Abstract
Background
Gabapentin and pregabalin were originally introduced as anticonvulsant medications but are now also prescribed on- and off-label for multiple medical disorders, especially for pain management. The national opioid crisis has led to increased use of non-opioid pain medications, including gabapentinoids, which has been associated with changing patterns of adverse events associated with these medications. This study investigated the characteristics and trends of gabapentin and pregabalin exposures reported to US poison centers from 2012 to 2022.
Methods
National Poison Data System data involving gabapentin and pregabalin exposures for 2012 to 2022 were analyzed.
Results
There were 124,161 exposures involving gabapentin and pregabalin as the primary substance reported to US poison centers during the study period. Most exposures involved gabapentin (85.9%), females (59.4%), single-substance exposures (62.9%), or occurred at a residence (97.2%). Suspected suicides accounted for 45.2% of exposures. Most exposures were associated with a minor effect (27.4%) or no effect (34.0%), while 22.1% experienced a serious medical outcome, including 96 fatalities. The rate of gabapentin and pregabalin exposures per one million US population increased by 236.1% from 22.7 in 2012 to 76.5 in 2019 (P < 0.001), followed by a non-significant decrease to 68.5 in 2022 (P = 0.068).
Conclusions
The rate of gabapentin and pregabalin exposures reported to US poison centers increased by more than 230% from 2012 to 2019 before plateauing from 2019 to 2022. The observed rate trend was driven primarily by gabapentin exposures and by cases associated with suspected suicide. Although most exposures were associated with a minor or no effect, 22% of individuals experienced a serious medical outcome, including 96 fatalities. These findings contribute to the discussion of rescheduling gabapentin as a federally controlled substance, which is the current status of pregabalin. Prevention of suicide associated with gabapentin and pregabalin merits special attention.
Keywords: Gabapentin, Pregabalin, Gabapentinoid, Toxicity, Suicide
Background
Gabapentin and pregabalin are gamma-aminobutyric acid analogs that were originally introduced as anticonvulsant medications. They are also prescribed for post-herpetic neuralgia, fibromyalgia, and neuropathic pain (Faryar et al. 2019; Klein-Schwartz et al. 2003; Reynolds et al. 2020; Tharp et al. 2019; Pfizer 2017, 2018). In addition, gabapentin is used off-label for mood disorders, mild alcohol withdrawal, alcohol use disorder, migraine headache, chronic pain, and post-operative pain management (Klein-Schwartz et al. 2003; Reynolds et al. 2020; Ghai et al. 2011; Miller and Price 2009; Sirven 2010). Gabapentin is the most commonly prescribed of the gabapentinoids and was the seventh most commonly prescribed medication in the United States (US) in 2019 (Mattson et al. 2022). The crisis of opioid misuse nationally has led to increased use of non-opioid medications, including gabapentinoids, for pain management (Goins et al. 2021). Unlike pregabalin, gabapentin is not a federally controlled substance under the Controlled Substances Act; however, it is a scheduled substance in at least seven states (Peckham et al. 2018; Sharkey 2023; US Drug Enforcement Administration 2023).
Adverse effects of gabapentin include dizziness, ataxia, tremors, and respiratory depression (Faryar et al. 2019; Pfizer 2017; Wills et al. 2014). Likewise, adverse effects of pregabalin include dizziness, somnolence and sedation (Pfizer 2018; Ghai et al. 2011). Co-use of gabapentin or pregabalin with other substances, including opioids, is common and may potentiate effects, such as euphoria and respiratory depression (Faryar et al. 2019; Mattson et al. 2022; Peckham et al. 2018; US Food and Drug Administration 2020). Based on this concern, the US Food and Drug Administration (FDA) issued a drug safety communication in December 2019, which required manufacturers to add warnings about respiratory depression to the prescribing information for gabapentinoids (US Food and Drug Administration 2020).
Previous studies on gabapentin and pregabalin have had limitations in their scope, including some focusing on only regional data (Faryar et al. 2019; Klein-Schwartz et al. 2003; Wills et al. 2014), specific age groups (Faryar et al. 2019; Reynolds et al. 2020; Wills et al. 2014; Toce et al. 2022), or fatalities (Tharp et al. 2019; Greene et al. 2023). The most recent study to publish gabapentin-related reporting trends to poison centers (PCs) nationally included data through 2017 (Reynolds et al. 2020). The objective of this study is to update our knowledge about the characteristics and trends of exposures involving gabapentin and pregabalin reported to US PCs from 2012 to 2022.
Methods
Data sources
Deidentified study data were obtained from the National Poison Data System (NPDS), which is a database maintained by America’s Poison Centers (America’s Poison Centers 2023). The database collects information in near real-time from calls to PCs in the US and its territories. Inter-censal and post-censal population estimates obtained from the US Census Bureau from 2012 to 2022 were used to calculate population-based exposure rates, including age-specific and sex-specific rates (US Census Bureau 2010, 2020). This study was determined to be exempt from approval by the institutional review board of the authors’ institution.
Case selection
Data for cases involving exposures to gabapentin and pregabalin (based on NPDS generic codes) reported to US PCs from January 1, 2012, through December 31, 2022, were obtained from the NPDS. Cases were excluded from the study if (1) the medical outcome was coded as “confirmed non-exposure” (n = 1,290) or “unrelated effect” (n = 14,519) or (2) the reason for exposure was coded as “adverse reaction-food” (n = 43), “unintentional-bite/sting” (n = 24), or “unintentional-food poisoning” (n = 42). In addition, fatal cases were excluded from the study if the relative contribution to fatality for gabapentin or pregabalin was determined by America’s Poison Centers to be “clearly not responsible” or “probably not responsible” (n = 14). This yielded a total of 228,233 exposure cases.
Study variables
Age groups were categorized as (1) ≤ 5 years, (2) 6–19 years, (3) 20–29 years, (4) 30–39 years, (5) 40–49 years, (6) 50–59 years, and (7) ≥ 60 years. Based on NPDS categories, the reason for exposure was grouped as (1) unintentional-general, (2) unintentional-therapeutic error, (3) unintentional-other (includes unintentional-environmental, unintentional-occupational, and unintentional-misuse), (4) unintentional-unknown, (5) intentional-suspected suicide, (6) intentional-misuse, (7) intentional-abuse, (8) intentional-unknown, and (9) other (includes adverse reaction-drug, adverse reaction-other, other-contamination/tampering, other-malicious, and other-withdrawal). Exposure sites were grouped as (1) residence (includes own residence and other residence), (2) other (includes school, healthcare facility, workplace, public area, and restaurant/food service), and (3) unknown.
Based on NPDS categories, the highest level of health care received was grouped as (1) no healthcare facility treatment received (includes the NPDS categories “managed on site” [not in a healthcare facility] and “other” management site), (2) treated/evaluated and released, (3) admitted to a critical care unit (CCU), (4) admitted to a non-CCU, (5) admitted to a psychiatric facility, (6) refused referral/did not arrive at healthcare facility, and (7) unknown (includes patient lost to follow-up and left against medical advice). For the purpose of analysis, admitted to a CCU and admitted to a non-CCU were combined into medical admission.
Based on NPDS categories, medical outcome was grouped as (1) no effect, (2) minor effect (minimally bothersome symptoms that generally resolve rapidly with no residual disability), (3) moderate effect (more pronounced, prolonged, or systemic than minor symptoms), (4) major effect (symptoms are life-threatening or result in significant disability or disfigurement), (5) death, (6) not followed (includes minimal clinical effects possible and judged as non-toxic exposure), and (7) unknown (includes unable to follow [potentially toxic exposure]). For the purpose of analysis, moderate effect, major effect, and death were combined into “serious medical outcome.” Additional variables included substance category (gabapentin or pregabalin), sex, year, and exposure type (single-substance or multiple-substance).
Statistical analysis
Data were analyzed using IBM Statistics SPSS 28 for Windows (IBM Corp, Armonk, NY) and SAS 9.4 (SAS Institute Inc, Cary, NC) statistical software. Exposures involving gabapentin or pregabalin as the primary substance (including both single-substance and multiple-substance exposures) were used to analyze the general characteristics of gabapentin and pregabalin exposures. A primary substance is the substance most likely responsible for the observed clinical effects as determined by the specialists in poison information at a PC. Trends over time were determined using all reported exposures (including primary and non-primary substance exposures). Piecewise and simple linear regression models were used, as appropriate, to determine the statistical significance of trends by evaluating whether the null hypothesis of slope = 0 could be rejected. Break points for piecewise analyses were determined based on scatter plots. Statistical significance was determined at α = 0.05. Other analyses included the calculation of risk ratios (RRs) with 95% confidence intervals (CIs).
Results
General characteristics
There were 124,161 exposures for which gabapentin or pregabalin was the primary substance. Most involved females (59.4%), were single-substance exposures (62.9%), occurred at a residence (97.2%), or were already in or en route to a healthcare facility when the PC was contacted (Table 1). Gabapentin accounted for 85.9% and pregabalin accounted for 14.1% of these exposures (Table 2).
Table 1.
Characteristics of exposures involving gabapentin or pregabalin as the primary substance reported to the national poison data system by age group, 2012–2022
| Characteristics | Age group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ≤ 5 years n (%)a |
6–19 years n (%)a |
20–29 years n (%)a |
30–39 years n (%)a |
40–49 years n (%)a |
50–59 years n (%)a |
≥ 60 years n (%)a |
Unknown | Total n (%)a |
|
| Sex | |||||||||
| Male | 7,935 (51.1) | 3,747 (36.9) | 6,860 (44.0) | 8,332 (42.2) | 7,246 (38.1) | 7,004 (38.7) | 7,293 (35.3) | 1,849 | 50,266 (40.6) |
| Female | 7,599 (48.9) | 6,398 (63.1) | 8,722 (56.0) | 11,403 (57.8) | 11,762 (61.9) | 11,094 (61.3) | 13,375 (64.7) | 3,098 | 73,451 (59.4) |
| Unknown | 45 | 11 | 16 | 16 | 14 | 8 | 20 | 314 | 444 |
| Type of exposure | |||||||||
| Single-substance | 13,498 (86.6) | 6,851 (67.5) | 8,838 (56.7) | 10,733 (54.3) | 10,052 (52.8) | 10,085 (55.7) | 14,049 (67.9) | 4,122 | 78,228 (62.9) |
| Multiple-substance | 2,081 (13.4) | 3,305 (32.5) | 6,760 (43.4) | 9,018 (45.7) | 8,970 (47.2) | 8,021 (44.3) | 6,639 (32.1) | 1,139 | 45,933 (37.1) |
| Reason for exposure | |||||||||
| Unintentional | 15,524 (99.8) | 2,549 (25.2) | 1,879 (12.2) | 2,741 (14.1) | 3,499 (18.7) | 5,185 (29.2) | 13,044 (64.3) | 2,552 | 46,973 (38.5) |
| Unintentional–general | 14,946 (96.1) | 554 (5.5) | 328 (2.1) | 385 (2.0) | 427 (2.3) | 450 (2.5) | 865 (4.3) | 342 | 18,297 (15.0) |
| Unintentional–therapeutic error | 548 (3.5) | 1,917 (19.0) | 1,447 (9.4) | 2,193 (11.3) | 2,939 (15.7) | 4,580 (25.8) | 11,947 (58.9) | 2,139 | 27,710 (22.7) |
| Unintentional–otherb | 27 (0.2) | 64 (0.6) | 88 (0.6) | 115 (0.6) | 99 (0.5) | 116 (0.7) | 185 (0.9) | 55 | 749 (0.6) |
| Unintentional–unknown | 3 (0.0) | 14 (0.1) | 16 (0.1) | 48 (0.2) | 34 (0.2) | 39 (0.2) | 47 (0.2) | 16 | 217 (0.2) |
| Intentional | 2 (0.0) | 7,430 (73.9) | 13,143 (85.7) | 16,263 (83.9) | 14,730 (78.9) | 11,920 (67.1) | 6,020 (29.7) | 1,943 | 71,451 (58.5) |
| Intentional–suspected suicide | 2 (0.0) | 5,854 (58.2) | 9,867 (64.3) | 12,522 (64.6) | 11,942 (63.9) | 9,527 (53.7) | 4,240 (20.9) | 1,251 | 55,205 (45.2) |
| Intentional–misuse | 0 (0.0) | 418 (4.2) | 1,128 (7.4) | 1,412 (7.3) | 1,191 (6.4) | 1,140 (6.4) | 1,120 (5.5) | 227 | 6,636 (5.4) |
| Intentional–abuse | 0 (0.0) | 844 (8.4) | 1,495 (9.7) | 1,459 (7.5) | 855 (4.6) | 576 (3.2) | 276 (1.4) | 195 | 5,700 (4.7) |
| Intentional–unknown | 0 (0.0) | 314 (3.1) | 653 (4.3) | 870 (4.5) | 742 (4.0) | 677 (3.8) | 384 (1.9) | 270 | 3,910 (3.2) |
| Otherc | 22 (0.1) | 86 (0.9) | 320 (2.1) | 388 (2.0) | 445 (2.4) | 627 (3.5) | 1,220 (6.0) | 584 | 3,692 (3.0) |
| Unknown | 31 | 91 | 256 | 359 | 348 | 374 | 404 | 182 | 2,045 |
| Management site | |||||||||
| Managed on site (not in a healthcare facility) | 10,414 (67.4) | 2,107 (20.9) | 1,724 (11.1) | 2,361 (12.0) | 3,049 (16.1) | 4,542 (25.2) | 11,622 (56.7) | 2,787 | 38,606 (31.3) |
| Individual already in (en route to) a healthcare facility | 3,475 (22.5) | 6,979 (69.1) | 12,325 (79.4) | 15,771 (80.2) | 14,641 (77.3) | 12,360 (68.6) | 7,474 (36.5) | 688 | 73,713 (60.0) |
| Individual referred by poison center to a healthcare facility | 1,509 (9.8) | 915 (9.1) | 1,371 (8.8) | 1,407 (7.2) | 1,145 (6.0) | 1,002 (5.6) | 1,152 (5.6) | 1,477 | 9,978 (8.1) |
| Other | 56 (0.4) | 93 (0.9) | 108 (0.7) | 122 (0.6) | 109 (0.6) | 126 (0.7) | 251 (1.2) | 108 | 973 (0.8) |
| Unknown | 125 | 62 | 70 | 90 | 78 | 76 | 189 | 201 | 891 |
| Highest level of health care received | |||||||||
| No healthcare facility treatment receivedd | 10,470 (69.9) | 2,200 (23.0) | 1,832 (12.8) | 2,483 (13.5) | 3,158 (17.6) | 4,668 (27.1) | 11,873 (60.1) | 2,895 | 39,579 (34.0) |
| Treated/evaluated and released | 3,950 (26.4) | 3,155 (33.0) | 4,795 (33.5) | 5,450 (29.6) | 4,679 (26.0) | 3,866 (22.5) | 2,894 (14.7) | 257 | 29,046 (25.1) |
| Admitted | 352 (2.4) | 4,037 (39.3) | 7,349 (51.3) | 10,092 (55.0) | 9,830 (54.8) | 8,431 (49.0) | 4,655 (23.6) | 221 | 44,967 (39.0) |
| Admitted to a critical care unit | 144 (1.0) | 724 (7.6) | 1,949 (13.6) | 3,136 (17.1) | 3,178 (17.7) | 2,750 (16.0) | 1,546 (7.8) | 60 | 13,487 (11.7) |
| Admitted to a non-critical care unit | 204 (1.4) | 763 (8.0) | 1,466 (10.2) | 2,218 (12.1) | 2,132 (11.9) | 2,169 (12.6) | 1,709 (8.7) | 51 | 10,712 (9.3) |
| Admitted to a psychiatric facility | 4 (0.0) | 2,550 (26.7) | 3,934 (27.5) | 4,738 (25.8) | 4,520 (25.2) | 3,512 (20.4) | 1,400 (7.1) | 110 | 20,768 (18.0) |
| Refused referral/did not arrive at healthcare facility | 204 (1.4) | 162 (1.7) | 353 (2.5) | 363 (2.0) | 297 (1.7) | 252 (1.5) | 326 (1.7) | 420 | 2,377 (2.0) |
| Unknowne | 603 | 602 | 1,269 | 1,363 | 1,058 | 889 | 940 | 1,468 | 8,192 |
| Medical outcome | |||||||||
| No effect | 7,410 (49.6) | 2,675 (27.7) | 3,217 (22.2) | 3,731 (20.1) | 3,502 (19.4) | 3,139 (18.1) | 3,462 (17.4) | 421 | 27,557 (23.6) |
| Minor effect | 1,129 (7.6) | 3,389 (35.1) | 5,331 (36.8) | 6,473 (34.9) | 6,065 (33.6) | 5,215 (30.1) | 3,867 (19.4) | 468 | 31,937 (27.4) |
| Serious medical outcome | 179 (1.2) | 1,729 (17.9) | 4,010 (27.6) | 5,883 (31.7) | 5,583 (30.9) | 4,935 (28.4) | 3,147 (15.8) | 193 | 25,659 (22.1) |
| Moderate effect | 167 (1.1) | 1,536 (15.9) | 3,373 (23.3) | 4,891 (26.4) | 4,601 (25.5) | 4,065 (23.4) | 2,589 (13.0) | 167 | 21,389 (18.3) |
| Major effect | 12 (0.1) | 191 (2.0) | 630 (4.3) | 972 (5.2) | 964 (5.3) | 845 (4.9) | 537 (2.7) | 23 | 4,174 (3.6) |
| Death | 0 (0.0) | 2 (0.0) | 7 (0.0) | 20 (0.1) | 18 (0.1) | 25 (0.1) | 21 (0.1) | 3 | 96 (0.1) |
| Not followedf | 6,219 (42.1) | 1,851 (19.4) | 1,930 (13.8) | 2,469 (12.9) | 2,905 (16.1) | 4,050 (23.7) | 9,417 (47.2) | 2,579 | 31,420 (27.0) |
| Unknowng | 642 | 512 | 1,110 | 1,195 | 967 | 767 | 795 | 1,600 | 7,588 |
| Total (row %) | 15,579 (12.6) | 10,156 (8.2) | 15,598 (12.6) | 19,751 (16.0) | 19,022 (15.4) | 18,106 (14.7) | 20,688 (16.8) | 5,261 | 124,161 (100.0) |
aColumn percentages may not add to 100.0% due to rounding error
b Includes unintentional-environmental, unintentional-occupational, and unintentional-misuse
cIncludes other-contamination/tampering, other-malicious, other-withdrawal, adverse reaction-drug or other
d Includes cases for which management site was recorded “managed on site (not in a healthcare facility)” or “other”
e Includes patient lost to follow-up/ left against medical advice/unknown
f Includes “not followed (minimal clinical effects possible)” and “not followed (judged as a non-toxic exposure)”
g Includes “unable to follow (judged as a potentially toxic exposure)”
Table 2.
Characteristics of exposures involving gabapentin or pregabalin as the primary substance reported to the national poison data system by bubstance, 2012–2022
| Characteristics | Substance | ||
|---|---|---|---|
| Gabapentin n (%)a |
Pregabalin n (%)a |
Total n (%)a |
|
| Sex | |||
| Male | 43,525 (41.0) | 6,741 (38.6) | 50,266 (40.6) |
| Female | 62,731 (59.0) | 10,720 (61.4) | 73,451 (59.4) |
| Unknown | 383 | 61 | 444 |
| Age group | |||
| ≤5 years | 12,750 (12.5) | 2,829 (16.9) | 15,579 (13.1) |
| 6-19 years | 8,892 (8.7) | 1,264 (7.5) | 10,156 (18.5) |
| 20-29 years | 14,038 (13.7) | 1,560 (9.3) | 15,598 (13.1) |
| 30-39 years | 17,385 (17.0) | 2,366 (14.1) | 19,751 (16.6) |
| 40-49 years | 16,322 (16.0) | 2,700 (16.1) | 19,022 (16.0) |
| 50-59 years | 15,340 (15.0) | 2,766 (16.5) | 18,106 (15.2) |
| ≥60 years | 17,411 (17.1) | 3,277 (19.6) | 20,688 (17.4) |
| Unknown | 4,501 | 760 | 5,261 |
| Type of exposure | |||
| Single-substance | 66,819 (62.7) | 11,409 (65.1) | 77,228 (63.0) |
| Multiple-substance | 39,820 (37.3) | 6,113 (34.9) | 45,933 (37.0) |
| Reason for exposure | |||
| Unintentional | 39,480 (37.6) | 7,493 (43.5) | 46,973 (38.5) |
| Unintentional–general | 15,136 (14.4) | 3,161 (18.4) | 18,297 (15.0) |
| Unintentional–therapeutic error | 23,527 (22.4) | 4,183 (24.3) | 27,710 (22.7) |
| Unintentional–otherb | 642 (0.6) | 107 (0.6) | 749 (0.6) |
| Unintentional–unknown | 175 (0.2) | 42 (0.2) | 217 (0.2) |
| Intentional | 62,498 (59.6) | 8,953 (52.1) | 70,451 (58.5) |
| Intentional–suspected suicide | 48,701 (46.4) | 6,504 (37.8) | 54,205 (45.2) |
| Intentional–misuse | 5,617 (5.4) | 1,019 (5.9) | 6,636 (5.4) |
| Intentional–abuse | 4,848 (4.6) | 852 (5.0) | 5,700 (4.7) |
| Intentional–unknown | 3,332 (3.2) | 578 (3.4) | 3,910 (3.2) |
| Otherc | 2,939 (2.8) | 753 (4.4) | 3,692 (3.0) |
| Unknown | 1,722 | 323 | 2,045 |
| Management site | |||
| Managed on site (not in a healthcare facility) | 32,887 (31.1) | 5,719 (32.9) | 38,606 (31.3) |
| Individual already in (en route to) a healthcare facility | 63,709 (60.2) | 10,004 (57.5) | 73,713 (59.8) |
| Individual referred by poison center to a healthcare facility | 8,466 (8.0) | 1,512 (8.7) | 9,978 (8.1) |
| Other | 822 (0.8) | 151 (0.9) | 973 (0.8) |
| Unknown | 755 | 136 | 891 |
| Highest level of health care received | |||
| No healthcare facility treatment receivedd | 33,709 (33.9) | 5,870 (35.8) | 39,579 (34.1) |
| Treated/evaluated and released | 24,916 (25.0) | 4,130 (25.2) | 29,046 (25.0) |
| Admitted | 38,954 (39.1) | 6,033 (36.7) | 44,967 (38.7) |
| Admitted to a critical care unit | 11,242 (11.3) | 2,245 (13.7) | 13,487 (11.6) |
| Admitted to a non-critical care unit | 9,129 (9.2) | 1,583 (9.6) | 10,712 (9.2) |
| Admitted to a psychiatric facility | 18,583 (18.6) | 2,205 (13.4) | 20,768 (17.9) |
| Refused referral/did not arrive at healthcare facility | 1,996 (2.0) | 381 (2.3) | 2,377 (2.0) |
| Unknowne | 7,084 | 1,108 | 8,192 |
| Medical outcome | |||
| No effect | 23,874 (23.8) | 3,683 (22.4) | 27,557 (23.6) |
| Minor effect | 27,631 (27.6) | 4,306 (26.2) | 31,937 (27.4) |
| Serious medical outcome | 21,505 (21.4) | 4,154 (25.2) | 25,659 (22.0) |
| Moderate effect | 17,964 (17.9) | 3,425 (20.8) | 21,389 (18.3) |
| Major effect | 3,452 (3.4) | 722 (4.4) | 4,174 (3.6) |
| Death | 89 (0.1) | 7 (0.0) | 96 (0.1) |
| Not followedf | 27,135 (27.1) | 4,285 (26.1) | 31,420 (26.9) |
| Unknowng | 6,494 | 1,094 | 7,588 |
| Total (row %) | 106,639 (85.9) | 17,522 (14.1) | 124,161 (100.0) |
aColumn percentages may not add to 100.0% due to rounding error
bIncludes unintentional-environmental, unintentional-occupational, and unintentional-misuse
cIncludes other-contamination/tampering, other-malicious, other-withdrawal, adverse reaction-drug or other
dIncludes cases for which management site was recorded “managed on site (not in a healthcare facility)” or “other”
eIncludes patient lost to follow-up/ left against medical advice/ unknown
fIncludes “not followed (minimal clinical effects possible)” and “not followed (judged as a non-toxic exposure)”
gIncludes “unable to follow (judged as a potentially toxic exposure)”
Reason for exposure
Suspected suicide was the most common (45.2%) reason for exposure involving gabapentin or pregabalin as the primary substance for all individuals combined (Table 1). However, the reason for exposure varied among age groups. Unintentional-general was the most common reason (96.1%) among ≤ 5-year-olds, therapeutic errors accounted for most (58.9%) exposures among individuals ≥ 60 years old, and suspected suicides represented most exposures among the other age groups.
Among the 55,205 suspected suicide exposures, 28.0% were associated with a serious medical outcome, including 53 fatalities. Exposures related to suspected suicides were more likely to be associated with a serious medical outcome (RR: 2.63, 95% CI: 2.57–2.70) than exposures related to all other reasons for exposure combined. Suspected suicides were also more likely to be associated with admission to a CCU or non-CCU (RR: 3.07, 95% CI: 3.00-3.15) than all other reasons for exposure combined.
Single-substance and multiple-substance exposures
Most (62.9%) exposures involved a single substance, which was especially true among children ≤ 5 years old (86.6%) (Table 1). Single-substance exposures accounted for more than three-fourths (78.6%) of unintentional exposures, including 83.5% of unintentional-general exposures and 75.7% of therapeutic errors. Single-substance exposures were less predominant (52.2%) among intentional exposures, including intentional-misuse (69.8%), intentional-abuse (55.6%), and suspected suicide (49.5%). Multiple-substance exposures with gabapentin or pregabalin as the primary substance were more likely to be associated with a serious medical outcome (RR: 2.53, 95% CI: 2.47–2.58) or admission to a CCU or non-CCU (RR: 2.40, 95% CI: 2.35–2.46) than single-substance exposures. Among these 45,933 multiple-substance exposures, the top five most commonly involved substances (in addition to gabapentin or pregabalin, which were the primary substances) were (1) ethanol (alcoholic beverages) (25.9%), (2) benzodiazepines (18.2%), (3) atypical antipsychotics (7.5%), (4) trazodone (4.5%), and (5) ibuprofen (3.2%).
Highest level of health care received
Although most exposures reported to a US PC did not receive care at a healthcare facility (34.0%) or were treated/evaluated and released (25.1%); 11.7% were admitted to a CCU and 9.3% were admitted to a non-CCU (Table 1). Individuals ≤ 5 years old or ≥ 60 years old were less likely to be admitted to a CCU or non-CCU (RR: 0.39, 95% CI: 0.38–0.41) than the other age groups combined. Individuals exposed to gabapentin were less likely to be admitted to a CCU or non-CCU (RR: 0.88, 95% CI: 0.85–0.90) and more likely to be admitted to a psychiatric facility (RR: 1.39, 95% CI: 1.33–1.45) than individuals exposed to pregabalin.
Medical outcome
Most reported exposures were associated with a minor effect (27.4%) or no effect (34.0%), while 22.1% experienced a serious medical outcome. The age group with the greatest proportion of serious medical outcomes was 30-39-year-olds (31.7%), followed by individuals 40–49 years old (30.9%) and 50–59 years old (28.4%) (Table 1). Individuals ≤ 5 years old or ≥ 60 years old were less likely to experience a serious medical outcome than individuals in the other age groups combined (RR: 0.34, 95% CI: 0.33–0.35). Individuals exposed to gabapentin were less likely to experience a serious medical outcome than those exposed to pregabalin (RR: 0.85, 95% CI: 0.83–0.87).
There were 96 fatalities reported during the study period, including 25 among individuals 50–59 years old, followed by individuals ≥ 60 years old (n = 21), 30–39 years old (n = 20), and 40–49 years old (n = 18). Fifty-two decedents were female, 58 were associated with multiple-substance exposures, 53 were identified as suspected suicide, and 89 involved gabapentin as the primary substance (including 36 as the single-substance). Among the 81 fatalities that had a relative contribution to fatality determination by America’s Poison Centers, gabapentin or pregabalin was undoubtedly responsible for the death in 11 (13.6%), probably responsible in 37 (45.7%), contributory in 17 (21.0%), and unknown in 16 (19.8%).
Trends
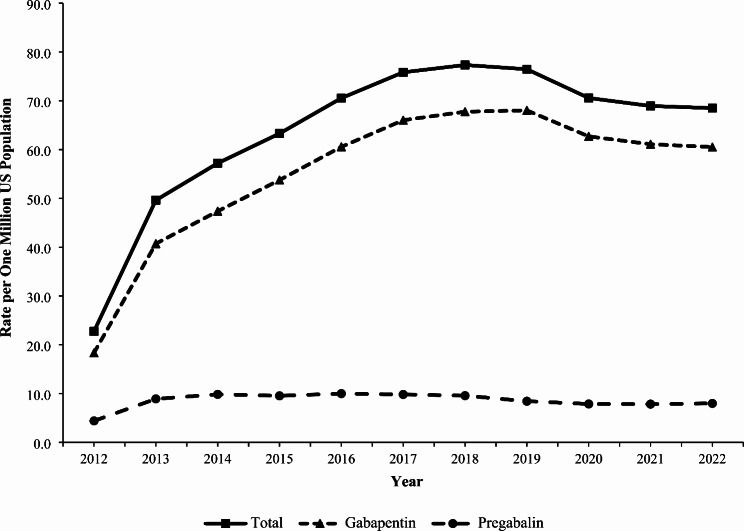
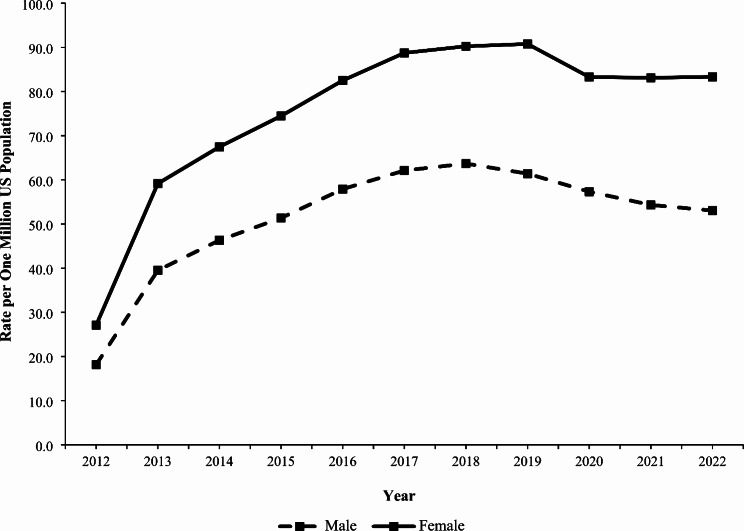
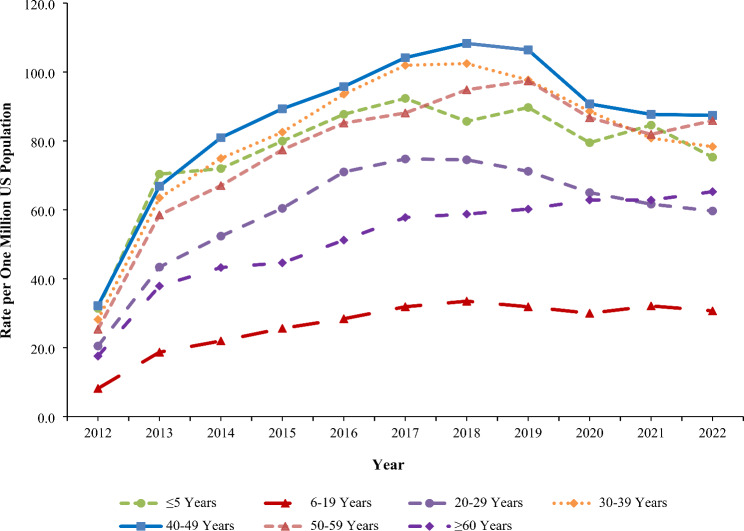
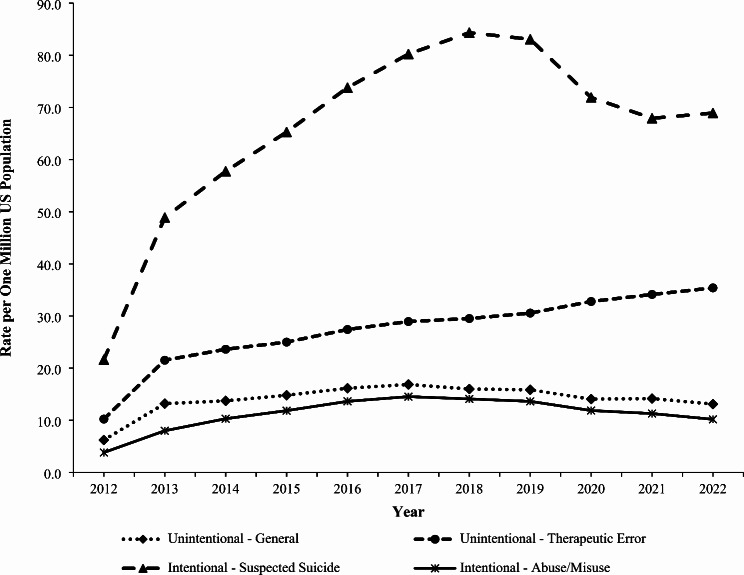
The rate of exposure per one million US population involving gabapentin or pregabalin (as either the primary or non-primary substance) reported to US PCs increased by 236.1% from 22.7 in 2012 to 76.5 in 2019 (P < 0.001), followed by a non-significant decrease to 68.5 in 2022 (P = 0.068) (Fig. 1). The rate of gabapentin exposures alone increased 270.8% from 18.3 in 2012 to 68.0 in 2019 (P < 0.001), followed by a decrease to 60.5 in 2022 (P = 0.052). In contrast, the rate of pregabalin exposures did not change significantly during the study period (P = 0.782) (Fig. 1). The rate trend for gabapentin and pregabalin exposures was similar for females and males, except males experienced a 13.6% decline from 2019 to 2022 (P = 0.038). Females experienced a higher rate than males throughout the study period, with a growing difference between the rates observed over time (Fig. 2). There was variation of the rate trends among age groups, with the highest rates observed among 40-49-year-olds and 30-39-year-olds (Fig. 3). Although the rates of intentional misuse or abuse and unintentional-general exposures remained relatively unchanged, the rate of therapeutic errors increased consistently during the study period (P < 0.001). The rate of suspected suicide increased by 290.1% from 21.6 in 2012 to 84.4 in 2018 (P < 0.001) and then decreased by 18.3% to 68.9 in 2022 (P = 0.007) (Fig. 4).
Fig. 1.
Annual Rate of Gabapentin and Pregabalin Exposures Reported to the National Poison Data System by Substance, 2012–2022
Fig. 2.
Annual Rate of Gabapentin and Pregabalin Exposures Reported to the National Poison Data System by Sex, 2012–2022
Fig. 3.
Annual Rate of Gabapentin and Pregabalin Exposures Reported to the National Poison Data System by Age Group, 2012–2022
Fig. 4.
Annual Rate of Gabapentin and Pregabalin Exposures Reported to the National Poison Data System by Selected Reason for Exposure, 2012–2022
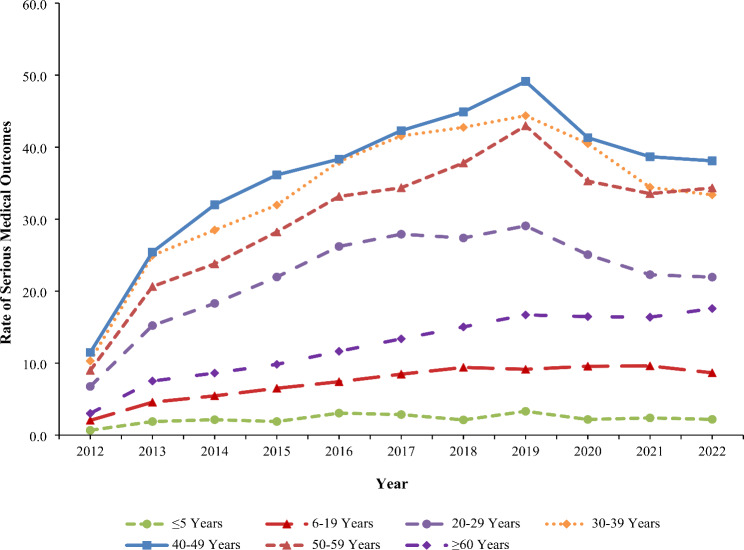
The rate trends of serious medical outcomes varied by age group. The rate remained constant among children ≤ 5 years old (P = 0.119) but increased significantly among 6-19-year-olds (P < 0.001) and ≥ 60-year-olds (P < 0.001) from 2012 to 2022 (Fig. 5). For the other age groups (i.e., individuals 20–29, 30–39, 40–49, and 50–59 years old), the rate of a serious medical outcome increased significantly from 2012 to 2019, followed by a significant decrease from 2019 to 2022 (P-values < 0.05) (Fig. 5).
Fig. 5.
Annual Rate of Serious Medical Outcomes per One Million US Population Associated with Gabapentin and Pregabalin Exposures Reported to the National Poison Data System by Age Group, 2012–2022
Discussion
During the 11-year study period, US PCs received 124,161 reports of gabapentin or pregabalin exposure as the primary substance, which averaged 6,208 exposures annually. There was a female predominance, which is consistent with findings from previous studies (Faryar et al. 2019; Reynolds et al. 2020) Suspected suicide was the most common reason for exposure in this study, accounting for 45% of exposures, which aligns with previous research (Faryar et al. 2019; Klein-Schwartz et al. 2003; Reynolds et al. 2020; Toce et al. 2022). Suspected suicides represented more than half of exposures among individuals 6–59 years old. The trend for the rate of suspected suicide was a driver of the trend observed for gabapentin and pregabalin exposures during the study period. Recommendations to help prevent these suicides include prescriber education, increased screening for co-occurring substance use disorders, mood disorders, and suicidal ideation, and judicious prescribing practices for gabapentin and pregabalin (Farah et al. 2023).
Approximately 10% of gabapentin and pregabalin exposures in this study were attributed to intentional misuse or abuse. Previous reports have identified the intentional misuse or abuse of gabapentin and pregabalin and associated consequences (Tharp et al. 2019; Mattson et al. 2022; Goins et al. 2021; Peckham et al. 2018a, 2018b; Hagg et al. 2020; Schifano 2014). In particular, the concurrent use of opioids and gabapentinoids has been linked to an increased likelihood of hospitalization and opioid-related fatalities, which prompted an update to the Beers criteria by the American Geriatric Society, advising caution against dual therapy among older adults (Goins et al. 2021; Peckham et al. 2018a; American American Geriatrics Society 2019; Gomes et al. 2017). Although pregabalin is a federally controlled substance, gabapentin is not, which has led to calls for the US Drug Enforcement Administration to schedule gabapentin the same as pregabalin (Peckham et al. 2018a, 2018b). Gabapentin is a scheduled drug in some states and legislation has been introduced in a number of other states to reclassify or monitor its use (Sharkey 2023; Peckham et al. 2018b; Throckmorton et al. 2018).
The rate of exposures reported to US PCs involving gabapentin and pregabalin increased from 2012 to 2019 and then decreased slightly from 2019 to 2022. This trend generally parallels the number of gabapentin prescriptions dispensed nationally, which increased by 70.4% from 2011 to 2015, followed by a slower increase of 20.0% from 2015 to 2019 and then 3.4% from 2019 to 2021 (US Drug Enforcement Administration 2023). The reasons for the observed trend are likely multifactorial and cannot be determined by this study. Potential factors may include a 2019 drug safety communication by the FDA that required warnings about respiratory depression be added to the prescribing information for gabapentinoids (US Food and Drug Administration 2020), scheduling of gabapentin as a controlled substance in a number of states (Sharkey 2023), the US opioid crisis (Spencer et al. 2002), and the COVID-19 pandemic. The rate of suspected suicide was a driver of the observed overall rate trend for gabapentin and pregabalin exposures, and it was similar to that for suicide-related fatalities (from all causes) in the US, which increased from 2001 to 2018, declined in 2019 and 2020, before increasing again in 2021 (Garnett and Curtin 2001).
In this study, pregabalin was more likely than gabapentin to be associated with a serious medical outcome or admission to a CCU or non-CCU, which may be attributable to multiple factors, including pregabalin’s higher potency, quicker rate of absorption, and greater bioavailability compared with gabapentin (Schifano 2014). Although statistically significant, the differences between the two medications were not large and should be interpreted with caution, especially given that the dosage of gabapentin versus that of pregabalin was not accounted for in this study, not all exposures were captured by the NPDS, and not all captured exposures were followed to a known outcome. Regardless, because gabapentin accounted for 86% of exposures in this study, its outcomes were important in terms of comparative frequency.
The reason for exposure among children ≤ 5 years old was usually “unintentional-general,” which represents exploratory behaviors. As young children gain mobility, they explore their environment without recognizing potential danger. Most medical outcomes in this age group were minor or no effects; however, approximately 1% were associated with a serious medical outcome. Prevention strategies for these incidents include (1) storing these medications out of reach and sight of children, preferably in a locked location, (2) using child-resistant containers, and (3) having the poison helpline number (800-222-1222) available in case an exposure does occur (US Centers for Disease Control and Prevention 2023).
Among individuals ≥ 60 years old, therapeutic errors were the most predominant reason for exposure. The likelihood of these errors occurring is exacerbated by the greater number of prescription medications used by older adults (National Institute on Drug Abuse 2020). The use of pill organizers may help prevent common errors; however, these should be child-resistant to mitigate unintended child exposures. The use of medication reminders and tracking systems, and when necessary, having a second adult regularly conduct medication reconciliation or assist in medication administration to older adults, may be helpful (Lavan et al. 2016).
Careful consideration is advised when prescribing gabapentinoids, especially to high-risk individuals, such as persons using opioids, persons with substance use disorders, older adults, and those susceptible to respiratory depression (US Food and Drug Administration 2020; Hagg et al. 2020; Chincholkar et al. 2020). To mitigate the possibility of adverse effects, clinicians should closely monitor patient response, and patients should promptly report any side effects (Chincholkar et al. 2020). The risk for intentional misuse and abuse should also be monitored by the prescribing clinician and other healthcare personnel and family members involved in the patient’s care (Hagg et al. 2020; Chincholkar et al. 2020).
Study limitations
This study has several limitations. Because the NPDS is a passive surveillance system, this study underestimates the incidence of gabapentin and pregabalin exposures. Exposures may be treated in emergency departments, urgent care facilities, or other healthcare settings without a PC being contacted, and some exposures may not receive medical care at all. The proportion of all true exposures captured by the NPDS may change over time. There also may be bias regarding which cases are reported to a PC, based on an individual’s age, severity of the exposure, or other factors (Hoffman 2007). Data are self-reported and cannot be completely verified by the PCs or America’s Poison Centers. Cases reported to the NPDS do not necessarily represent a poisoning or overdose. Repeat exposures involving the same individual were not identifiable because we did not have access to personal identifiers. Medication dose was not analyzed in this study. Although NPDS protocols for quality control and follow-up are used, data errors and missing data may occur. Despite these limitations, the NPDS provides a large national database useful for investigating the characteristics and trends of gabapentin and pregabalin exposures in the US.
Conclusions
The rate of gabapentin and pregabalin exposures reported to US PCs increased by more than 230% from 2012 to 2019 before plateauing from 2019 to 2022. The observed rate trend was driven primarily by gabapentin exposures and by cases associated with suspected suicide. Although most exposures were associated with a minor or no effect, 22% of individuals experienced a serious medical outcome, including 96 fatalities. These findings contribute to the discussion of rescheduling gabapentin as a federally controlled substance, which is the current status of pregabalin. Prevention of suicide associated with gabapentin and pregabalin merits special attention.
Acknowledgements
Not applicable.
Abbreviations
- CCU
Critical Care Unit
- CI
Confidence Interval
- FDA
Food and Drug Administration
- NPDS
National Poison Data System
- PC
Poison Center
- RR
Risk Ratio
- US
United States
Author contributions
EC contributed to the conception and design of the study, conducted data analyses, and contributed to interpretation of data; she drafted the article, approved the final version to be published, and agrees to be accountable for all aspects of the work. SK conducted data analyses and contributed to interpretation of data; she reviewed and revised the article critically for important intellectual content, approved the final version to be published, and agrees to be accountable for all aspects of the work. NR, HH, HS, JY, and MZ contributed to the interpretation of data; they reviewed and revised the article critically for important intellectual content, approved the final version to be published, and agree to be accountable for all aspects of the work. NR also contributed to acquisition of data. GS contributed to the conception and design of the study and interpretation of data; he assisted in drafting the article and reviewed and revised the article critically for important intellectual content, approved the final version to be published, and agrees to be accountable for all aspects of the work.
Funding
First author, EC, received a student scholar research stipend from the Child Injury Prevention Alliance while she worked on this study. The interpretations and conclusions in this article do not necessarily represent those of the funding organization. The funding organization was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data availability
Data analyzed in this study were from the National Poison Data System, which is a proprietary database owned and managed by America’s Poison Centers. Data requests should be submitted to America’s Poison Centers at: https://www.aapcc.org/national-poison-data-system.
Declarations
Ethics approval and consent to participate
This study was deemed exempt from approval by the institutional review board of the Abigail Wexner Research Institute at Nationwide Children’s Hospital. All methods were performed in accordance with the ethical standards as laid down in the Declaration of Helsinki and its later amendments or comparable ethical standards. All data were de-identified and written informed consent was not obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Disclaimer by America’s Poison Centers
America’s Poison Centers maintains the National Poison Data System (NPDS), which houses de-identified case records of self-reported information collected from callers during exposure management and poison information calls managed by the country’s poison control centers (PCCs). NPDS data do not reflect the entire universe of exposures to a particular substance as additional exposures may go unreported to PCCs; accordingly, NPDS data should not be construed to represent the complete incidence of US exposures to any substance(s). Exposures do not necessarily represent a poisoning or overdose, and America’s Poison Centers is not able to completely verify the accuracy of every report. Findings based on NPDS data do not necessarily reflect the opinions of America’s Poison Centers.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- American Geriatrics Society. American Geriatrics Society 2019 updated AGS Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–94. 10.1111/jgs.15767. [DOI] [PubMed] [Google Scholar]
- America’s Poison Centers. National Poison Data System. 2023. https://www.aapcc.org/national-poison-data-system. Accessed 18 Apr 2024.
- Chincholkar M, Gabapentinoids. Pharmacokinetics, pharmacodynamics and considerations for clinical practice. Br J Pain. 2020;14(2):104–14. 10.1177/2049463720912496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah R, Saumitra SV, Cole RJ, et al. Suspected suicide attempts by self-poisoning among persons aged 10–19 years during the COVID-19 pandemic — United States, 2020–2022. Morb Mortal Wkly Rep. 2023;72(16):426–30. 10.15585/mmwr.mm7216a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faryar KA, Webb AN, Bhandari B, Price TG, Bosse GM. Trending gabapentin exposures in Kentucky after legislation requiring use of the state prescription drug monitoring program for all opioid prescriptions. Clin Toxicol. 2019;57(6):398–403. 10.1080/15563650.2019.1687902. [DOI] [PubMed] [Google Scholar]
- Garnett MF, Curtin SC. Suicide mortality in the United States, 2001–2021. NCHS Data Brief No. 464. Updated April 2023. Accessed April 18, 2024. https://www.cdc.gov/nchs/data/databriefs/db464.pdf [PubMed]
- Ghai A, Gupta M, Hooda S, Singla D, Wadhera R. A randomized controlled trial to compare pregabalin with gabapentin for postoperative pain in abdominal hysterectomy. Saudi J Anaesth. 2011;5(3):252–7. 10.4103/1658-354X.84097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goins A, Patel K, Alles SRA. The gabapentinoid drugs and their abuse potential. Pharmacol Ther. 2021;227:107926. 10.1016/j.pharmthera.2021.107926. [DOI] [PubMed] [Google Scholar]
- Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case–control study. PLoS Med. 2017;14(10):e1002396. 10.1371/journal.pmed.1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene SC, Wyatt K, Cates AL, Weiss S. Anticonvulsant fatalities reported to the American Association of Poison Control Centers 2000–2019. Seizure. 2023;106:1–6. 10.1016/j.seizure.2023.01.010. [DOI] [PubMed] [Google Scholar]
- Hagg S, Jonsson AK, Ahlner J. Current evidence on abuse and misuse of gabapentinoids. Drug Saf. 2020;43(12):1235–54. 10.1007/s40264-020-00985-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RS. Understanding the limitations of retrospective analyses of poison center data. Clin Toxicol. 2007;45(8):943–5. 10.1080/15563650701233370. [DOI] [PubMed] [Google Scholar]
- Klein-Schwartz W, Shepherd JG, Gorman S, Dahl B. Characterization of gabapentin overdose using a poison center case series. J Toxicol Clin Toxicol. 2003;41(1):11–5. 10.1081/clt-120018265. [DOI] [PubMed] [Google Scholar]
- Lavan AH, Gallagher PF, O’Mahony D. Methods to reduce prescribing errors in elderly patients with multimorbidity. Clin Interv Aging. 2016;11:857–66. 10.2147/CIA.S80280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson CL, Chowdhury F, Gilson TP. Notes from the field: Trends in gabapentin detection and involvement in drug overdose deaths — 23 states and the District of Columbia, 2019–2020. Morb Mortal Wkly Rep. 2022;71(19):664–6. 10.15585/mmwr.mm7119a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A, Price G. Gabapentin toxicity in renal failure: The importance of dose adjustment. Pain Med. 2009;10(1):190–2. 10.1111/j.1526-4637.2008.00492.x. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. Substance use in older adults. DrugFacts. Updated July 2020. Accessed April 18. 2024. https://nida.nih.gov/publications/drugfacts/substance-use-in-older-adults-drugfacts
- Peckham AM, Fairman KA, Sclar DA. All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: A retrospective cohort analysis of a commercially insured US population. Drug Saf. 2018a;41(2):213–28. 10.1007/s40264-017-0595-1. [DOI] [PubMed] [Google Scholar]
- Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: The case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy. 2018b;11:109–16. 10.2147/RMHP.S168504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizer. Neurontin (gabapentin) drug label. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf. Accessed 11 Oct 2024.
- Pfizer. Lyrica (pregabalin) drug label. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021446s035,022488s013lbl.pdf. Accessed 11 Oct 2024.
- Reynolds K, Kaufman R, Korenoski A, Fennimore L, Shulman J. Trends in gabapentin and baclofen exposures reported to U.S. poison centers. Clin Toxicol. 2020;58(7):763–72. 10.1080/15563650.2019.1687902. [DOI] [PubMed] [Google Scholar]
- Schifano F. Misuse and abuse of pregabalin and gabapentin: Cause for concern? CNS Drugs. 2014;28:491–6. 10.1007/s40263-014-0164-4. [DOI] [PubMed] [Google Scholar]
- Sharkey L. Is gabapentin a narcotic or controlled substance? Healthline. Updated May 3, 2023. Accessed October 11, 2024. https://www.healthline.com/health/is-gabapentin-a-narcotic
- Sirven JI. New uses for older drugs: The tales of aspirin, thalidomide, and gabapentin. Mayo Clin Proc. 2010;85(6):508–11. 10.4065/mcp.2010.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer MR, Garnett MF, Minino AM. Drug overdose deaths in the United States, 2002–2022. NCHS Data Brief No. 491. Updated March 2024. Accessed April 18, 2024. https://www.cdc.gov/nchs/data/databriefs/db491.pdf
- Tharp AM, Hobron K, Wright T. Gabapentin-related deaths: Patterns of abuse and postmortem levels. J Forensic Sci. 2019;64(4):1105–11. 10.1111/1556-4029.14021. [DOI] [PubMed] [Google Scholar]
- Throckmorton DC, Gottlieb S, Woodcock J. The FDA and the next wave of drug abuse— proactive pharmacovigilance. N Engl J Med. 2018;379(3):205–7. 10.1056/NEJMp1806486. [DOI] [PubMed] [Google Scholar]
- Toce MS, Hudgins JD, Yuskaitis CJ, Monuteaux MC, Bourgeois FT. National assessment of anti-epileptic drug exposures among pre-teens and adolescents, 2000–2020. Clin Toxicol. 2022;60(6):681–7. 10.1080/15563650.2021.2023747. [DOI] [PubMed] [Google Scholar]
- US Drug Enforcement Administration. Gabapentin (Neurontin). Updated January 2023. Accessed October 11. 2024. https://www.deadiversion.usdoj.gov/drug_chem_info/gabapentin.pdf
- US Food and Drug Administration. FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR). Updated January 30, 2020. Accessed April 18, 2024. https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-serious-breathing-problems-seizure-and-nerve-pain-medicines-gabapentin-neurontin
- US Census Bureau. Annual estimates of the resident population by single year of age and sex for the United States: April 1, 2010 to July 1, 2020. Updated October 8, 2021. Accessed April 18. 2024. https://www.census.gov/programs-surveys/popest/technical-documentation/research/evaluation-estimates/2020-evaluation-estimates/2010s-national-detail.html
- US Census Bureau. Annual estimates of the resident population by single year of age and sex for the United States: April 1, 2020 to July 1, 2022. Updated June 25, 2024. Accessed July 8, 2024. https://www.census.gov/data/tables/time-series/demo/popest/2020s-national-detail.html
- US Centers for Disease Control and Prevention. Adverse drug events in children. Updated February 23. 2023. Accessed April 18, 2024. https://www.cdc.gov/medicationsafety/parents_childrenadversedrugevents.html#:~:text=To%20reduce%20the%20risk%20of%20harm%20from%20adverse,a%20place%20young%20children%20cannot%20reach%20or%20see
- Wills B, Reynolds P, Chu E, Murphy C, Cumpston K, Stromberg P, Rose R. Clinical outcomes in newer anticonvulsant overdose: A poison center observational study. J Med Toxicol. 2014;10(3):254–60. 10.1007/s13181-014-0384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analyzed in this study were from the National Poison Data System, which is a proprietary database owned and managed by America’s Poison Centers. Data requests should be submitted to America’s Poison Centers at: https://www.aapcc.org/national-poison-data-system.