulmonary exercise testing parameters affecting each of these slopes in 122 patients with all Global Initiative for Chronic Obstructive Lung Disease (GOLD) COPD grades selected from our database.
CO2 slope is more useful than OUES in clinical use for the pathophysiological evaluation of COPD. Then, we investigated the cardiopulmonary exercise testing parameters affecting each of these slopes in 122 patients with all Global Initiative for Chronic Obstructive Lung Disease (GOLD) COPD grades selected from our database.
Results
Compared with the GOLD I-II group (n = 51), peak 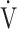 O2 (p < 0.0001), OUES (p = 0.0161),
O2 (p < 0.0001), OUES (p = 0.0161), 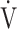 E at peak exercise (p < 0.0001), and percutaneous oxygen saturation (SpO2) at peak exercise (p = 0.0004) were significantly lower in the GOLD III-IV group (n = 71). The GOLD III-IV group was divided into two groups by the exertional decrease in SpO2 from rest to peak exercise: 3% or less (the non-desaturation group: n = 23), or greater than 3% (the desaturation group: n = 48). OUES correlated only weakly with peak
E at peak exercise (p < 0.0001), and percutaneous oxygen saturation (SpO2) at peak exercise (p = 0.0004) were significantly lower in the GOLD III-IV group (n = 71). The GOLD III-IV group was divided into two groups by the exertional decrease in SpO2 from rest to peak exercise: 3% or less (the non-desaturation group: n = 23), or greater than 3% (the desaturation group: n = 48). OUES correlated only weakly with peak 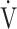 O2,
O2, 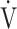 E at peak exercise, and the difference between inspired and expired mean O2 concentrations (ΔFO2) at peak exercise, i.e., an indicator of oxygen consumption ability throughout the body, in the GOLD III-IV group with exertional hypoxemia. In contrast, the
E at peak exercise, and the difference between inspired and expired mean O2 concentrations (ΔFO2) at peak exercise, i.e., an indicator of oxygen consumption ability throughout the body, in the GOLD III-IV group with exertional hypoxemia. In contrast, the 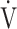 E-
E-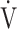 CO2 slope was significantly correlated with ΔFO2 at peak exercise, regardless of the COPD grade and exertional desaturation. Across all COPD stages, there was no correlation between the
CO2 slope was significantly correlated with ΔFO2 at peak exercise, regardless of the COPD grade and exertional desaturation. Across all COPD stages, there was no correlation between the 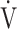 E-
E-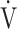 CO2 slope and
CO2 slope and 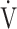 E at peak exercise, and stepwise analysis identified peak
E at peak exercise, and stepwise analysis identified peak 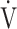 O2 (p = 0.0345) and ΔFO2 (p < 0.0001) as variables with a greater effect on the
O2 (p = 0.0345) and ΔFO2 (p < 0.0001) as variables with a greater effect on the 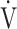 E-
E-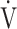 CO2 slope.
CO2 slope.
Conclusions
The OUES may be less useful in advanced COPD with exertional hypoxemia. The 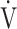 E-
E-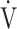 CO2 slope, which is independent of
CO2 slope, which is independent of 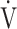 E, focuses on oxygen consumption ability and exercise tolerance in COPD, regardless of the exertional hypoxemia level and COPD grade. Therefore, the
E, focuses on oxygen consumption ability and exercise tolerance in COPD, regardless of the exertional hypoxemia level and COPD grade. Therefore, the 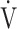 E-
E-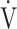 CO2 slope might be useful in establishing or evaluating tailor-made therapies for individual patient’s pathologies in COPD as an indicator focusing on oxygen consumption ability.
CO2 slope might be useful in establishing or evaluating tailor-made therapies for individual patient’s pathologies in COPD as an indicator focusing on oxygen consumption ability.
Supplementary Information
The online version contains supplementary material available at10.1186/s12890-024-03312-2.
Keywords: Oxygen, Carbon dioxide, Exercise tolerance, Gas exchange, Pulmonary rehabilitation, Ventilatory efficiency
Background
With the aging of the population, there is an urgent need to improve and maintain exercise tolerance irrespective of disease severity in chronic obstructive pulmonary disease (COPD) patients [1–3]. In cardiopulmonary exercise testing (CPET) using expiratory gas analysis, all parameters are calculated from directly measured ventilation flow and carbon dioxide (CO2) and oxygen (O2) concentrations [4]. As shown in the online supplementary Methods, oxygen uptake (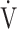 O2), is calculated using the following equation:
O2), is calculated using the following equation:
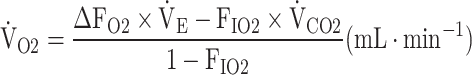 |
where FIO2 and FEO2 are inspired and expired mean O2 concentrations, respectively, 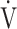 E is minute ventilation, and
E is minute ventilation, and 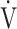 CO2 is carbon dioxide output. Based on the above equation and defining ΔFO2 = FIO2 − FEO2,
CO2 is carbon dioxide output. Based on the above equation and defining ΔFO2 = FIO2 − FEO2, 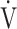 O2 is considered dependent on
O2 is considered dependent on 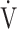 E and ΔFO2. ΔFO2 is considered to reflect the ability of oxygen consumption processed by the entire body, including the muscles [4, 5], as shown in online supplementary Figure S1. Given that the primary indicator of exercise tolerance in CPET is peak
E and ΔFO2. ΔFO2 is considered to reflect the ability of oxygen consumption processed by the entire body, including the muscles [4, 5], as shown in online supplementary Figure S1. Given that the primary indicator of exercise tolerance in CPET is peak 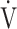 O2 [4, 6], information on the individual contributions of
O2 [4, 6], information on the individual contributions of 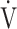 E or ΔFO2 to exercise tolerance, might be important when formulating treatment plans by considering which of the two factors has greater potential for improving exercise intolerance. However, this assumption has not been adequately evaluated in COPD.
E or ΔFO2 to exercise tolerance, might be important when formulating treatment plans by considering which of the two factors has greater potential for improving exercise intolerance. However, this assumption has not been adequately evaluated in COPD.
We previously reported that ΔFO2, which is directly measured in incremental exercise at peak exercise, has a strong negative correlation with ventilatory efficiency, indicated (1) by the 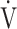 E-
E-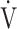 CO2 slope, which indicates the degree of CO2 clearance during exercise [7, 8], and (2) by the
CO2 slope, which indicates the degree of CO2 clearance during exercise [7, 8], and (2) by the 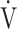 E/
E/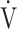 CO2 nadir, which is defined as the lowest value of the ratio between
CO2 nadir, which is defined as the lowest value of the ratio between 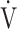 E and
E and 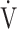 CO2 during exercise, but is not well correlated with
CO2 during exercise, but is not well correlated with 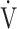 E/
E/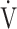 CO2 intercept, that is, the Y intercept from the
CO2 intercept, that is, the Y intercept from the 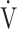 E versus
E versus 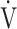 CO2 regression line [9]. Furthermore, the only indicators that were independent of
CO2 regression line [9]. Furthermore, the only indicators that were independent of 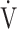 E at peak exercise were ΔFO2 and the
E at peak exercise were ΔFO2 and the 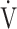 E-
E-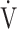 CO2 slope [9]. Therefore, of the three ventilatory efficiency indicators, this study focused on the
CO2 slope [9]. Therefore, of the three ventilatory efficiency indicators, this study focused on the 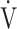 E-
E-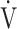 CO2 slope. We also previously reported that, after pulmonary rehabilitation in patients with advanced COPD, the increased ΔFO2 at peak exercise in incremental exercise was correlated with the improvement in not only ventilatory efficiency, but also peak
CO2 slope. We also previously reported that, after pulmonary rehabilitation in patients with advanced COPD, the increased ΔFO2 at peak exercise in incremental exercise was correlated with the improvement in not only ventilatory efficiency, but also peak 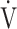 O2. However, the increase in
O2. However, the increase in 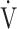 E does not affect the improvement in the
E does not affect the improvement in the 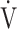 E-
E-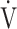 CO2 slope [10]. Furthermore, no significant correlation was confirmed between the change in
CO2 slope [10]. Furthermore, no significant correlation was confirmed between the change in 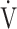 E and that in ΔFO2 at peak exercise following pulmonary rehabilitation. Interestingly, the calculated ratio of oxygen uptake to ventilation (i.e.,
E and that in ΔFO2 at peak exercise following pulmonary rehabilitation. Interestingly, the calculated ratio of oxygen uptake to ventilation (i.e., 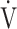 O2/
O2/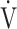 E), which is almost equivalent, but not equal, to actually measured ΔFO2, is known as Oxygen Uptake Efficiency, and its highest plateau (OUEP) during incremental exercise has been reported as a useful factor for assessing the severity of dysfunction and as a prognostic factor for mortality and morbidity in patients with chronic heart failure, though it has not been evaluated in COPD [11]. More in depth knowledge of specialized indicators that reflect the ability of oxygen consumption to increase ΔFO2 during exercise separately from ventilatory ability to increase
E), which is almost equivalent, but not equal, to actually measured ΔFO2, is known as Oxygen Uptake Efficiency, and its highest plateau (OUEP) during incremental exercise has been reported as a useful factor for assessing the severity of dysfunction and as a prognostic factor for mortality and morbidity in patients with chronic heart failure, though it has not been evaluated in COPD [11]. More in depth knowledge of specialized indicators that reflect the ability of oxygen consumption to increase ΔFO2 during exercise separately from ventilatory ability to increase 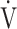 E would be useful for establishing therapeutic strategies and assessing exercise therapy targeting peripheral muscles not only in COPD patients, but in all patients for whom exercise therapy is indicated.
E would be useful for establishing therapeutic strategies and assessing exercise therapy targeting peripheral muscles not only in COPD patients, but in all patients for whom exercise therapy is indicated.
On the other hand, the Oxygen Uptake Efficiency Slope (OUES) in the regression equation 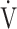 O2 = OUES × log10
O2 = OUES × log10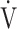 E + intercept is considered an objective estimate of cardiopulmonary functional reserve even at submaximal exercise. OUES has been proposed as a surrogate for maximal oxygen uptake (
E + intercept is considered an objective estimate of cardiopulmonary functional reserve even at submaximal exercise. OUES has been proposed as a surrogate for maximal oxygen uptake (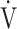 O2 max.) based on the concept of OUES [12], because OUES has the ability to interrogate responses at submaximal exercise, which can be beneficial in patients whose peak
O2 max.) based on the concept of OUES [12], because OUES has the ability to interrogate responses at submaximal exercise, which can be beneficial in patients whose peak 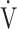 O2 is low (i.e., due to premature exercise termination because of symptoms or failure of
O2 is low (i.e., due to premature exercise termination because of symptoms or failure of 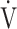 O2 to increase due to oxygen extraction difficulties). Although the physiological meaning of OUES has been highlighted in healthy subjects [13], children [12], and adult patients with cardiac disease [14], it has not been evaluated in COPD. Traditionally, OUES and OUEP have been used for outcome prediction in cardiovascular patients in whom respiratory limitation is not an issue. Given the advantage of the logarithm step, OUES emphasizes the physical condition in
O2 to increase due to oxygen extraction difficulties). Although the physiological meaning of OUES has been highlighted in healthy subjects [13], children [12], and adult patients with cardiac disease [14], it has not been evaluated in COPD. Traditionally, OUES and OUEP have been used for outcome prediction in cardiovascular patients in whom respiratory limitation is not an issue. Given the advantage of the logarithm step, OUES emphasizes the physical condition in 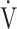 E in the early exercise phase. Therefore it might be a different exercise indicator from the later exercise phase evaluation of COPD such as the
E in the early exercise phase. Therefore it might be a different exercise indicator from the later exercise phase evaluation of COPD such as the 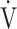 E-
E-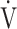 CO2 slope [7, 8] and peak
CO2 slope [7, 8] and peak 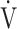 O2, because the severity of ventilatory disorders due to airflow limitation is greater at the end of exercise. In other words, the cardiopulmonary functional reserve suggested by OUES in advanced COPD with severe airflow limitation might be an overestimation that cannot actually be reached.
O2, because the severity of ventilatory disorders due to airflow limitation is greater at the end of exercise. In other words, the cardiopulmonary functional reserve suggested by OUES in advanced COPD with severe airflow limitation might be an overestimation that cannot actually be reached.
We hypothesized that, in COPD patients with exertional dyspnea, the 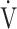 E-
E-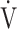 CO2 slope is more useful than OUES in understanding their exercise pathophysiology and formulating a specific treatment plan for each patient based on that pathophysiology. In this study, we evaluated patients with all Global Initiative for Chronic Obstructive Lung Disease (GOLD) COPD grades, to examine the clinical implications of the
CO2 slope is more useful than OUES in understanding their exercise pathophysiology and formulating a specific treatment plan for each patient based on that pathophysiology. In this study, we evaluated patients with all Global Initiative for Chronic Obstructive Lung Disease (GOLD) COPD grades, to examine the clinical implications of the 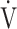 E-
E-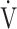 CO2 slope and OUES, and to determine which of the pathophysiological indicators, especially ventilatory variables and ΔFO2 during exercise are affected by them.
CO2 slope and OUES, and to determine which of the pathophysiological indicators, especially ventilatory variables and ΔFO2 during exercise are affected by them.
Methods
Subjects
This was a retrospective study using existing clinical records. Of a cumulative total of 1212 patients who required evaluation of their exertional dyspnea during routine medical care and underwent CPET to understand their exercise pathophysiology or before treatment such as pulmonary rehabilitation at the NHO Osaka Toneyama Medical Centre from January 2015 to August 2022, 122 patients diagnosed with COPD ranging from mild to very severe according to the GOLD criteria, were selected and their data were retrospectively analyzed. Patients were excluded if: (1) they were diagnosed with bronchial asthma, active infection, or severe heart disease; (2) had a history of lung resection; (3) there were changes in their drug regimen within 4 weeks before CPET; or (4) they were unstable due to other reasons. This study was performed in accordance with the Declaration of Helsinki and was approved by the institutional review board of the NHO Osaka Toneyama Medical Center (approval number: TNH-A-2022018). Each patient provided, written, informed consent before CPET evaluation during routine medical care, with the understanding that CPET was performed because it was considered necessary in daily clinical practice, and that the data obtained may be used in clinical research or other settings.
Pulmonary function tests (PFTs)
Post-bronchodilator spirometry was performed (CHESTAC 8800; CHEST M.I. Inc., Tokyo, Japan) according to the recommendations of the American Thoracic Society [15]. PFTs were performed within 2 weeks before or after CPET.
Cardiopulmonary exercise testing (CPET)
A CPET system (Aero monitor AE310S; Minato Medical Science Co., Ltd, Osaka, Japan) was used for symptom-limited incremental exercise tests performed in 1-minute or 2-minute stages with a 10-watt step protocol using an electrically braked cycle ergometer (CV-1000SS; Lode, Groningen, The Netherlands) [10, 16]. Calibrations for ventilatory measurement and gas measurements (CO2 and O2) were performed before each CPET. Pre-exercise resting measurements were obtained during the steady-state period after at least 3 min of rest after preparation for CPET. Ventilatory variables were measured on a breath-by-breath basis using a face mask, and they are presented as 30-s averages at rest, at 2-min intervals during exercise, and at the end of exercise. Dyspnea levels were assessed at rest, during the last 15 s of each exercise stage, and at the end of exercise using a 10-point modified Borg category-ratio scale. ΔFO2 was calculated from the difference between FIO2 and FEO2 measured during exercise. As measurements, the maximum value of ΔFO2 during exercise (ΔFO2 max) and ΔFO2 at peak exercise were examined. In addition, from January 2015 to August 2022, CPET was performed using the same expiratory gas analyzer based on the above protocol without any changes. The 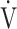 E-
E-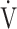 CO2 slope and
CO2 slope and 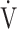 E/
E/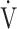 CO2 nadir were calculated and defined as previously described [9]. Based on the assumption that log-transforming
CO2 nadir were calculated and defined as previously described [9]. Based on the assumption that log-transforming 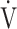 E will make the relationship between
E will make the relationship between 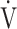 E and
E and 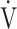 O2 more linear than using
O2 more linear than using 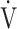 E, as shown in Fig. 1 and online supplemental Figure S2, OUES was calculated as the slope of the regression line relating
E, as shown in Fig. 1 and online supplemental Figure S2, OUES was calculated as the slope of the regression line relating 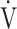 O2 (mL ⋅ min− 1) to the common logarithm of
O2 (mL ⋅ min− 1) to the common logarithm of 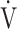 E (L ⋅ min− 1) by the equation:
E (L ⋅ min− 1) by the equation: 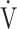 O2 (mL ⋅ min− 1) = slope × log 10
O2 (mL ⋅ min− 1) = slope × log 10
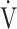 E (L ⋅ min− 1) + intercept, where the constant ‘slope’ is OUES, using breath-by-breath gas-exchange measurements [12]. Basically, to avoid possible irregular breathing patterns, data from the 3-min resting period, from the first minute of exercise, and from a plateau in
E (L ⋅ min− 1) + intercept, where the constant ‘slope’ is OUES, using breath-by-breath gas-exchange measurements [12]. Basically, to avoid possible irregular breathing patterns, data from the 3-min resting period, from the first minute of exercise, and from a plateau in 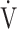 O2 were excluded from the calculation of OUES. The anaerobic threshold (AT) was determined using the V-slope method [4]. Predicted maximal voluntary ventilation (MVV) was calculated as FEV1 × 35 [4]. Predicted maximum heart rate was calculated as 220–age in years [4].
O2 were excluded from the calculation of OUES. The anaerobic threshold (AT) was determined using the V-slope method [4]. Predicted maximal voluntary ventilation (MVV) was calculated as FEV1 × 35 [4]. Predicted maximum heart rate was calculated as 220–age in years [4].
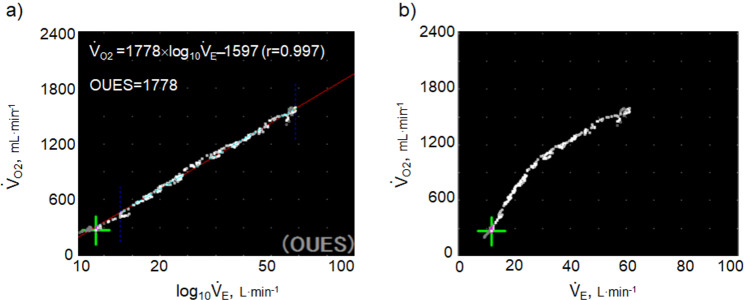
Fig. 1.
Calculation of OUES. a) The relationship between oxygen uptake (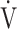 O2: mL ⋅ min−1) and the common logarithm of minute ventilation (
O2: mL ⋅ min−1) and the common logarithm of minute ventilation (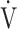 E: L ⋅ min−1) (b) the relationship between
E: L ⋅ min−1) (b) the relationship between 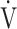 O2 (mL ⋅ min−1) and
O2 (mL ⋅ min−1) and 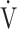 E (L ⋅ min−1).
E (L ⋅ min−1). 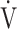 O2 and
O2 and 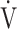 E obtained from breath-by-breath gas-exchange measurements in one COPD patient with GOLD I (75 years old, 64.2 kg) were used. OUES was calculated as the slope of the regression line relating
E obtained from breath-by-breath gas-exchange measurements in one COPD patient with GOLD I (75 years old, 64.2 kg) were used. OUES was calculated as the slope of the regression line relating 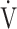 O2 (mL ⋅ min− 1) to the common logarithm of
O2 (mL ⋅ min− 1) to the common logarithm of 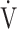 E (L ⋅ min− 1) by the equation:
E (L ⋅ min− 1) by the equation: 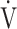 O2 = slope × log 10
O2 = slope × log 10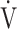 E + intercept. where the constant ‘slope’ is the OUES. Basically, to avoid possible irregular breathing patterns, data from the 3-min resting period, from the first minute of exercise, and from a plateau in
E + intercept. where the constant ‘slope’ is the OUES. Basically, to avoid possible irregular breathing patterns, data from the 3-min resting period, from the first minute of exercise, and from a plateau in 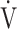 O2 were excluded from the calculation of the OUES. Green cross: exercise starting point; blue crosses: period during which OUES was calculated
O2 were excluded from the calculation of the OUES. Green cross: exercise starting point; blue crosses: period during which OUES was calculated
Statistical analysis
Variables are presented as means (standard deviation) or medians (interquartile range) depending on the normality of the distribution (Shapiro–Wilk test) unless otherwise stated. Depending on the normality of the distribution, for continuous variables, (1) an unpaired t-test or the Wilcoxon rank-sum test was used for comparison between patients with GOLD grades I-II and III-IV, and (2) univariate analysis using Pearson’s correlation or Spearman’s rank correlation coefficient was used to evaluate correlations between clinical variables. R squared was used to confirm how close the data were to the fitted regression line. The chi-squared test was used for categorical variables. Bidirectional stepwise variable selection was performed to determine variables more correlated with the 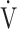 E-
E-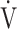 CO2 slope of the exertional variables related to oxygen uptake including COPD grade, which was divided into GOLD grades I-II and III-IV, and the desaturation level at peak exercise, with variance inflation factors (VIFs) less than 3. In the stepwise regression models, the p values were set at 0.20 for variables to both enter and stay. A p value of less than 0.05 was considered significant. Statistical analyses were performed using JMP software, version 11 (SAS Institute Inc., Cary, NC, USA).
CO2 slope of the exertional variables related to oxygen uptake including COPD grade, which was divided into GOLD grades I-II and III-IV, and the desaturation level at peak exercise, with variance inflation factors (VIFs) less than 3. In the stepwise regression models, the p values were set at 0.20 for variables to both enter and stay. A p value of less than 0.05 was considered significant. Statistical analyses were performed using JMP software, version 11 (SAS Institute Inc., Cary, NC, USA).
Results
The study sample consisted of 122 patients distributed across all GOLD stages (Table 1). There were no significant differences in age, sex, smoking history, and the choice of inhalation therapy between the GOLD I-II and III-IV groups, although BMI (p = 0.0322) was lower, and especially, the frequency of triple inhalation therapy (p = 0.0008) was higher in the GOLD III- IV group than in the GOLD I-II group (Table 1).
Table 1.
Baseline characteristics of COPD patients classified according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria
| All COPD patients (n = 122) | GOLD spirometric severity | |||
|---|---|---|---|---|
| I + II (n = 51) | III + IV (n = 71) | p-value | ||
| Age, y | 74 (6) [75 (70; 78)] | 75 (5) | 73 (6) [74 (70; 78)] | 0.2253 |
| Sex, male/female (n) | 112/10 | 47/4 | 65/6 | 0.9040 |
| BMI, kg ⋅ m− 2 | 21.5 (3.3) | 22.2 (3.1) | 21.0 (3.4) | 0.0322 |
| Pulmonary function test | ||||
| FEV1, L | 1.27 (0.58) [1.09 (0.86; 1.58)] | 1.81 (0.52) [1.71 (1.49; 2.14)] | 0.88 (0.19) | < 0.0001 |
| %FEV1, % predicted | 50.8 (22.4) [45.6 (35.2; 63.7)] | 72.0 (18.0) [67.9 (57.6; 82.6)] | 35.6 (8.4) | < 0.0001 |
| FEV1/FVC, % | 42.2 (12.2) [40.6 (31.9;49.3)] | 51.8 (10.0) [50.0 (43.8; 60.3)] | 35.3 (8.2) [32.9 (29.9; 40.0)] | < 0.0001 |
| VC, L | 3.05 (0.77) | 3.50 (0.68) | 2.72 (0.65) | < 0.0001 |
| %VC, % | 99.0 (23.1) | 113.8 (122.1) | 88.5 (20.6) | < 0.0001 |
| IC, L | 1.96 (0.52) | 2.27 (0.48) | 1.73 (0.43) | < 0.0001 |
| Medications (n) | ||||
| LAMA | 102 | 37 | 65 | 0.0052 |
| LABA | 92 | 31 | 61 | 0.0015 |
| ICS | 64 | 21 | 43 | 0.0344 |
| Triple inhalation therapy | 45 | 10 | 35 | 0.0008 |
Data are presented as means (standard deviation) or [medians (interquartile range: 25th percentile to 75th percentile)], unless otherwise stated. BMI: body mass index; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; IC: inspiratory capacity; ICS: inhaled corticosteroid; LABA: long-acting β2-agonist; LAMA: long-acting muscarinic antagonist; VC: vital capacity. Medications are presented separately. Pulmonary function tests were performed after bronchodilator administration
Peak exercise variables
Incremental exercise parameters at peak exercise in COPD patients in the GOLD I-II and III-IV groups are shown in Table 2. Compared with the GOLD I-II group, peak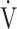 O2 (p < 0.0001), OUES (p = 0.0161), O2 pulse (p < 0.0001), percutaneous oxygen saturation (SpO2) (p = 0.0004) at peak exercise, and
O2 (p < 0.0001), OUES (p = 0.0161), O2 pulse (p < 0.0001), percutaneous oxygen saturation (SpO2) (p = 0.0004) at peak exercise, and 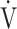 E (p < 0.0001) at peak exercise were significantly lower in the GOLD III-IV group with reduced breathing reserve, as expected by 1-
E (p < 0.0001) at peak exercise were significantly lower in the GOLD III-IV group with reduced breathing reserve, as expected by 1-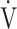 E/maximal voluntary ventilation (MVV). Reduced
E/maximal voluntary ventilation (MVV). Reduced 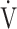 E in the GOLD III-IV group was caused by expiratory airflow limitation, indicated by lower values of tidal volume (VT) (p < 0.0001), respiratory frequency (fR) (p = 0.0079), and inspiratory duty cycle (TI/Ttot, where Ti is the inspiratory time of a single breath in seconds and Ttot is the total time of a single respiratory cycle in seconds) (p < 0.0001). There were no significant differences in ΔFO2 max during exercise, ΔFO2 at peak exercise, and the
E in the GOLD III-IV group was caused by expiratory airflow limitation, indicated by lower values of tidal volume (VT) (p < 0.0001), respiratory frequency (fR) (p = 0.0079), and inspiratory duty cycle (TI/Ttot, where Ti is the inspiratory time of a single breath in seconds and Ttot is the total time of a single respiratory cycle in seconds) (p < 0.0001). There were no significant differences in ΔFO2 max during exercise, ΔFO2 at peak exercise, and the 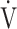 E-
E-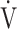 CO2 slope between the GOLD I-II and III-IV groups. However, the dependence of ΔFO2 at peak exercise on peak
CO2 slope between the GOLD I-II and III-IV groups. However, the dependence of ΔFO2 at peak exercise on peak 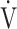 O2, expressed as the square of the correlation coefficient, was highest in the GOLD IV group (GOLD I (n = 18): r2 = 0.21; GOLD II (n = 33): r2 = 0.29; GOLD III (n = 51): r2 = 0.12; GOLD IV (n = 20): r2 = 0.68). In contrast, OUES (p = 0.0161) was lower in the GOLD III-IV group than in the GOLD I-II group.
O2, expressed as the square of the correlation coefficient, was highest in the GOLD IV group (GOLD I (n = 18): r2 = 0.21; GOLD II (n = 33): r2 = 0.29; GOLD III (n = 51): r2 = 0.12; GOLD IV (n = 20): r2 = 0.68). In contrast, OUES (p = 0.0161) was lower in the GOLD III-IV group than in the GOLD I-II group.
Table 2.
Incremental exercise variables in COPD patients classified according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria
| GOLD spirometric severity | |||
|---|---|---|---|
| I + II (n = 51) | III + IV (n = 71) | p-value | |
| At peak exercise | |||
| Dyspnea, Borg scale | 5.6 (1.8) [5.0 (4.0; 7.0)] | 6.0 (1.9) [6.0 (5.0; 8.0)] | 0.2126 |
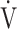 O2, mL⋅min−1 ⋅ kg−1 O2, mL⋅min−1 ⋅ kg−1
|
14.9 (3.8) [15.2 (11.7; 17.3)] | 10.6 (2.3) | < 0.0001 |
| R | 1.10 (0.10) | 1.01 (0.08) [1.01 (0.96; 1.05)] | < 0.0001 |
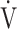 E, L ⋅ min−1 E, L ⋅ min−1
|
43.7 (12.6) | 28.0 (6.6) | < 0.0001 |
| VT, mL | 1397 (320) | 987 (239) | < 0.0001 |
| fR, breaths ⋅ min− 1 | 31.7 (6.1) | 28.9 (5.3) | 0.0079 |
| TI/Ttot | 0.41 (0.05) [0.41 (0.38; 0.43)] | 0.34 (0.05) | < 0.0001 |
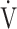 E/ E/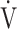 O2 O2
|
49.7 (10.1) | 48.0 (7.4) [47.0 (44.0; 51.2)] | 0.2892 |
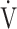 E/ E/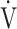 CO2 CO2
|
45.1 (8.7) | 47.8 (8.4) [46.8 (42.7; 51.0)] | 0.1623 |
1−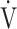 E/MVV, % E/MVV, % |
29.2 (15.4) | 8.4 (19.0) [11.9 (2.9; 19.4)] | < 0.0001 |
| HR, beats ⋅ min− 1 | 124 (17.6) | 112 (16.8) | 0.0005 |
| HR/predicted maximum HR, % | 80.8 (12.4) | 80.0 (10.7) | 0.7007 |
| SpO2, % | 93 (4) [95 (91; 97)] | 91 (5) [92 (87; 94)] | 0.0004 |
| O2 pulse, mL ⋅ beats− 1 | 7.3 (1.9) | 5.4 (1.6) [5.1 (4.2; 6.4)] | < 0.0001 |
| OUES | 1319 (420) | 1135 (440) [1115 (880; 1328)] | 0.0161 |
| ΔFO2, % | 2.58 (0.50) [2.52 (2.28; 2.74)] | 2.59 (0.38) | 0.5014 |
| During exercise | |||
| ΔFO2 max., % | 2.88 (0.57) [2.78 (2.47; 3.11)] | 2.72 (0.41) | 0.2062 |
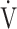 E- E-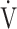 CO2 slope CO2 slope |
39.1 (8.9) | 40.3 (10.9) [39.2 (34.2; 43.2)] | 0.8073 |
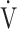 E/ E/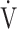 CO2 nadir CO2 nadir |
43.1 (7.9) | 46.8 (7.6) [46.0 (41.0; 50.0)] | 0.0294 |
| Number of patients reaching AT, n (%) | 49 (96) | 51 (72) | 0.0006 |
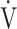 O2 at AT, mL ⋅ min−1 O2 at AT, mL ⋅ min−1
|
601 (178) [575 (490; 670)] | 481(104) | 0.0001 |
| Number of patients reaching RCP, n (%) | 19 (37) | 7 (10) | 0.0003 |
Data are presented as means (standard deviation) or [medians (interquartile range: 25th percentile to 75th percentile)], unless otherwise stated. AT: anaerobic threshold; ΔFO2: difference between inspired mean oxygen concentration and expired mean oxygen concentration; ΔFO2 max.: the highest value during exercise; fR: breathing frequency; HR: heart rate; O2 pulse: 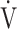 O2/HR; predicted maximum HR: 220−age (y); OUES: oxygen uptake efficiency slope (see the Methods for details); R: gas exchange ratio; RCP: respiratory compensation point; TI/Ttot: inspiratory duty cycle;
O2/HR; predicted maximum HR: 220−age (y); OUES: oxygen uptake efficiency slope (see the Methods for details); R: gas exchange ratio; RCP: respiratory compensation point; TI/Ttot: inspiratory duty cycle; 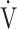 CO2: carbon dioxide output;
CO2: carbon dioxide output; 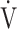 E: minute ventilation; and
E: minute ventilation; and 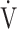 E/
E/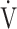 CO2-slope: the slope was determined by linear regression analysis of
CO2-slope: the slope was determined by linear regression analysis of 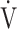 E to
E to 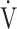 CO2 obtained during exercise (see the Methods for details);
CO2 obtained during exercise (see the Methods for details); 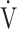 E/
E/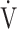 CO2-nadir: the lowest value during exercise (see the Methods for details);
CO2-nadir: the lowest value during exercise (see the Methods for details); 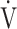 O2: oxygen uptake; VT: tidal volume. Estimated maximal voluntary ventilation (MVV) (L·min− 1) was equal to forced expiratory volume in one second (FEV1)×35
O2: oxygen uptake; VT: tidal volume. Estimated maximal voluntary ventilation (MVV) (L·min− 1) was equal to forced expiratory volume in one second (FEV1)×35
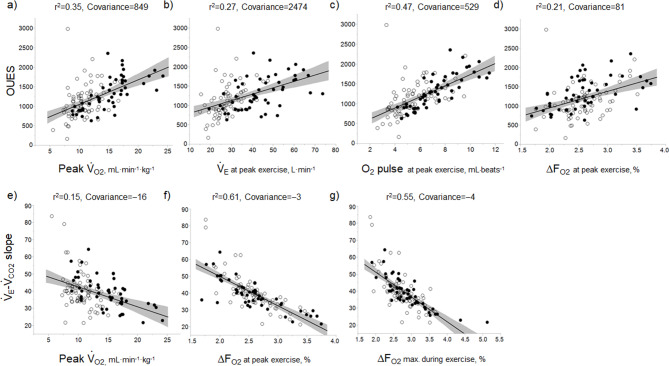
Correlations of OUES with exercise variables
In all GOLD grades, and in the GOLD I-II and GOLD III-IV groups, OUES was not strongly correlated with peak 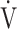 O2,
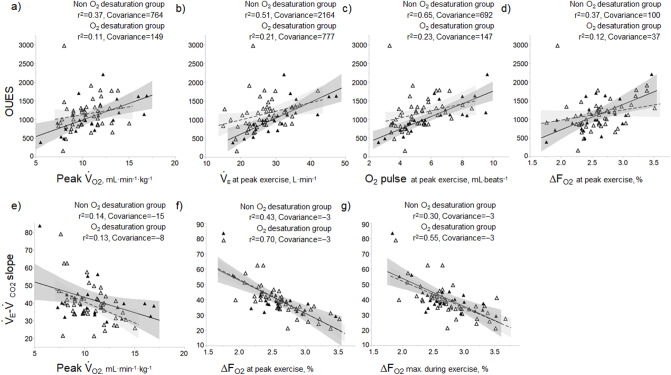
O2, 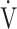 E, ventilatory efficiency, ΔFO2 at peak exercise, and resting pulmonary function; the square of the correlation coefficient was not greater than 0.5 in all groups. (Table 3; Fig. 2a-d). The GOLD III-IV group was divided into two groups depending on the exertional decrease from rest to peak exercise in SpO2 into the non-desaturation group (3% or less, n = 23) and the desaturation group (greater than 3%, n = 48), as shown in Table 4. The correlation coefficients of OUES with exertional variables, such as peak
E, ventilatory efficiency, ΔFO2 at peak exercise, and resting pulmonary function; the square of the correlation coefficient was not greater than 0.5 in all groups. (Table 3; Fig. 2a-d). The GOLD III-IV group was divided into two groups depending on the exertional decrease from rest to peak exercise in SpO2 into the non-desaturation group (3% or less, n = 23) and the desaturation group (greater than 3%, n = 48), as shown in Table 4. The correlation coefficients of OUES with exertional variables, such as peak 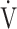 O2,
O2, 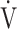 E, oxygen pulse, and ΔFO2 at peak exercise, were higher in the non-desaturation group than in the desaturation group (Fig. 3a-d). In the desaturation group of the GOLD III-IV group,
E, oxygen pulse, and ΔFO2 at peak exercise, were higher in the non-desaturation group than in the desaturation group (Fig. 3a-d). In the desaturation group of the GOLD III-IV group, 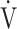 E at peak exercise (p = 0.0124) was significantly lower, and O2-pulse at peak exercise (p = 0.0818) tended to be lower than in the non-desaturation group, but peak
E at peak exercise (p = 0.0124) was significantly lower, and O2-pulse at peak exercise (p = 0.0818) tended to be lower than in the non-desaturation group, but peak 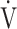 O2 (p = 0.4505) and ΔFO2 at peak exercise (p = 0.2839) were not (Table 4; Fig. 4).
O2 (p = 0.4505) and ΔFO2 at peak exercise (p = 0.2839) were not (Table 4; Fig. 4).
Table 3.
Square of the correlation coefficient of parameters related to OUES in COPD patients (n = 122)
| All COPD patients | GOLD I-II (n = 51) | GOLD III-IV (n = 71) | |
|---|---|---|---|
| Peak incremental exercise parameters | |||
| Dyspnoea, Borg scale | 0.02 | 0.00 | 0.03 |
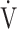 O2, mL ⋅ min−1 ⋅ kg O2, mL ⋅ min−1 ⋅ kg |
0.35 | 0.60 | 0.19 |
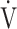 E, L ⋅ min−1 E, L ⋅ min−1
|
0.27 | 0.25 | 0.26 |
| VT, mL | 0.27 | 0.30 | 0.19 |
| fR, breaths ⋅ min− 1 | 0.01 | 0.01 | 0.00 |
| TI/Ttot | 0.03 | 0.01 | 0.00 |
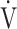 E/ E/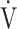 CO2 CO2
|
0.26 | 0.31 | 0.14 |
| HR, beats ⋅ min− 1 | 0.05 | 0.09 | 0.01 |
| O2 pulse, mL ⋅ beats− 1 | 0.47 | 0.66 | 0.35 |
| ΔFO2, % | 0.21 | 0.33 | 0.17 |
| During exercise | |||
| ΔFO2 max., % | 0.18 | 0.34 | 0.07 |
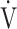 E− E−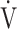 CO2 slope CO2 slope |
0.25 | 0.29 | 0.20 |
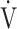 E/ E/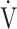 CO2 nadir CO2 nadir |
0.22 | 0.28 | 0.08 |
AT (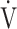 O2), mL ⋅ min−1 O2), mL ⋅ min−1
|
0.39 | 0.46 | 0.33 |
| Pulmonary function and others | |||
| FEV1, L | 0.16 | 0.19 | 0.12 |
| VC, L | 0.19 | 0.08 | 0.07 |
| FVC, L | 0.18 | 0.07 | 0.14 |
| IC, L | 0.17 | 0.16 | 0.10 |
| Age, y | 0.00 | 0.00 | 0.02 |
| BMI, kg ⋅ m− 2 | 0.14 | 0.13 | 0.15 |
AT: anaerobic threshold; BMI: body mass index; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; ΔFO2: difference between inspired mean oxygen concentration and expired mean oxygen concentration; ΔFO2 max.: the highest ΔFO2 value during exercise; fR: breathing frequency; HR: heart rate; IC: inspiratory capacity; O2 pulse: 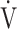 O2/HR; OUES: oxygen uptake efficiency slope (see the Methods for details); TI/Ttot: inspiratory duty cycle; VC: vital capacity;
O2/HR; OUES: oxygen uptake efficiency slope (see the Methods for details); TI/Ttot: inspiratory duty cycle; VC: vital capacity; 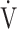 CO2: carbon dioxide output;
CO2: carbon dioxide output; 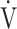 E: minute ventilation;
E: minute ventilation; 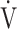 E/
E/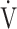 CO2-nadir: the lowest value during exercise (see the Methods for details); and
CO2-nadir: the lowest value during exercise (see the Methods for details); and 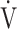 E−
E−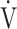 CO2-slope: the slope was determined by linear regression analysis of
CO2-slope: the slope was determined by linear regression analysis of 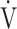 E to
E to 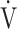 CO2 obtained during exercise (see the Methods for details);
CO2 obtained during exercise (see the Methods for details); 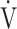 O2: oxygen uptake; VT: tidal volume
O2: oxygen uptake; VT: tidal volume
Fig. 2.
In all COPD stages, correlations of the oxygen uptake efficiency slope (OUES) (upper panel) and the 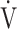 E/
E/ CO2 slope (bottom panel) with exertional variables during cardiopulmonary exercise testing. Correlations of OUES with peak
CO2 slope (bottom panel) with exertional variables during cardiopulmonary exercise testing. Correlations of OUES with peak  O2 (a),
O2 (a),  E at peak exercise (b), O2 pulse at peak exercise (c), and ΔFO2 at peak exercise (d) are shown. Correlations of the
E at peak exercise (b), O2 pulse at peak exercise (c), and ΔFO2 at peak exercise (d) are shown. Correlations of the  E/
E/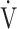 CO2 slope with peak
CO2 slope with peak 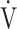 O2 (e), ΔFO2 at peak exercise (f), and ΔFO2 max. during exercise (g) are shown. ΔFO2: difference between inspired and expired mean oxygen concentrations; ΔFO2 max.: highest ΔFO2 value during exercise; O2 pulse:
O2 (e), ΔFO2 at peak exercise (f), and ΔFO2 max. during exercise (g) are shown. ΔFO2: difference between inspired and expired mean oxygen concentrations; ΔFO2 max.: highest ΔFO2 value during exercise; O2 pulse: 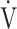 O2/heart rate;
O2/heart rate; 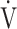 E: minute ventilation;
E: minute ventilation; 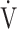 O2: oxygen uptake. Closed circle: Global Initiative for Chronic Obstructive Lung Disease (GOLD) I-II group (n = 51). Open circle: GOLD III-IV group (n = 71). The shaded area indicates the CI. The correlation coefficient (r) was obtained in the analysis of all COPD stages
O2: oxygen uptake. Closed circle: Global Initiative for Chronic Obstructive Lung Disease (GOLD) I-II group (n = 51). Open circle: GOLD III-IV group (n = 71). The shaded area indicates the CI. The correlation coefficient (r) was obtained in the analysis of all COPD stages
Table 4.
Incremental exercise variables in GOLD III-IV COPD patients divided into two groups by the desaturation level at peak exercise
| Non-desaturation group: 3% or less (n = 23) | Desaturation group: greater than 3% (n = 48) | p-value | |
|---|---|---|---|
| At peak exercise | |||
| Dyspnea, Borg scale | 5.7 (1.7) | 6.2 (1.9) [6.5 (5.0; 8.0)] | 0.2520 |
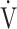 O2, mL⋅min−1 ⋅ kg−1 O2, mL⋅min−1 ⋅ kg−1
|
11.0 (3.0) | 10.5 (1.9) | 0.4505 |
| R | 1.02 (0.10) [1.02 (0.95; 1.07)] | 1.00 (0.07) | 0.7119 |
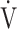 E, L ⋅ min−1 E, L ⋅ min−1
|
30.7 (7.2) | 26.6 (5.9) | 0.0124 |
| VT, mL | 1029 (240) | 967 (238) [917 (794; 1082)] | 0.2796 |
| fR, breaths ⋅ min− 1 | 30.3 (5.2) | 28.2 (5.2) | 0.1058 |
| TI/Ttot | 0.35 (0.06) | 0.34 (0.04) | 0.2806 |
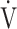 E/ E/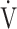 O2 O2
|
49.5 (8.1) | 47.3 (7.0) | 0.2310 |
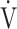 E/ E/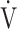 CO2 CO2
|
48.9 (9.8) [48.0 (43.0; 52.0)] | 47.3 (7.6) [46.0 (41.4; 51.0)] | 0.6055 |
1−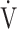 E/MVV, % E/MVV, % |
2.2 (15.9) [10.2 (−8.1; 17.8)] | 11.3 (13.9) [12.7 (3.3; 20.3)] | 0.2169 |
| HR, beats ⋅ min− 1 | 111 (17) | 113 (17) | 0.5799 |
| HR/predicted maximum HR, % | 78.8 (10.4) | 80.6 (11.0) | 0.5280 |
| SpO2, % | 95 (2) | 88 (5) | ND |
| O2 pulse, mL ⋅ beats− 1 | 6.0 (2.1) | 5.1 (1.3) | 0.0818 |
| OUES | 1086 (437) | 1158 (444) [1129 (880; 1345)] | 0.4317 |
| ΔFO2, % | 2.52 (0.39) | 2.63 (0.37) | 0.2839 |
| During exercise | |||
| ΔFO2 max., % | 2.68 (0.43) | 2.73 (0.41) | 0.6083 |
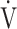 E- E-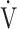 CO2 slope CO2 slope |
41.7 (11.4) [39.1 (34.6; 44.8)] | 39.6 (10.7) [39.3 (34.1; 42.8)] | 0.5972 |
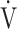 E/ E/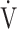 CO2 nadir CO2 nadir |
47.4 (8.1) | 46.5 (7.3) [45.5 (41.0; 49.0)] | 0.6387 |
| Number of patients reaching AT, n (%) | 16 (70) | 35 (73) | 0.7689 |
 O2 at AT, mL ⋅ min−1 O2 at AT, mL ⋅ min−1
|
515 (144) | 464(78) | 0.1109 |
Data are presented as means (standard deviation) or [medians (interquartile range: 25th percentile to 75th percentile)], unless otherwise stated. AT: anaerobic threshold; ΔFO2: difference between inspired mean oxygen concentration and expired mean oxygen concentration; ΔFO2 max.: the highest value during exercise; fR: breathing frequency; HR: heart rate; O2 pulse:  O2/HR; predicted maximum HR: 220−age (y); ND: not done; OUES: oxygen uptake efficiency slope (see the Methods for details); R: gas exchange ratio; RCP: respiratory compensation point; TI/Ttot: inspiratory duty cycle;
O2/HR; predicted maximum HR: 220−age (y); ND: not done; OUES: oxygen uptake efficiency slope (see the Methods for details); R: gas exchange ratio; RCP: respiratory compensation point; TI/Ttot: inspiratory duty cycle;  CO2: carbon dioxide output;
CO2: carbon dioxide output;  E: minute ventilation; and
E: minute ventilation; and 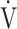 E/
E/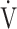 CO2-slope: the slope was determined by linear regression analysis of
CO2-slope: the slope was determined by linear regression analysis of 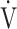 E to
E to 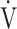 O2 obtained during exercise (see the Methods for details);
O2 obtained during exercise (see the Methods for details); 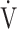 E/
E/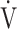 CO2-nadir: the lowest value during exercise (see the Methods for details);
CO2-nadir: the lowest value during exercise (see the Methods for details); 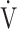 O2: oxygen uptake; VT: tidal volume. Estimated maximal voluntary ventilation (MVV) (L·min− 1) was equal to forced expiratory volume in one second (FEV1)×35
O2: oxygen uptake; VT: tidal volume. Estimated maximal voluntary ventilation (MVV) (L·min− 1) was equal to forced expiratory volume in one second (FEV1)×35
Fig. 3.
In COPD Global Initiative for Chronic Obstructive Lung Disease (GOLD) III and IV stages, the correlations of oxygen uptake efficiency slope (OUES) (upper panel) and the 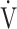 E/
E/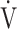 CO2 slope (bottom panel) with exertional variables during cardiopulmonary exercise testing classified by oxygen (O2) desaturation level. Correlations of OUES with peak
CO2 slope (bottom panel) with exertional variables during cardiopulmonary exercise testing classified by oxygen (O2) desaturation level. Correlations of OUES with peak 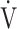 O2 (a),
O2 (a), 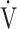 E at peak exercise (b), O2 pulse at peak exercise (c), and ΔFO2 at peak exercise (d) are shown. Correlations of the
E at peak exercise (b), O2 pulse at peak exercise (c), and ΔFO2 at peak exercise (d) are shown. Correlations of the 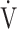 E/
E/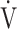 CO2 slope with peak
CO2 slope with peak 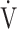 O2 (e), ΔFO2 at peak exercise (f), and ΔFO2 max. during exercise (g) are shown. ΔFO2: difference between inspired and expired mean oxygen concentrations; ΔFO2 max.: highest ΔFO2 value during exercise; O2 pulse:
O2 (e), ΔFO2 at peak exercise (f), and ΔFO2 max. during exercise (g) are shown. ΔFO2: difference between inspired and expired mean oxygen concentrations; ΔFO2 max.: highest ΔFO2 value during exercise; O2 pulse: 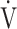 O2/heart rate;
O2/heart rate; 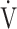 E: minute ventilation;
E: minute ventilation; 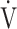 O2: oxygen uptake. Closed triangle and solid line: group with O2 desaturation of 3% or less during exercise (n = 23). Open triangle and dotted line: group with O2 desaturation greater than 3% during exercise (n = 48). The shaded area indicates the CI. The correlation coefficient (r) was obtained in the analysis for each group
O2: oxygen uptake. Closed triangle and solid line: group with O2 desaturation of 3% or less during exercise (n = 23). Open triangle and dotted line: group with O2 desaturation greater than 3% during exercise (n = 48). The shaded area indicates the CI. The correlation coefficient (r) was obtained in the analysis for each group
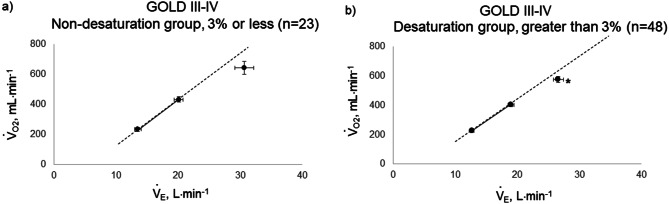
Fig. 4.
Relationship between oxygen uptake (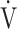 O2: mL ⋅ min−1) and minute ventilation (
O2: mL ⋅ min−1) and minute ventilation (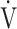 E: L ⋅ min−1) in (a) GOLD III-IV without exertional desaturation, 3% or less decrease (n = 23) and (b) GOLD III-IV with exertional desaturation, greater than 3% decrease (n = 48). Plots from left to right at rest, at 2 min, and at peak exercise. To see whether it is inappropriate to represent the relationship of
E: L ⋅ min−1) in (a) GOLD III-IV without exertional desaturation, 3% or less decrease (n = 23) and (b) GOLD III-IV with exertional desaturation, greater than 3% decrease (n = 48). Plots from left to right at rest, at 2 min, and at peak exercise. To see whether it is inappropriate to represent the relationship of 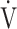 O2 to
O2 to 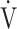 E as a line, the dotted line is a straight line connecting the resting and 2-minute plots. GOLD: Global Initiative for Chronic Obstructive Lung Disease. Mean ± standard error. *: p < 0.05 between the non-desaturation and desaturation groups in
E as a line, the dotted line is a straight line connecting the resting and 2-minute plots. GOLD: Global Initiative for Chronic Obstructive Lung Disease. Mean ± standard error. *: p < 0.05 between the non-desaturation and desaturation groups in 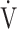 E (x-component)
E (x-component)
Correlation of the 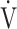 E-
E-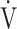 CO2 slope with exercise variables
CO2 slope with exercise variables
In all GOLD grades, and in the GOLD I-II and GOLD III-IV groups, there was no correlation between 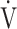 E-
E-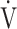 CO2 slope and
CO2 slope and 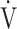 E at peak exercise ( Table 5). There were, however, correlations between the
E at peak exercise ( Table 5). There were, however, correlations between the 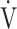 E-
E-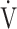 CO2 slope and oxygen uptake-related variables (peak
CO2 slope and oxygen uptake-related variables (peak 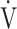 O2,
O2, 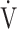 O2 at the AT, OUES, ΔFO2 at peak exercise, and maximum ΔFO2 during exercise) (Table 3 5; Fig. 2e-g). In the GOLD III-IV group, the correlation coefficients of the
O2 at the AT, OUES, ΔFO2 at peak exercise, and maximum ΔFO2 during exercise) (Table 3 5; Fig. 2e-g). In the GOLD III-IV group, the correlation coefficients of the 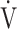 E-
E-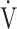 CO2 slope with exertional variables, such as peak
CO2 slope with exertional variables, such as peak 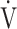 O2, ΔFO2 at peak exercise, and maximal ΔFO2 during exercise were almost the same between the groups with and without exertional desaturation (Fig. 3e-g). Of the four exercise variables related to oxygen uptake (peak
O2, ΔFO2 at peak exercise, and maximal ΔFO2 during exercise were almost the same between the groups with and without exertional desaturation (Fig. 3e-g). Of the four exercise variables related to oxygen uptake (peak 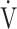 O2,
O2, 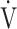 O2 at the AT, OUES, ΔFO2 at peak exercise), and including COPD grade and SpO2 at peak exercise, investigations of more influential variables that correlated with the
O2 at the AT, OUES, ΔFO2 at peak exercise), and including COPD grade and SpO2 at peak exercise, investigations of more influential variables that correlated with the 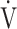 E-
E-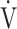 CO2 slope across all GOLD grades were performed using stepwise variable selection. The analysis identified peak
CO2 slope across all GOLD grades were performed using stepwise variable selection. The analysis identified peak 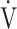 O2 (F value = 4.6, p=0.0345) and ΔFO2 at peak exercise (F value = 148.4, p < 0.0001) as more influential variables correlated with the
O2 (F value = 4.6, p=0.0345) and ΔFO2 at peak exercise (F value = 148.4, p < 0.0001) as more influential variables correlated with the 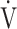 E-
E-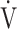 CO2 slope.
CO2 slope.
Table 5.
Square of the correlation coefficient of parameters related to 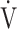 E-
E-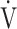 co2 slope in COPD patients (n = 122)
co2 slope in COPD patients (n = 122)
| All COPD patients | GOLD I-II (n = 51) | GOLD III-IV (n = 71) | |
|---|---|---|---|
| Peak incremental exercise parameters | |||
| Dyspnoea, Borg scale | 0.00 | 0.03 | 0.00 |
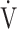 O2, mL ⋅ min−1 ⋅ kg O2, mL ⋅ min−1 ⋅ kg |
0.15 | 0.34 | 0.14 |
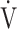 E, L ⋅ min−1 E, L ⋅ min−1
|
0.00 | 0.00 | 0.00 |
| VT, mL | 0.04 | 0.07 | 0.06 |
| fR, breaths ⋅ min− 1 | 0.05 | 0.04 | 0.06 |
| TI/Ttot | 0.00 | 0.02 | 0.00 |
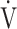 E/ E/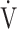 O2 O2
|
0.57 | 0.55 | 0.60 |
| HR, beats ⋅ min− 1 | 0.13 | 0.23 | 0.11 |
| O2 pulse, mL ⋅ beats− 1 | 0.08 | 0.13 | 0.06 |
| ΔFO2, % | 0.61 | 0.57 | 0.63 |
| During exercise | |||
| ΔFO2 max., % | 0.55 | 0.69 | 0.45 |
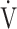 E/ E/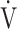 CO2 nadir CO2 nadir |
0.61 | 0.81 | 0.54 |
AT (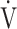 O2), mL ⋅ min−1 O2), mL ⋅ min−1
|
0.07 | 0.14 | 0.09 |
| Pulmonary function and others | |||
| FEV1, L | 0.00 | 0.04 | 0.00 |
| IC, L | 0.00 | 0.01 | 0.00 |
| Age, y | 0.00 | 0.00 | 0.01 |
| BMI, kg ⋅ m− 2 | 0.02 | 0.00 | 0.04 |
AT: anaerobic threshold; BMI: body mass index; FEV1: forced expiratory volume in one second; ΔFO2: difference between inspired mean oxygen concentration and expired mean oxygen concentration; ΔFO2 max.: the highest ΔFO2 value during exercise; fR: breathing frequency; HR: heart rate; IC: inspiratory capacity; O2 pulse: 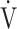 O2/HR; TI/Ttot: inspiratory duty cycle;
O2/HR; TI/Ttot: inspiratory duty cycle; 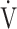 CO2: carbon dioxide output;
CO2: carbon dioxide output; 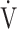 E: minute ventilation;
E: minute ventilation; 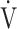 E/
E/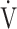 CO2-nadir: the lowest value during exercise (see the Methods for details);
CO2-nadir: the lowest value during exercise (see the Methods for details); 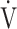 E-
E-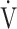 CO2-slope: the slope was determined by linear regression analysis of
CO2-slope: the slope was determined by linear regression analysis of 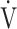 E to
E to 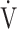 CO2 obtained during exercise (see the Methods for details);
CO2 obtained during exercise (see the Methods for details); 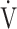 O2: oxygen uptake; VT: tidal volume
O2: oxygen uptake; VT: tidal volume
Discussion
We investigated the clinical implications of OUES and the 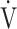 E-
E-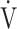 CO2 slope as indicators of pathophysiological conditions during incremental exercise in patients with all COPD grades. The main findings of this study were as follows. First, OUES was not strongly correlated across all GOLD grades with exercise tolerance, ventilatory variables, ventilatory efficiency, and oxygen consumption ability. In particular, these correlations were weaker in the GOLD III-IV group with exertional oxygen desaturation. In contrast, the
CO2 slope as indicators of pathophysiological conditions during incremental exercise in patients with all COPD grades. The main findings of this study were as follows. First, OUES was not strongly correlated across all GOLD grades with exercise tolerance, ventilatory variables, ventilatory efficiency, and oxygen consumption ability. In particular, these correlations were weaker in the GOLD III-IV group with exertional oxygen desaturation. In contrast, the 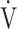 E-
E-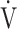 CO2 slope was specifically correlated with oxygen consumption ability and exercise tolerance regardless of COPD grade and exertional oxygen desaturation, having only a minimal correlation with
CO2 slope was specifically correlated with oxygen consumption ability and exercise tolerance regardless of COPD grade and exertional oxygen desaturation, having only a minimal correlation with 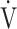 E.
E.
Admittedly, though OUES and the 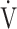 E-
E-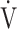 CO2 slope are commonly used indicators of gas exchange efficiency evaluated by CPET, there have been no reports of their clinical use in COPD patients based on the differences between the two indicators. In particular, little is known about what OUES really means. Based on the formula,
CO2 slope are commonly used indicators of gas exchange efficiency evaluated by CPET, there have been no reports of their clinical use in COPD patients based on the differences between the two indicators. In particular, little is known about what OUES really means. Based on the formula, 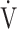 O2 = OUES × log10
O2 = OUES × log10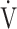 E + intercept, given that the value of log10
E + intercept, given that the value of log10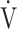 E is smaller than that of the actually measured value of
E is smaller than that of the actually measured value of 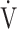 E, and that OUES represents the absolute rate of increase in
E, and that OUES represents the absolute rate of increase in 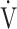 O2 per log10
O2 per log10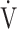 E, even if minor ventilatory abnormalities occur up to submaximal exercise before reaching peak exercise, OUES allows detection of the resultant ventilatory variations more sensitively than does the
E, even if minor ventilatory abnormalities occur up to submaximal exercise before reaching peak exercise, OUES allows detection of the resultant ventilatory variations more sensitively than does the 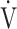 E-
E-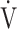 CO2 slope, which is evaluated using the actual measured value of
CO2 slope, which is evaluated using the actual measured value of 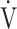 E. Indeed, Barron et al. [17] reported that OUES, rather than the
E. Indeed, Barron et al. [17] reported that OUES, rather than the 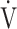 E-
E-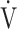 CO2 slope, was useful for differentiating between heart failure requiring excessive ventilatory compensation and COPD resulting in reduced ventilatory ability with the same exercise tolerance, which might indicate that OUES is more sensitive to the exertional ventilatory response during exercise. In the present study, regardless of COPD severity, compared with the
CO2 slope, was useful for differentiating between heart failure requiring excessive ventilatory compensation and COPD resulting in reduced ventilatory ability with the same exercise tolerance, which might indicate that OUES is more sensitive to the exertional ventilatory response during exercise. In the present study, regardless of COPD severity, compared with the 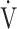 E-
E-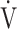 CO2 slope, OUES correlated with
CO2 slope, OUES correlated with 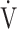 E at peak exercise, although the correlation was not strong (Tables 3 and 5). Certainly, in the population investigated in the present study, OUES may have been extensively, but never strongly, correlated with not only
E at peak exercise, although the correlation was not strong (Tables 3 and 5). Certainly, in the population investigated in the present study, OUES may have been extensively, but never strongly, correlated with not only 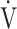 E and ΔFO, but also with peak
E and ΔFO, but also with peak 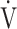 O2, and O2-pulse as an indicator of cardiac function. However, if COPD progressed to GOLD III and IV with exertional hypoxia, OUES correlated only weakly with the above exercise variables (Fig. 3). In the GOLD III-IV group with exertional hypoxia,
O2, and O2-pulse as an indicator of cardiac function. However, if COPD progressed to GOLD III and IV with exertional hypoxia, OUES correlated only weakly with the above exercise variables (Fig. 3). In the GOLD III-IV group with exertional hypoxia, 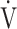 E at peak exercise was lower, and O2-pulse tended to be lower, but there were no significant differences in HR at peak exercise and HR at peak exercise/predicted maximal HR between the groups with and without exertional hypoxia (Table 4). These findings may not suggest that cardiac function is decreasing in the group with exertional hypoxia, but they may suggest that exertional hypoxia is involved as one of the reasons for the reduced O2-pulse during exercise [4]. Furthermore, though Fig. 1 and online supplement Figure S2 demonstrate that the relationship between
E at peak exercise was lower, and O2-pulse tended to be lower, but there were no significant differences in HR at peak exercise and HR at peak exercise/predicted maximal HR between the groups with and without exertional hypoxia (Table 4). These findings may not suggest that cardiac function is decreasing in the group with exertional hypoxia, but they may suggest that exertional hypoxia is involved as one of the reasons for the reduced O2-pulse during exercise [4]. Furthermore, though Fig. 1 and online supplement Figure S2 demonstrate that the relationship between 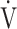 O2 and
O2 and 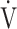 E during exercise is such that using the log transformation of
E during exercise is such that using the log transformation of 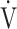 E was valid in the present study, a lack of increase in
E was valid in the present study, a lack of increase in 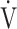 E during exercise in advanced COPD might decrease the utility of the logarithmic conversion of
E during exercise in advanced COPD might decrease the utility of the logarithmic conversion of 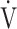 E, because the relationship between
E, because the relationship between 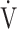 E and
E and 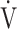 O2 was more linear in the desaturation group of the GOLD III-IV group (Fig. 4). Given that most advanced COPD is associated with reduced
O2 was more linear in the desaturation group of the GOLD III-IV group (Fig. 4). Given that most advanced COPD is associated with reduced 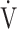 E and exertional hypoxia, the clinical utility of OUES, especially in advanced COPD may be less.
E and exertional hypoxia, the clinical utility of OUES, especially in advanced COPD may be less.
In advanced COPD, due to the existing ventilatory disorders, even with maximal ventilatory effort only reduced 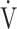 E can be achieved, which is a factor that limits exercise performance and reduces exercise tolerance [18, 19]. In such cases, to enable higher
E can be achieved, which is a factor that limits exercise performance and reduces exercise tolerance [18, 19]. In such cases, to enable higher 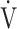 O2, the subjects often rely on the compensatory increase in ΔFO2 due to the reduction in FEO2, that is, higher oxygen consumption ability throughout the body [9, 10]. In the present study, the dependence of ΔFO2 on peak
O2, the subjects often rely on the compensatory increase in ΔFO2 due to the reduction in FEO2, that is, higher oxygen consumption ability throughout the body [9, 10]. In the present study, the dependence of ΔFO2 on peak 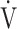 O2, expressed as the square of the correlation coefficient, was higher in the GOLD IV group than in the other stages. The following two conflicting findings in our previous studies might be very useful to suggest that specific CPET parameters related to exertional oxygen consumption ability would enable planning of treatment strategies and their evaluation. First, increasing ΔFO2 at peak exercise in pulmonary rehabilitation with exercise therapy in severe and very severe COPD patients led to improvement in both ventilatory efficiency and peak
O2, expressed as the square of the correlation coefficient, was higher in the GOLD IV group than in the other stages. The following two conflicting findings in our previous studies might be very useful to suggest that specific CPET parameters related to exertional oxygen consumption ability would enable planning of treatment strategies and their evaluation. First, increasing ΔFO2 at peak exercise in pulmonary rehabilitation with exercise therapy in severe and very severe COPD patients led to improvement in both ventilatory efficiency and peak 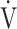 O2 [10]. Second, in contrast, a treatment specifically aimed at improving ventilation using expiratory pressure load training, resulted in improved ventilation without improving ΔFO2 at peak exercise in patients with COPD, improving peak
O2 [10]. Second, in contrast, a treatment specifically aimed at improving ventilation using expiratory pressure load training, resulted in improved ventilation without improving ΔFO2 at peak exercise in patients with COPD, improving peak 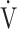 O2, but with no significant change in ventilatory efficiency [20]. When assessing exertional variables using CPET in many diseases, many reports have shown that the
O2, but with no significant change in ventilatory efficiency [20]. When assessing exertional variables using CPET in many diseases, many reports have shown that the 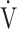 E-
E-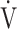 CO2 slope is related to exercise intolerance [8, 21]. So far, the
CO2 slope is related to exercise intolerance [8, 21]. So far, the 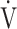 E-
E- CO2 slope has been considered to mainly reflect lung efficiency as a physiological mechanism. However, the
CO2 slope has been considered to mainly reflect lung efficiency as a physiological mechanism. However, the  E-
E- CO2 slope was also found to be affected by the oxygen consumption ability resulting from circulatory disturbance, in addition to excessive ventilation in the lung [22], and Nayor et al. [23] reported that
CO2 slope was also found to be affected by the oxygen consumption ability resulting from circulatory disturbance, in addition to excessive ventilation in the lung [22], and Nayor et al. [23] reported that  E/
E/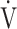 CO2 nadir is related to the arteriovenous O2 difference at peak exercise, suggesting that ventilatory efficiency correlates with the oxygen extraction ability of peripheral muscles. In the present study, ΔFO2 at peak exercise correlated with ventilatory efficiency not only across all COPD grades (Table 5), but also in advanced COPD, regardless of exertional hypoxemia (Fig. 3). In addition, stepwise variable analysis, including the COPD grade and the SpO2 level at peak exercise, identified ΔFO2 at peak exercise rather than peak
CO2 nadir is related to the arteriovenous O2 difference at peak exercise, suggesting that ventilatory efficiency correlates with the oxygen extraction ability of peripheral muscles. In the present study, ΔFO2 at peak exercise correlated with ventilatory efficiency not only across all COPD grades (Table 5), but also in advanced COPD, regardless of exertional hypoxemia (Fig. 3). In addition, stepwise variable analysis, including the COPD grade and the SpO2 level at peak exercise, identified ΔFO2 at peak exercise rather than peak 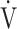 O2 as a more influential variable correlated with the
O2 as a more influential variable correlated with the 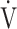 E-
E-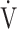 CO2 slope across all GOLD grades. Although this needs to be studied in other diseases that result in excess ventilation during exercise, the above findings suggest that, at least in ventilation-limited diseases such as COPD, the
CO2 slope across all GOLD grades. Although this needs to be studied in other diseases that result in excess ventilation during exercise, the above findings suggest that, at least in ventilation-limited diseases such as COPD, the 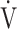 E-
E-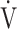 CO2 slope is a specific indicator of oxygen consumption ability that is independent of ventilatory ability to increase
CO2 slope is a specific indicator of oxygen consumption ability that is independent of ventilatory ability to increase 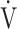 E. Given that (1) for advanced COPD with exertional hypoxia, assessment of exercise variables by OUES appears to have limitations, as shown in the present study, and that (2) in advanced COPD, pulmonary rehabilitation is often required to focus on improving oxygen consumption ability rather than ventilation due to the presence of a severe ventilatory disorder, the
E. Given that (1) for advanced COPD with exertional hypoxia, assessment of exercise variables by OUES appears to have limitations, as shown in the present study, and that (2) in advanced COPD, pulmonary rehabilitation is often required to focus on improving oxygen consumption ability rather than ventilation due to the presence of a severe ventilatory disorder, the 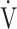 E-
E-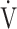 CO2 slope might be useful for planning and assessing personalized pulmonary rehabilitation.
CO2 slope might be useful for planning and assessing personalized pulmonary rehabilitation.
This study has some limitations. First, since the present study involved COPD patients at a single center, a multicenter evaluation including not only COPD patients, but also those with many other diseases, is needed to confirm the physiological mechanisms reflected by the 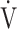 E-
E-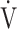 CO2 slope and OUES. Second, assessment of blood circulation, such as by echocardiography or right heart catheterization, would have been useful [24, 25]. Given that there was no strong relationship between the arterial desaturation level and ΔFO2 at peak exercise in the non-desaturation group and the desaturation group in the GOLD III-IV group in the present study, and that among the variables related to ΔFO2, FIO2 is almost constant at 21%, and, therefore, ΔFO2 depends primarily on FEO2, it was assumed that FEO2 is probably closely related to mixed venous oxygen pressure, which is obtained by right heart catheterization. This assumption has not been fully investigated and requires further study. Third, treatment strategies targeted at improving oxygen consumption ability are underdeveloped [3, 26, 27]. If such treatments become available, the importance of the
CO2 slope and OUES. Second, assessment of blood circulation, such as by echocardiography or right heart catheterization, would have been useful [24, 25]. Given that there was no strong relationship between the arterial desaturation level and ΔFO2 at peak exercise in the non-desaturation group and the desaturation group in the GOLD III-IV group in the present study, and that among the variables related to ΔFO2, FIO2 is almost constant at 21%, and, therefore, ΔFO2 depends primarily on FEO2, it was assumed that FEO2 is probably closely related to mixed venous oxygen pressure, which is obtained by right heart catheterization. This assumption has not been fully investigated and requires further study. Third, treatment strategies targeted at improving oxygen consumption ability are underdeveloped [3, 26, 27]. If such treatments become available, the importance of the 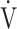 E-
E-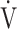 CO2 slope in relation to oxygen consumption ability might become more apparent. Increasing muscle mass while improving oxygen extraction ability, rather than simply increasing muscle mass as the endpoint, is necessary to improve exercise intolerance, which, as of now, still remains an unresolved issue.
CO2 slope in relation to oxygen consumption ability might become more apparent. Increasing muscle mass while improving oxygen extraction ability, rather than simply increasing muscle mass as the endpoint, is necessary to improve exercise intolerance, which, as of now, still remains an unresolved issue.
Conclusions
We demonstrated the characteristics of OUES and the 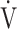 E-
E-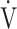 CO2 slope as indicators of pathophysiological exertional conditions in patients with all COPD grades. OUES is a comprehensive exercise index extensively involving pulmonary, cardiovascular, and muscle crosstalk in the body in COPD, but its association with them was never strong, and it may not be useful in cases of advanced COPD with exertional hypoxemia. In contrast, the
CO2 slope as indicators of pathophysiological exertional conditions in patients with all COPD grades. OUES is a comprehensive exercise index extensively involving pulmonary, cardiovascular, and muscle crosstalk in the body in COPD, but its association with them was never strong, and it may not be useful in cases of advanced COPD with exertional hypoxemia. In contrast, the 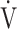 E-
E-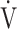 CO2 slope is an index that is independent of ventilatory ability, and it assesses oxygen consumption ability and exercise tolerance in COPD cases, regardless of the exertional hypoxemia level and COPD grade. Therefore, the
CO2 slope is an index that is independent of ventilatory ability, and it assesses oxygen consumption ability and exercise tolerance in COPD cases, regardless of the exertional hypoxemia level and COPD grade. Therefore, the 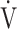 E-
E-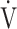 CO2 slope might be useful in establishing tailor-made pulmonary rehabilitation strategies for individual pathologies in COPD, serving as an indicator that focuses more on oxygen consumption ability independent of ventilatory ability.
CO2 slope might be useful in establishing tailor-made pulmonary rehabilitation strategies for individual pathologies in COPD, serving as an indicator that focuses more on oxygen consumption ability independent of ventilatory ability.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank J. Ikeda, and S. Ito for help with the CPET measurements.
Abbreviations
- CPET
Cardiopulmonary exercise testing
- ΔFO2
Difference between inspired and expired mean oxygen concentrations
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- ICS
Inhaled corticosteroid
- LABA
Long-acting β2-agonist
- LAMA
Long-acting muscarinic antagonist
- OUES
Oxygen-uptake efficiency slope
-
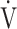 E-
E- CO2 slope
CO2 slope Minute ventilation-carbon dioxide production slope
Author contributions
All authors contributed substantially to this article. M.K. was responsible for the study conception and design. Y. H., M.K., K.K., M.S., M.Y., N.Y., H.K., H.H., F.M., M.T., Y.R., S.S., N.T., M.T., T.K., and K.H. were responsible for data acquisition, analysis and interpretation. Y.H. was responsible for drafting the article. Y. H., M.K., K.K., M.S., M.Y., N.Y., H.K., H.H., F.M., M.T., Y. R., S.S., N.T., M.T., T.K., and K.H. were responsible for revising the article. Each author approved the submission of this manuscript for publication.
Funding
This work was supported by a Grant-in-Aid for Clinical Research from the National Hospital Organization (number, not applicable). The funder had no role in the study design, data collection and analysis, or preparation of the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The institutional review board of the National Hospital Organization Osaka Toneyama Medical Center approved the study protocol (approval number: TNH-R-2022018) and the protocol was in accordance with the Declaration of Helsinki for experiments involving human subjects. The patients/participants provided their written informed consent for study participation before cardiopulmonary exercise testing.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Armstrong M, Vogiatzis I. Personalized exercise training in chronic lung diseases. Respirology. 2019;24(9):854–62. [DOI] [PubMed] [Google Scholar]
- 2.Riley CM, Sciurba FC. Diagnosis and Outpatient Management of Chronic Obstructive Pulmonary Disease: a review. JAMA. 2019;321(8):786–97. [DOI] [PubMed] [Google Scholar]
- 3.Sepúlveda-Loyola W, Osadnik C, Phu S, Morita AA, Duque G, Probst VS. Diagnosis, prevalence, and clinical impact of Sarcopenia in COPD: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2020;11(5):1164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wasserman K, Hansen J, Sue D, Stringer W, Sietsema K, Sun X-G. Principles of exercise testing and interpretation: including pathophysiology and clinical applications. Philadelphia: Lippincott Williams and Wilkins; 2012. [Google Scholar]
- 5.Laviolette L, Laveneziana P. Exercise Testing in the prognostic evaluation of patients with lung and heart diseases. In: Clinical Exercise Testing (ERS Monograph). edn. Edited by Palange P, Laveneziana P, Neder JA, Ward SA. Sheffield: European Respiratory Society; 2018: 222–234.
- 6.Ward SA. Determinants of the physiological systems’ response to muscular exercise in healthy subjects. In: Clinical Exercise Testing (ERS Monograph). edn. Edited by Palange P, Laveneziana P, Neder JA, Ward SA. Sheffield: European Respiratory Society; 2018: 1–33.
- 7.Neder JA, Berton DC, Arbex FF, Alencar MC, Rocha A, Sperandio PA et al. Physiological and clinical relevance of exercise ventilatory efficiency in COPD. Eur Respir J 2017, 49(3). [DOI] [PubMed]
- 8.Weatherald J, Sattler C, Garcia G, Laveneziana P. Ventilatory response to exercise in cardiopulmonary disease: the role of chemosensitivity and dead space. Eur Respir J 2018, 51(2). [DOI] [PubMed]
- 9.Miki K, Tsujino K, Maekura R, Matsuki T, Miki M, Hashimoto H, et al. Oxygen extraction based on Inspiratory and Expiratory Gas Analysis identifies Ventilatory Inefficiency in Chronic Obstructive Pulmonary Disease. Front Physiol. 2021;12:703977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyazaki A, Miki K, Maekura R, Tsujino K, Hashimoto H, Miki M et al. Increased oxygen extraction by Pulmonary Rehabilitation improves Exercise Tolerance and Ventilatory Efficiency in Advanced Chronic Obstructive Pulmonary Disease. J Clin Med 2022, 11(4). [DOI] [PMC free article] [PubMed]
- 11.Sun XG, Hansen JE, Stringer WW. Oxygen uptake efficiency plateau best predicts early death in heart failure. Chest. 2012;141(5):1284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baba R, Nagashima M, Goto M, Nagano Y, Yokota M, Tauchi N, et al. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol. 1996;28(6):1567–72. [DOI] [PubMed] [Google Scholar]
- 13.Baba R, Kubo N, Morotome Y, Iwagaki S. Reproducibility of the oxygen uptake efficiency slope in normal healthy subjects. J Sports Med Phys Fit. 1999;39(3):202–6. [PubMed] [Google Scholar]
- 14.Davies LC, Wensel R, Georgiadou P, Cicoira M, Coats AJ, Piepoli MF, et al. Enhanced prognostic value from cardiopulmonary exercise testing in chronic heart failure by non-linear analysis: oxygen uptake efficiency slope. Eur Heart J. 2006;27(6):684–90. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152(3):1107–36. [DOI] [PubMed] [Google Scholar]
- 16.Radtke T, Crook S, Kaltsakas G, Louvaris Z, Berton D, Urquhart DS et al. ERS statement on standardisation of cardiopulmonary exercise testing in chronic lung diseases. Eur Respir Rev 2019, 28(154). [DOI] [PMC free article] [PubMed]
- 17.Barron A, Francis DP, Mayet J, Ewert R, Obst A, Mason M, et al. Oxygen Uptake Efficiency Slope and Breathing Reserve, not anaerobic threshold, discriminate between patients with Cardiovascular Disease over Chronic Obstructive Pulmonary Disease. JACC Heart Fail. 2016;4(4):252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagawa H, Miki K, Kitada S, Miki M, Yoshimura K, Oshitani Y, et al. Dyspnea and the varying pathophysiologic manifestations of Chronic Obstructive Pulmonary Disease evaluated by Cardiopulmonary Exercise Testing with arterial blood analysis. Front Physiol. 2018;9:1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Donnell DE, Milne KM, James MD, de Torres JP, Neder JA. Dyspnea in COPD: new mechanistic insights and management implications. Adv Ther. 2020;37(1):41–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miki K, Tsujino K, Fukui M, Miki M, Kitajima T, Sumitani H, et al. Laryngeal widening and adequate ventilation by expiratory pressure load training improve aerobic capacity in COPD: a randomised controlled trial. Thorax. 2023;79(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips DB, Collins S, Stickland MK. Measurement and interpretation of Exercise Ventilatory Efficiency. Front Physiol. 2020;11:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mezzani A, Giordano A, Komici K, Corrà U. Different determinants of ventilatory inefficiency at different stages of reduced ejection Fraction Chronic Heart failure natural history. J Am Heart Assoc 2017, 6(5). [DOI] [PMC free article] [PubMed]
- 23.Nayor M, Xanthakis V, Tanguay M, Blodgett JB, Shah RV, Schoenike M, et al. Clinical and Hemodynamic Associations and Prognostic Implications of Ventilatory Efficiency in patients with preserved left ventricular systolic function. Circ Heart Fail. 2020;13(5):e006729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holverda S, Bogaard HJ, Groepenhoff H, Postmus PE, Boonstra A, Vonk-Noordegraaf A. Cardiopulmonary exercise test characteristics in patients with chronic obstructive pulmonary disease and associated pulmonary hypertension. Respiration. 2008;76(2):160–7. [DOI] [PubMed] [Google Scholar]
- 25.Torres-Castro R, Gimeno-Santos E, Vilaró J, Roqué-Figuls M, Moisés J, Vasconcello-Castillo L et al. Effect of pulmonary hypertension on exercise tolerance in patients with COPD: a prognostic systematic review and meta-analysis. Eur Respir Rev 2021, 30(160). [DOI] [PMC free article] [PubMed]
- 26.Hill K, Cavalheri V, Mathur S, Roig M, Janaudis-Ferreira T, Robles P, et al. Neuromuscular electrostimulation for adults with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;5(5):Cd010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pavitt MJ, Lewis A, Buttery SC, Fernandez BO, Mikus-Lelinska M, Banya WAS, et al. Dietary nitrate supplementation to enhance exercise capacity in hypoxic COPD: EDEN-OX, a double-blind, placebo-controlled, randomised cross-over study. Thorax. 2022;77(10):968–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.