Abstract
The northern house mosquito, Culex pipiens, employs diapause as an essential survival strategy during winter, inducing important phenotypic changes such as enhanced stress tolerance, lipid accumulation, and extended longevity. During diapause, the cessation of reproductive development represents another distinctive phenotypic change, underlining the need for adjusted modulation of gene expressions within the ovary. Although considerable advancements in screening gene expression profiles in diapausing and non-diapausing mosquitoes, there remains a gap in tissue-specific transcriptomic profiling that could elucidate the complicated formation of diverse diapause features in Cx. pipiens. Here, we filled this gap by utilizing RNA sequencing, providing a detailed examination of gene expression patterns in the fat body and ovary during diapause compared to non-diapause conditions. Functional annotation of upregulated genes identified associations with carbohydrate metabolism, stress tolerance, immunity, and epigenetic regulation. The validation of candidate genes using quantitative real-time PCR verified the differentially expressed genes identified in diapausing mosquitoes. Our findings contribute novel insights into potential regulators during diapause in Cx. pipiens, thereby opening possible avenues for developing innovative vector control strategies.
Keywords: Culex pipiens, RNA-seq, Fat body, Diapause, Ovary
1. Introduction
Insects employ several strategies to maintain their survival in the face of adverse environmental changes during seasonal transitions. Diapause serves as an alternative developmental program in Cx. pipiens, triggered specifically by photoperiod. Diapause triggers phenotypic alterations that result in increased energy stores, reduced growth and metabolism, improved stress tolerance, and an extended lifespan (Denlinger and Armbruster, 2014). The development arrest of most diapausing insects continues until environmental changes occur or endogenous regulations take place (Koštál, 2006). The utilization of the conventional model organism Drosophila melanogaster in diapause research has proven to be ineffective, mostly because of its inconspicuous diapause characteristics (Emerson et al., 2009; Schmidt et al., 2005). Researchers employed RNA sequencing in non-model insect organisms to enhance the comprehension of transcriptome disparities between diapausing and non-diapausing insects. This led to significant progress in revealing unique gene expression patterns throughout all stages of diapause (Poupardin et al., 2015; Santos et al., 2018).
Culex pipiens, commonly known as the northern house mosquito, is a major vector that transmits the West Nile virus. In late summer and early fall, adult female Cx. pipiens enter a state of reproductive diapause in response to the shortened daylight hours. In these mosquitoes, photoperiod is the main environmental cue that initiates diapause. It affects key circadian clock genes and the genes that control them, such as cycle (cyc), clock (clk), timeless (tim), and period (per) (Dhungana et al., 2023; Meireles-Filho and Kyriacou, 2013; Meuti et al., 2015). In Cx. pipiens, fat hypertrophy happens when insulin signaling is blocked and the forkhead transcription factor (FOXO) is upregulated (Sim et al., 2015; Sim and Denlinger, 2013, 2008a).
The adipose tissue is the primary location for the storage and metabolism of energy. Adipocytes not only regulate energy metabolism, but also participate in the creation of hemolymph proteins, some of which are involved in the processes of morphogenesis, lipid transport, and the development of egg follicle cells (Arrese and Soulages, 2010). Cx. pipiens employs a method of enhancing fat storage and inhibiting lipid metabolism to minimize energy usage during diapause. To accomplish this, adult females need to actively search for more sources of carbohydrates and improve their ability to digest them, while also avoiding using their energy reserves for physical development (Hahn and Denlinger, 2011). In Cx. pipiens, the fat reserves that persist after the end of diapause are used for the development of eggs (Zhou and Miesfeld, 2009).
In addition to energy storage, diapausing insects also reduce their respiratory metabolism to minimize expenditure which they experience in response to temperature variations, and the cold temperatures during winter greatly enhance the suppression of metabolic activity (Denlinger, 1986). Diapausing individuals also suppress their somatic growth, resulting in decreased protein synthesis and transcription, which contribute to increased levels of energy reserves (Denlinger et al., 2012). The ovary is crucial in managing the reproduction of Cx. pipiens by producing ecdysteroid and overseeing the maturation of eggs. Research has shown that the reproductive process of female insects belonging to the Diptera and Lepidoptera species depends on the secretion of ecdysone by their ovaries (Brown et al., 2009). In mosquitoes, bloodmeal cues the brain to trigger ecdysteroid production in the ovary, which then undergoes conversion into its active form, 20-hydroxyecdysone (20E), within the hemolymph. Afterward, 20E stimulates the synthesis of vitellogenin (Vg) in the adipose tissue. The generated vitellogenin (Vg) is delivered to the ovary, where it is utilized for the formation of yolk, which ultimately leads to the maturation of eggs (Roy et al., 2016). During diapause, female Cx. pipiens undergo significant phenotypic changes to cope with severe weather conditions and shorter daylight periods. These adaptations primarily involve an increase in fat storage and the halt of ovarian growth. Thus, we expect that there will be modified controls of gene expression in both the fat body and ovaries of diapausing mosquitoes.
Previous research has shown that changes in phenotype can happen in energy metabolism and reproductive development during diapause (Kang et al., 2021; Sim et al., 2015; Sim and Denlinger, 2008b). In Colorado potato beetles, tissue specific transcriptomic changes occur during diapause, especially in the fat body and the flight muscle (Lebenzon et al., 2021). Transcriptomic screening in pupae of butterfly Pieris napi also unveiled preprogrammed gene expression regulations that are more pronounced in the head and abdomen (Pruisscher et al., 2022). However, it is still not clear how these changes are controlled at the transcriptome level in Cx. pipiens. In this study, we conducted a thorough transcriptomic analysis of the fat bodies and ovaries in diapausing Cx. pipiens using RNA-seq. Diapausing Cx. pipiens substantially accumulate lipid storage during the first 6 days post-eclosion and significant storage of glycogen does not happen until 9 days after eclosion (Zhou and Miesfeld, 2009). Thus, we utilized mosquitoes 7-10 days old for this study. Our objective was to discover new genes and analyze the metabolic pathways that could potentially influence the onset and regulation of diapause. We hypothesize that during diapause, genes and pathways related to immunity, stress tolerance, lipid synthesis, and lifespan extension are upregulated, while those associated with metabolism and reproduction are downregulated in diapausing mosquitoes. Our results indicate genes and pathways involved in glycolysis/gluconeogenesis, pyruvate metabolism, and cytochrome P450 metabolism have altered expression levels during diapause in the fat body and ovary. Our study demonstrates the diapause's innate adaptive metabolic flexibility, exposing dynamic changes in transcriptional regulation throughout the period.
2. Material and Methods
2.1. Mosquito rearing
The Cx. pipiens colony was developed in September 2000 from larvae collected in Columbus, OH; additional field-collected mosquitoes from Dr. Megan Meuti's laboratory at Ohio State University supplemented the colony in 2009 and 2022. The Cx. pipiens colony was reared under a 15h:9h light:dark (L:D) photoperiod at 25°C and 75% relative humidity (RH) as previously described (Sim, 2008). Hatched larvae from blood-fed adult females were transferred into plastic trays containing distilled water at a density of 300 individuals per tray. Larvae were provided with TetraMin® fish flakes and pupae were placed in a water cup inside a cage with 10% sucrose solution for emerging adults. To induce diapause, mosquito larvae were transferred at 2nd instar stage to an 18°C chamber with 75% relative humidity and 9h:15h light:dark photoperiod. Non-diapause mosquitoes used for RNA sequencing and qRT-PCR validations were extracted from our main insect colony and reared at 18°C with 75% relative humidity and 15h:9h light:dark photoperiod. Diapause status was confirmed by measuring primary follicle and germarium lengths, and ovarian development stage was determined based on described methods (Christophers, 1911).
2.2. Total RNA Extraction
Four distinct groups were utilized for the extraction of total RNA. The samples consisted of fat bodies and ovaries obtained from females, either in a diapausing or non-diapausing state. Mosquitoes were collected 7-10 days post eclosion. Each biological replicate was collected from three batches of 30 mosquitoes using TRIzol (Life Technologies, Carlsbad, CA, USA). Total RNA purity was tested using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
2.3. Library Preparation and Sequencing
The libraries used were built with TruSeq RNA Library Prep Kit v2 according to the manufacturer protocol (Illumina, San Diego, CA, USA). Briefly, the samples were subjected to a two-step purification process employing magnetic beads with poly-T oligo attached. This was followed by fragmentation of the materials and priming for cDNA synthesis. cDNA was synthesized employing reverse transcriptase and random primers, forming double stranded (ds) cDNA, which was subsequently collected using Ampure XP beads (Beckman Coulter, Pasadena, CA, USA). The ds cDNA underwent end repaired to convert any resulting overhangs into blunt ends, followed by the adenylation of the 3’ end of the adaptors for pair-ended ligation. Subsequently, paired adapters were ligated to ds cDNA, selectively amplified by PCR. Following quality control, bridge amplification was performed, which was then loaded into an Illumina HiSeqX platform. A single molecular array was synthesized using reverse termination, generating unique clusters of nucleotides strands which were loaded for extension and imaging. The resulting clusters were extended with nucleotides containing reversible fluorophores, thus generating clusters that gave a unified signal.
2.4. Bioinformatics Analysis
For Illumina sequencing reads, the adapters used during the library construction were removed from the reads using Trimmomatic (Bolger et al., 2014). To avoid lower quality reads on the alignment, all reads were trimmed to 100 bp using the FASTX Toolkit v0.0.13 (Hannon, 2010) resulting in Phred-Quality-Scores greater than 30. Reads were aligned to Cx. quinquefasciatus genome (JHB2020) publicly available at VectorBase using HISAT2 v2.2099 (Kim et al., 2015). Read counts were generated using featureCounts (Liao et al., 2014). Summarized reads from featureCounts were then used in the differential expression analysis with DeSEQ2 (Love et al., 2014). Transcript abundance differences were visualized between D and ND conditions with volcano plots based on the read count and a statistical significance threshold (FDR p<0.05). Subsequently, Gene Ontology (GO) enrichment analyses were carried out using ShinyGO (Ge et al., 2020) with an FDR cutoff set at 0.05. To use ShinyGO, we first converted our genes with Vectorbase IDs into Uniprot IDs from the Vectorbase website, then the Uniprot IDs were used to generate resulting GO terms. Metabolic pathway enrichment analyses were conducted based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database available on Vectorbase (https://vectorbase.org) with the adjusted p-value cutoff at 0.05.
2.5. qRT-PCR validation of RNA sequencing data
For qRT-PCR gene expression validation, we extracted total RNAs separately from the fat bodies and ovaries of both D and ND samples using TRIzol reagent (Life Technologies, Carlsbad, CA, USA). Each extraction consisted of samples from around 30 mosquitoes that were 7-10 days old after adult eclosion. Genomic DNA was removed by using a Promega RQ1 DNAse kit as per manufacturer recommendations (Promega, Madison, WI, USA). About 1 μg RNA was reverse transcribed and amplified via superscript III RNase H-reverse transcriptase (Invitrogen, Carlsbad, CA, USA), per the manufacturer’s protocol. To measure relative expression levels, synthesized cDNAs were then used in the qPCR validation on the Rotor-Gene Q real-time PCR detection machine (QIAGEN, Germantown, MD, USA). Ct values generated from qRT-PCR validations were analyzed using the delta-delta Ct method. Ribosomal protein L19 (RpL19), an endogenous housekeeping gene, was used as an internal control. Transcript divergence from the qRT-PCR results was evaluated for statistical significance using the Student’s t-test. Primer information is reported in Tables S1 and S2.
2.6. Data deposition
The sequences reported in this paper have been deposited in the Sequence Read Archive (SRA) database (accession no., PRJNA1076830).
3. Results
3.1. Analysis of RNA-seq data
For diapausing ovary samples (DOV), Illumina HiSeq X sequencing gave a 16.4 Gbp total bases read with 81,486,075 read pairs. The average read length was 101 and the average quality score was 36. Non-diapausing ovary samples (NDOV) gave a total of 15.6 Gbp of reads, with 77,751,147 read pairs and an average read length of 101 and a quality score of 36. Diapausing fat body (DFB) samples produced 15.8 Gbp total bases read with 80,360,739 read pairs, an average read length of 101, and an average quality score of 36. The sequencing of non-diapausing fat body samples (NDFB) produced a total of 18.2 Gbp of reads, which were made up of 92,409,606 read pairs with an average length of 101 and a quality score of 36. After removing low-quality reads, eliminating poor-quality bases, and removing adaptor sequences, the fastq files were matched to the transcriptome of Cx. quinquefasciatus (JHB 2020, VectorBase). The DOV and NDOV readings exhibited alignment rates of 80.89% and 81.95% respectively. The alignment rate for the DFB readings was 84.09%, while the alignment rate for the NDFB values was 85.62%, demonstrating a significant degree of alignment overall. The summarized sequencing data and alignment information are reported in Table S3. Following read summarization and filtering, we examined the genes that were expressed differently under diapausing circumstances. An assessment of reads was performed by analyzing fold change differences and statistical significance. The results were then shown using volcano plots (Fig. 1A&1B). Among the 18,482 genes analyzed, 246 genes were found to be upregulated and 122 genes were downregulated in DFB. Additionally, 1,923 genes had similar expression levels, as shown in Figure 1C. In the case of DOV, there was an upregulation of 328 genes and a downregulation of just 27 genes. Additionally, 3,587 genes showed similar levels of expression (Fig. 1D).
Figure 1.
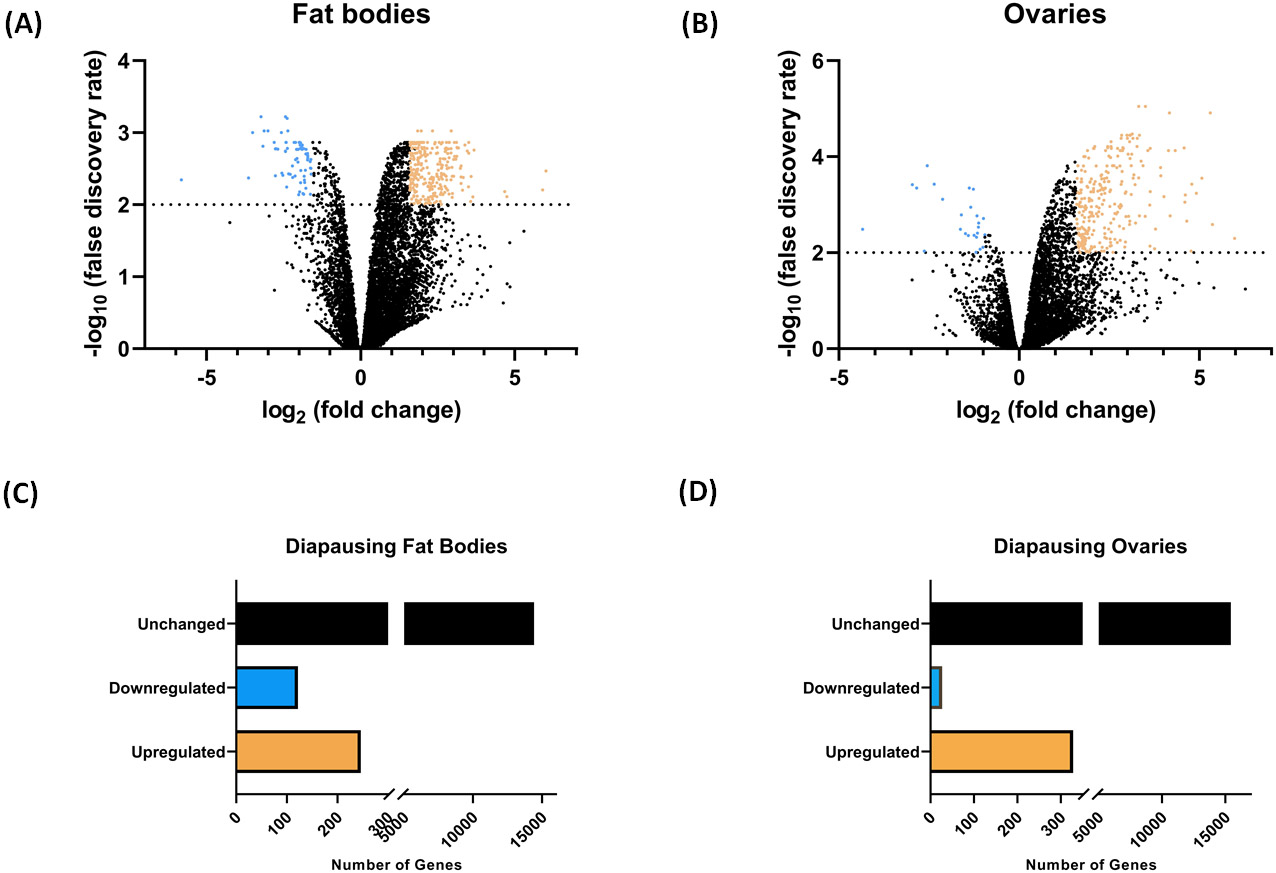
Summary statistics of differentially expressed genes in diapausing Cx. pipiens. Volcano plot illustrates logarithmic fold change values for comparison between DFB vs. NDFB (A) and DOV vs. NDOV (B), plotted against the negative log10 of the FDR values. Light orange dots indicate upregulated genes (FC ≥ 3), Blue dots indicate downregulated genes (FC ≤ 0.33), and black dots indicate genes not significantly altered between D and ND conditions. Bar graph depicts upregulated (FC≥3) and downregulated (FC≤0.33) genes in the fat bodies (C) and ovaries (D) of D females compared to the ND counterparts. The unchanged genes include those with non-zero expression levels that do not show significant differential expressions.
3.2. Analysis of gene ontology and KEGG pathways
To gain a comprehensive understanding of the biological processes linked to the genes that are differentially expressed in DFB, we performed gene ontology (GO) enrichment analysis on 246 upregulated genes in DFB. The clusters displayed a wide range of significant molecular functions and biological processes that play a crucial role in diapause formation. These include signal transduction, cell communication, cellular response to stimulus, and cellular developmental process (Fig. 2A). We also performed a GO enrichment analysis with genes downregulated in DFB (Table S8). The resulting clusters include molecular functions such as photoreceptor activity and serine-type endopeptidase activity (Figure S1). In addition, we performed a metabolic pathway enrichment analysis utilizing the Kyoto Encyclopedia of Genes and Genomes (KEGG) database in order to identify probable metabolic pathways associated with the elevated genes. We found that 246 upregulated genes in the DFB sample belonged to 8 pathways. These pathways included important metabolic processes for initiating diapause such as pyruvate metabolism and glycolysis/gluconeogenesis (Fig. 3A).
Figure 2.

Functional enrichment analysis shows significant clusters in biological processes consisting of upregulated genes in diapausing mosquito fat bodies (A) and ovaries (B). Top 20 most enriched processes are shown in figures. Complete GO analysis results are summarized in Table S5 & S6.
Figure 3.

Metabolic pathways significantly enriched based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database of genes from (A) Fat bodies and (B) Ovaries of diapausing mosquitoes 7-10 days post-eclosion. Diamonds symbolize the quantity of genes present in each pathway. Distinct pathways were demonstrated for fat bodies and ovarian tissues.
By employing the same approach, we utilized the Vectorbase database to conduct GO enrichment analysis on 328 gens upregulated in DOV. The generated clusters are associated with different biological processes such as serine-type peptidase, hydrolase, and proteolysis (Fig. 2B). Out of the 329 genes that were shown to be elevated in the DOV sample, 28 KEGG pathways were identified and assigned. Several pathways that have been identified include cytochrome P450 metabolism, steroid hormone biosynthesis, and starch and sucrose metabolism (Fig. 3B). Our efforts to find enriched GO terms and KEGG pathways in the downregulated genes of diapausing ovaries (Table S9) yielded no statistically significant results. This outcome is likely due to the small size of the downregulated gene list.
To identify the presence of genes exhibiting up or downregulation in both the fat body and ovary, we conducted a comparison between the up and downregulated gene lists from each tissue. Our results revealed 31 genes that were commonly upregulated in both the fat body and ovary, while only 2 genes exhibited downregulation in both tissues (Table S4). GO terms associated with the commonly upregulated genes encompass various biological processes, including small molecule catabolic process, cellular carbohydrate and sorbitol metabolic process (Figure S2). The commonly upregulated genes in both the fat body and ovary exhibited KEGG pathways related to glycolysis/gluconeogenesis, pyruvate metabolism, and glycine, serine, and threonine metabolism (Figure S3).
3.3. Validation of candidate genes using qRT-PCR
To verify the accuracy of our RNA-seq study, we chose to validate the upregulated genes that were discovered in our bioinformatic analysis of both DFB and DOV samples and are associated with the beginning and maintenance of diapause. This validation was done using qRT-PCR, and the results can be seen in Table 1 and Table 2. We obtained RNA samples from diapausing and non-diapausing mosquitoes during 7-10 days after they emerged from the pupal stage. Afterward, we synthesized complementary DNAs (cDNAs) from these RNA samples and conducted a quantitative reverse transcription polymerase chain reaction (qRT-PCR) experiment. All potential genes exhibited a greater mRNA abundance in diapausing conditions compared to non-diapausing conditions, with the exception of vitelline membrane protein 15a-3 (P < 0.05) (Fig. 4 & 5). Validation of genes from the DFB sample using qRT-PCR demonstrated a connection with histone demethylation, glucose metabolism, and immunological response. The genes found from the DOV sample exhibited associations with the breakdown of juvenile hormone, the ability to tolerate stress, and the creation of the vitelline membrane. These results show the reliability of bioinformatic analysis derived from our RNA-seq experiment.
Table 1.
Upregulated genes in DFB from RNA-seq and qRT-PCR validation
| Gene Name | Gene ID |
Vectorbase ID | Description/Activity | featureCounts Fold Change (FC) |
qRT-PCR FC |
|---|---|---|---|---|---|
| phosphoenolpyruvate carboxykinase | pepck | CQUJHB013202 | Gluconeogenesis | 17.85 | 1.43 |
| ATP-binding cassette sub-family G | atp | CQUJHB017700 | ABC-type transporter | 5.85 | 1.42 |
| aromatic-L-amino-acid decarboxylase | aadc | CQUJHB000109 | Dopamine synthesis | 4.34 | 7.75 |
| short chain acyl-CoA dehydrogenase | scad | CQUJHB017266 | Oxidoreductase activity | 3.81 | 1.74 |
| lysosomal alpha-mannosidase | laman | CQUJHB006140 | Carbohydrate metabolism | 3.79 | 2.68 |
| glycerol kinase | gk | CQUJHB018416 | Lipogenesis | 3.29 | 1.61 |
| sugar transporter | stp | CQUJHB015294 | Carbohydrate transport | 3.06 | 1.85 |
| UTY | uty | CQUJHB000550 | H3K27 demethylase | 1.38 | 2.39 |
| leukocyte elastase inhibitor | lei | CQUJHB013288 | Serine endopeptidase inhibitor | 1.21 | 3.99 |
Table 2.
Upregulated and downregulated genes in DOV from RNA-seq and qRT-PCR validation
| Gene Name | Gene ID | Vectorbase ID | Description/Activity | featureCounts Fold Change |
qRT- PCR FC |
|---|---|---|---|---|---|
| trypsin 3A1 | trypsin 3A1 | CQUJHB003219 | Serine peptidase | 40.42 | 31.35 |
| actin-1 | actin-1 | CQUJHB010858 | Cytoskeleton/Cell structure | 18.35 | 4.1 |
| chymotrypsin-2 | chymotrypsin-2 | CQUJHB005700 | Serine peptidase | 12.17 | 10.17 |
| myosin-VIIa | myo7a | CQUJHB000959 | Cytoskeleton | 7.57 | 4.57 |
| cytochrome P450 | cyp | CQUJHB006374 | Detoxification of xenobiotics | 6.37 | 4.47 |
| chitin synthase 2 | chs2 | CQUJHB007142 | Chitin synthase activity | 5.67 | 2.18 |
| CD109 antigen | CD109 | CQUJHB010439 | Endopeptidase inhibitor activity | 4.68 | 1.73 |
| leukocyte elastase inhibitor | serpinb1 | CQUJHB013288 | Serine endopeptidase inhibitor | 4.59 | 3.82 |
| fatty acid synthase | fasn | CQUJHB007902 | Fatty acid biosynthetic process | 3.58 | 1.53 |
| juvenile hormone esterase | jhe | CQUJHB008137 | Hydrolysis of juvenile hormone | 3 | 1.67 |
| vitelline membrane protein 15a-3 | vitelline membrane protein | CQUJHB000930 | Embryo development | 0.12 | 0.39 |
Figure 4.

qRT-PCR validation demonstrates the relative amounts of mRNA for selected genes in the RNA-seq study comparing fat body samples from D and ND females at 7-10 days following adult eclosion. A Student's t-test was conducted to analyze the expression levels of D and ND replicates for each gene. The significance levels were denoted as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Standard errors are depicted by error bars. Each gene was subjected to three biological replicates.
Figure 5.

qRT-PCR validation demonstrates the relative amounts of mRNA for selected genes in the RNA-seq analysis comparing ovary samples from D and ND females at 7-10 days following adult eclosion. A Student's t-test was conducted to compare the expression levels of D and ND replicates for each gene. Statistical significance was indicated by asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Standard errors are depicted by error bars. Each gene was subjected to three biological replicates.
Discussion
Culex pipiens utilizes diapause as a strategy to ensure its survival throughout the harsh winter season. Culex pipiens utilized an alternative developmental pathway characterized by specific phenotypic alterations, including the cessation of reproductive development, heightened fat storage, prolonged lifespan, and enhanced stress tolerance (Benoit and Denlinger, 2007; Bowen et al., 1988; Mitchell, 1981; Mitchell and Briegel, 1989; Rinehart et al., 2006). The adipose tissue and ovarian structures play crucial roles in the control of diapause. In insects, the fat body serves as the primary site for the synthesis of fatty acids and the production and storage of triglycerides (Hahn and Denlinger, 2011). Fat hypertrophy, which refers to the increase of fat body cells, is a significant hallmark of diapause in Cx. pipiens. Another feature is the cessation of ovarian development. Therefore, we expected to see significant alterations in the gene expression profiles of the fat body and ovaries between diapausing and non-diapausing mosquitoes. The goal of this study was to conduct a transcriptome screening to examine the gene expression patterns in diapausing and non-diapausing mosquitoes.
Our objective was to investigate the diverse transcriptional regulation of phenotypic and metabolic pathways that play a crucial role in diapause in Cx. pipiens. The results of our study are consistent with previous studies, suggesting that diapause is a dynamic and actively regulated process. This mechanism is well demonstrated by the activation and deactivation of many genes in diapausing mosquitoes (Denlinger and Armbruster, 2016). Through conducting GO enrichment analysis, we have shown that the upregulated genes in the diapausing fat body are linked to crucial biochemical processes that play a role in regulating diapause. The functions encompass serine-type endopeptidase and intracellular signal transmission. In addition, the metabolic pathways that have a high number of upregulated genes in the fat body are involved in several processes that lead to the onset of diapause, including glycolysis/gluconeogenesis and pyruvate metabolism. The downregulated genes in the DFB displayed GO terms associated with the catabolic processes of cellular carbohydrates and proteolysis, which are typically suppressed in diapausing mosquitoes. Furthermore, we observed an upregulation in the expression of genes associated with molecular activities such as oxidoreductase, alcohol dehydrogenase, and ATP-binding inside the diapausing ovary. By comparing the up and downregulated genes in both fat body and ovary, we identified 31 upregulated genes in both tissues while only 2 downregulated genes exist in both tissues. The GO analysis of the 31 commonly upregulated genes covered different biological processes such as cellular carbohydrate metabolic process and small molecule catabolic process.
We deliberately chose 20 genes among the upregulated genes in the fat body and the ovary due to their significance in regulating diapause to conduct qRT-PCR validation. The verified genes have either been identified in previous diapause studies or suggest a possible role in initiating diapause via modifying stress tolerance, immunity, energy conservation, histone modification, and reproductive development. A comprehensive and detailed analysis of each category of genes is provided in the subsequent sections.
Energy metabolism
Mosquito diapause is marked by the accumulation of energy reserves and decreased use of lipids (Denlinger and Armbruster, 2014). In our RNA-seq investigation, we have discovered many genes that are highly upregulated and have a connection to energy metabolism in Cx. pipiens. The enzyme phosphoenolpyruvate carboxykinase (PEPCK), involved in gluconeogenesis, cold shock tolerance, and appetite regulation, exhibited elevated expression in the DFB. The findings align with prior studies indicating an upsurge in the pepck expression in the Asian tiger mosquito Aedes albopictus and the flesh fly Sarcophaga bullata under diapausing condition (Poelchau et al., 2013; Spacht et al., 2018). The diapausing eggs of Bombyx mori exhibited increased expression of glycerol kinase (GK), a crucial enzyme involved in hepatic lipogenesis. This discovery validates the importance of GK in the use of glycerol (Kihara et al., 2009; Sriram et al., 2008). In addition, we noted a heightened gk expression in the fat body of diapausing mosquitoes, suggesting a substantial role in the storage of lipids during the initiation of diapause. In our prior work utilizing chromatin immunoprecipitation followed by sequencing (ChIP-seq), we revealed that the gene responsible for generating ATP-binding cassette G is directly controlled by FOXO, the principal controller of diapause in Cx. pipiens (Sim et al., 2015). Our transcriptome data consistently demonstrated an upregulation of this gene in DFB. This gene is involved in the transportation and use of lipids. Our research suggests a possible link with the onset of diapause. Our research has identified an additional gene, known as lysosomal alpha-mannosidase (LAMAN), that is increased in the DFB. LAMAN is an exoglycosidase enzyme that functions in the hydrolysis of intricate carbohydrates. The increased expression of lysosomal mannosidase in the gastrointestinal tract of Drosophila significantly aided in maintaining a balanced gut, preserving gut function, and prolonging lifespan (Tain et al., 2020). Our findings suggest that LAMAN may be responsible for certain unique diapause characteristics. Robich and Denlinger's (Rinehart et al., 2006) investigation revealed that fatty acid synthase, a crucial enzyme responsible for lipid synthesis, exhibited an elevation during the initial phase of diapause in Cx. pipiens. Our investigation revealed substantial expression of fatty acid synthase in the DOV of Cx. pipiens, in addition to its primary occurrence in the fat body. Our research shows that the ovaries of D mosquitoes have a similar role in promoting the production of fatty acids. Bombyxin B-1, an insect enzyme known to trigger ecdysone production, has been demonstrated to reduce carbohydrate storage in the silkworm Bombyx mori (Fullbright and Büllesbach, 2000). Our findings of significantly reduced expression of bombyxin B-1 in the fat body of diapausing mosquitoes suggests a similar regulatory role in Cx. pipiens, particularly in preparation for diapause formation. Further functional analysis of this protein will elucidate its specific role in diapause regulation. Chymotrypsin-2, trypsin 7, and trypsin 5G1, identified as downregulated in diapausing mosquito fat bodies in our study, aligns with previous findings by Robich and Denlinger (2005). Their study observed reduced expression of trypsin and chymotrypsin-like protease involved in blood digestion in diapausing Cx. pipiens. Our RNA-seq results validate these findings in the fat body, suggesting its potential involvement in the suppression of blood-feeding behavior in diapausing mosquitoes. Spartin is a protein participated in lipid transfer and lipid droplet breakdown (Chung et al., 2023). Our RNA-seq study identified Spartin to be downregulated in the fat body of diapausing mosquitoes, suggesting its role in lipid conservation during diapause in Cx. pipiens.
Stress tolerance and cellular immunity
Diapause is a critical mechanism that enables mosquitoes to survive during winter by enhancing their ability to tolerate various environmental challenges such as cold, desiccation, and starvation. Culex pipiens demonstrates adaptability by altering the expression of genes associated with stress tolerance and cellular immunity in order to endure harsh winter conditions. In our study, we observed elevated levels of gene expression for actin-1 and myosin-VIIa in the ovaries of diapausing mosquitoes. Myosin and actin work together synergistically to enhance muscle contractions. The results of our study are consistent with previous studies conducted on diapausing females in Cx. pipiens and Drosophila montana (Kankare et al., 2016; Kim et al., 2006). This suggests that these genes may have a significant impact on reinforcing the cytoskeleton, hence improving the ability to withstand low winter temperatures. Chitin synthase 2 is essential for chitin formation and serves as the primary support structure for the cytoskeleton of insects. Our qRT-PCR validation showed that the expression of chitin synthase 2 increased in the DOV compared to NDOV. In the parasitoid wasp Aphidius gifuensis, upregulation of chitin synthase in diapausing wasps reinforces larval cuticles, enhancing resistance to mechanical damage (Zhang et al., 2018). Given the halting of reproductive development in diapausing Cx. pipiens, we propose a similar stress tolerance function for chitin synthase in the ovary of diapausing mosquitoes. In addition, we have identified genes linked to stress tolerance in the adipose tissue. The leukocyte elastase inhibitor (LEI), a member of the serine protease inhibitors (serpins) family, plays a vital role in protecting cells against proteases during periods of stress (Torriglia et al., 2017). Serpins are a group of proteins that are extremely well-preserved and have a function in several biological processes. Insects rely on hemolymph serpins as a means of protecting themselves against illnesses (Zhang et al., 2013). Serpin7 plays a significant role in regulating egg diapause in Locusta migratoria by modulating the levels of polyphenol oxidase (Chen et al., 2020). Our research uncovered a significant increase in the expression of lei in both DFB and DOV, suggesting its potential involvement in enhancing stress tolerance during diapause. An additional gene, which is responsible for the production of acyl-CoA-dehydrogenase (ACAD), an enzyme involved in the initial steps of beta-oxidation of fatty acids, was shown to have enhanced activity in the DFB. The primary function of the human ACAD family is the degradation of lipids. However, this family consists of 11 members, some of which are also involved in the breakdown of amino acids (Swigoňová et al., 2009). Diapause is marked by a significant reduction in amino acid metabolism (Khodayari et al., 2013). Furthermore, the analysis of gene ontology (GO) demonstrated that acad is linked to oxidoreductase activity, which is correlated with enhanced stress tolerance during diapause in Cx. pipiens (King et al., 2021).
Trypsin and chymotrypsin belong to the endopeptidase superfamily and have significant functions in several biological processes. The blood-feeding mosquitoes utilize trypsin and chymotrypsin to effectively digest their blood meals. Robich and Denlinger (Robich and Denlinger, 2005) discovered a reduction in the expression of trypsin and chymotrypsin in diapausing Cx. pipiens. In contrast, our RNA-seq data revealed increased transcription levels of trypsin 3A1 and chymotrypsin-2 in the DOV. This difference can be attributed to the diverse roles played by different trypsin paralogs in Cx. pipiens, as well as the specific patterns of gene expression in individual organs. In addition, the use of both trypsin and chymotrypsin together has been widely employed as a mixture of enzymes that break down proteins, in order to assist in the restoration of tissues and accelerate the recovery of injuries (Shah and Mital, 2018). These endopeptidases possess anti-inflammatory and antioxidant properties, which suggests that they enhance stress resistance during diapause. CD109 is a cell surface antigen that is produced by immune cells and plays a vital role in important immunological responses. CD109 demonstrates substantial expression in the hemocytes of the larval stage of the cotton bollworm Helicoverpa armigera, thereby playing a vital role in cellular immunological responses (Jiang et al., 2020). Depletion of CD109 resulted in an increased bacterial count in the larval hemolymph and the cessation of hemocyte dispersion. In addition, we observed substantial levels of CD109 gene expression in the DOV, suggesting a comparable role in cellular immunity during diapause. Cytochrome P450 (CYP) is a vital set of enzymes that have a substantial impact on several biological processes. Their popularity is derived from their involvement in drug metabolism and the elimination of foreign compounds. Research has demonstrated that in insects, CYP (cytochrome P450) plays a crucial role in enhancing stress tolerance in older flies and enhancing the immune response in bumblebees (Pletcher et al., 2002; Riddell et al., 2014). Our work revealed a notable upsurge in the expression of the gene accountable for CYP in the ovaries of D mosquitoes, suggesting a possible common function in Cx. pipiens.
Initiation of diapause
Diapause start involves alterations in certain gene expressions, which are subsequently accompanied by changed diapausing behaviors that improve the mosquitoes' capacity to survive throughout winter seasons. Juvenile hormone (JH) acts as the principal regulator of insect growth and physiology. During the previtellogenic stage, the regulation of ovarian follicle cell development is controlled by the elevation of juvenile hormone (JH) levels (Zhu and Noriega, 2016). Diapause is a phase characterized by a decrease in the levels of juvenile hormone (JH), leading to the cessation of reproductive development. Juvenile hormone esterase (JHE) is an enzyme that functions to degrade juvenile hormone by hydrolysis. Our investigation uncovered an upsurge in the expression of the jhe gene in the DOV, which is probably accountable for the halt of ovarian growth. Aromatic L-amino acid decarboxylase (AADC) is an enzyme that plays a role in the last steps of dopamine synthesis (Allen et al., 2009). We noted an augmentation in the expression of this gene in the DFB. Dopamine is involved in starting and sustaining diapause, as shown by the elevated levels of dopamine reported in several species during diapause or diapause preparation (Isabel et al., 2001; Kostal et al., 1999; Noguchi and Hayakawa, 2001, 1997). Our data suggests that AADC also plays a function in beginning diapause in Cx. pipiens.
Reproduction
Ovarian development has significant cessation during diapause in Cx. pipiens. Vitelline membrane proteins serve as essential catalysts in the formation of the inner eggshell in insects. The eggshell is produced by follicular cells to provide vital protection to the embryo against physical damage and bacterial infection. In a CRISPR/Cas9 knockdown study by Zhai et al. (Zhai et al., 2022) in Plutella xylostella, the removal of a vitelline membrane protein reduced the hatchability of the eggs, which in turn caused the eggs to collapse. The qRT-PCR analysis revealed that the expression of vitelline membrane protein 15a-3 gene was downregulated in the diapausing individuals compared to the non-diapausing ones. This finding confirms the termination of reproductive development in diapausing Cx. pipiens, a defining trait of adult diapause in this mosquito species.
Epigenetic regulation
Increasing scientific studies offer evidence for the incorporation of various epigenetic mechanisms in the array of methods employed to regulate dormant stages of diapause in insects. Histone demethylase UTX removes methyl groups from the 27th lysine of H3. It targets H3K27me2 and H3K27me3 in particular (Hong et al., 2007). In our previous study (Wei et al., 2023), we observed a reduction in the level of H3K27me2 in the DFB. Our analysis of the transcriptome revealed that the utx gene has elevated expression levels in the DFB, suggesting its role in the reduction of H3K27me2 levels in the fat body. Excessive utx expression has been shown to lengthen life spans in C. elegans (Guillermo et al., 2021). Studies have shown that having a low level of H3K27me2/3 is associated with a longer and healthier lifespan, as well as an elevation in glycolysis (Lu et al., 2013; Ma et al., 2018; Siebold et al., 2010). Thus, the elevated utx expression in DFB suggests a potential association between prolonged lifespan and H3K27me2 levels in Cx. pipiens.
Conclusion
In sum, we analyzed the transcriptome profiles of the fat bodies and ovaries in diapausing mosquitoes Cx. pipiens and identified potential downstream targets that may regulate diapause features in this species. The present study provides the first thorough examination of changes in the transcriptome of the fat body and the ovary during diapause in Cx. pipiens, the main vector of the West Nile virus. The findings of our research highlight the significance of the fat body and ovary in regulating distinct aspects of diapause. We have discovered genes that are associated with several diapause characteristics, such as elevated fat storage, prolonged lifespan, enhanced capacity to cope with stress, and suspension of ovarian development. Additionally, functional characterizations of validated genes in this study will contribute to expanding our understanding of diapause regulation in Cx. pipiens.
Supplementary Material
Figure S1. Functional enrichment analysis shows significant clusters in molecular functions consisting of annotation clustering of downregulated genes in diapausing mosquito fat bodies. Complete GO analysis results are summarized in Table S10.
Figure S2. Functional enrichment analysis shows significant clusters in biological processes consisting of annotation clustering of commonly upregulated genes in both fat bodies and ovaries. Complete GO analysis results are summarized in Table S7.
Figure S3. Metabolic pathways significantly enriched based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database of genes upregulated in both fat bodies and ovaries of diapausing mosquitoes 7-10 days post-eclosion. Diamonds symbolize the quantity of genes present in each pathway.
Acknowledgement
This work was supported in part by the National Institutes of Health under grant number R15AI139861 National Science Foundation under grant number IOS-1944214.
References
- Allen GFG, Land JM, Heales SJR, 2009. A new perspective on the treatment of aromatic l-amino acid decarboxylase deficiency. Mol. Genet. Metab 97, 6–14. 10.1016/j.ymgme.2009.01.010 [DOI] [PubMed] [Google Scholar]
- Arrese EL, Soulages JL, 2010. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol 55, 207–225. 10.1146/annurev-ento-112408-085356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit JB, Denlinger DL, 2007. Suppression of water loss during adult diapause in the northern house mosquito, Culex pipiens. J. Exp. Biol 210, 217–226. 10.1242/jeb.02630 [DOI] [PubMed] [Google Scholar]
- Bowen MF, Davis EE, Haggart DA, 1988. A behavioural and sensory analysis of host-seeking behaviour in the diapausing mosquito Culex pipiens. J. Insect Physiol 34, 805–813. 10.1016/0022-1910(88)90155-2 [DOI] [Google Scholar]
- Brown MR, Sieglaff DH, Rees HH, 2009. Gonadal Ecdysteroidogenesis in Arthropoda: Occurrence and Regulation. Annu. Rev. Entomol 54, 105–125. 10.1146/annurev.ento.53.103106.093334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cui D, Ullah H, Hao K, Tu X, Zhang Z, 2020. Serpin7 controls egg diapause of migratory locust (Locusta migratoria) by regulating polyphenol oxidase. FEBS Open Bio 10, 707–717. 10.1002/2211-5463.12825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Park J, Lai ZW, Lambert TJ, Richards RC, Zhang J, Walther TC, Farese RV, 2023. The Troyer syndrome protein spartin mediates selective autophagy of lipid droplets. Nat Cell Biol 25, 1101–1110. 10.1038/s41556-023-01178-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger DL, 1986. Dormancy in Tropical Insects. Annu. Rev. Entomol 31, 239–264. 10.1146/annurev.en.31.010186.001323 [DOI] [PubMed] [Google Scholar]
- Denlinger DL, Armbruster PA, 2016. Molecular Physiology of Mosquito Diapause, in: Advances in Insect Physiology. Elsevier, pp. 329–361. 10.1016/bs.aiip.2016.05.002 [DOI] [Google Scholar]
- Denlinger DL, Armbruster PA, 2014. Mosquito Diapause. Annu. Rev. Entomol 59, 73–93. 10.1146/annurev-ento-011613-162023 [DOI] [PubMed] [Google Scholar]
- Denlinger DL, Yocum GD, Rinehart JP, 2012. 10 - Hormonal Control of Diapause, in: Gilbert LI (Ed.), Insect Endocrinology. Academic Press, San Diego, pp. 430–463. 10.1016/B978-0-12-384749-2.10010-X [DOI] [Google Scholar]
- Dhungana P, Wei X, Meuti M, Sim C, 2023. Identification of CYCLE targets that contribute diverse features of circadian rhythms in the mosquito Culex pipiens. Comp. Biochem. Physiol. Part D Genomics Proteomics 48, 101140. 10.1016/j.cbd.2023.101140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson KJ, Uyemura AM, McDaniel KL, Schmidt PS, Bradshaw WE, Holzapfel CM, 2009. Environmental control of ovarian dormancy in natural populations of Drosophila melanogaster. J. Comp. Physiol. A 195, 825–829. 10.1007/s00359-009-0460-5 [DOI] [PubMed] [Google Scholar]
- Fullbright G, Büllesbach EE, 2000. The Receptor Binding Conformation of Bombyxin Is Induced by Alanine(B15). Biochemistry 39, 9718–9724. 10.1021/bi000548i [DOI] [PubMed] [Google Scholar]
- Ge SX, Jung D, Yao R, 2020. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics 36, 2628–2629. 10.1093/bioinformatics/btz931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillermo ARR, Chocian K, Gavriilidis G, Vandamme J, Salcini AE, Mellor J, Woollard A, 2021. H3K27 modifiers regulate lifespan in C. elegans in a context-dependent manner. BMC Biol. 19, 59. 10.1186/s12915-021-00984-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn DA, Denlinger DL, 2011. Energetics of Insect Diapause. Annu. Rev. Entomol 56, 103–121. 10.1146/annurev-ento-112408-085436 [DOI] [PubMed] [Google Scholar]
- Hong S, Cho Y-W, Yu L-R, Yu H, Veenstra TD, Ge K, 2007. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc. Natl. Acad. Sci. U. S. A 104, 18439–18444. 10.1073/pnas.0707292104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabel G, Gourdoux L, Moreau R, 2001. Changes of biogenic amine levels in haemolymph during diapausing and non-diapausing status in Pieris brassicae L. Comp. Biochem. Physiol. A. Mol. Integr. Physiol 128, 117–127. 10.1016/S1095-6433(00)00284-1 [DOI] [PubMed] [Google Scholar]
- Jiang D, Du X, Wang C, Zhang S, Wang G, 2020. CD109 antigen-like gene is induced by ecdysone signaling and involved in the cellular immunity of Helicoverpa armigera. Biosci. Biotechnol. Biochem 84, 1183–1190. 10.1080/09168451.2020.1737504 [DOI] [PubMed] [Google Scholar]
- Kang DS, Kim S, Cotten MA, Sim C, 2021. Transcript Assembly and Quantification by RNA-Seq Reveals Significant Differences in Gene Expression and Genetic Variants in Mosquitoes of the Culex pipiens (Diptera: Culicidae) Complex. J. Med. Entomol 58, 139–145. 10.1093/jme/tjaa167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankare M, Parker DJ, Merisalo M, Salminen TS, Hoikkala A, 2016. Transcriptional Differences between Diapausing and Non-Diapausing D. montana Females Reared under the Same Photoperiod and Temperature. PLOS ONE 11, e0161852. 10.1371/journal.pone.0161852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodayari S, Moharramipour S, Larvor V, Hidalgo K, Renault D, 2013. Deciphering the metabolic changes associated with diapause syndrome and cold acclimation in the two-spotted spider mite Tetranychus urticae. PloS One 8, e54025. 10.1371/journal.pone.0054025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara F, Itoh K, Iwasaka M, Niimi T, Yamashita O, Yaginuma T, 2009. Glycerol kinase activity and glycerol kinase-3 gene are up-regulated by acclimation to 5 °C in diapause eggs of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol 39, 763–769. 10.1016/j.ibmb.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Kim M, Robich RM, Rinehart JP, Denlinger DL, 2006. Upregulation of two actin genes and redistribution of actin during diapause and cold stress in the northern house mosquito, Culex pipiens. J. Insect Physiol 52, 1226–1233. 10.1016/j.jinsphys.2006.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B, Ikenga A, Larsen M, Sim C, 2021. Suppressed expression of oxidoreductin-like protein, Oxidor, increases follicle degeneration and decreases survival during the overwintering diapause of the mosquito Culex pipiens. Comp. Biochem. Physiol. A. Mol. Integr. Physiol 257, 110959. 10.1016/j.cbpa.2021.110959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koštál V., 2006. Eco-physiological phases of insect diapause. J. Insect Physiol 52, 113–127. 10.1016/j.jinsphys.2005.09.008 [DOI] [PubMed] [Google Scholar]
- Kostal V, Noguchi H, Shimada K, Hayakawa Y, 1999. Dopamine and serotonin in the larval CNS of a drosophilid fly, Chymomyza costata: Are they involved in the regulation of diapause? Arch. Insect Biochem. Physiol 42, 147–162. [DOI] [PubMed] [Google Scholar]
- Lebenzon JE, Torson AS, Sinclair BJ, 2021. Diapause differentially modulates the transcriptomes of fat body and flight muscle in the Colorado potato beetle. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics 40, 100906. 10.1016/j.cbd.2021.100906 [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S, 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-X, 吕宇轩, Denlinger DL, Xu W-H, 徐卫华, 2013. Polycomb Repressive Complex 2 (PRC2) Protein ESC Regulates Insect Developmental Timing by Mediating H3K27me3 and Activating Prothoracicotropic Hormone Gene Expression. J. Biol. Chem 288, 23554–23564. 10.1074/jbc.M113.482497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Wang Hui, Cai Y, Wang Han, Niu K, Wu X, Ma H, Yang Y, Tong W, Liu F, Liu Z, Zhang Y, Liu R, Zhu Z-J, Liu N, 2018. Epigenetic drift of H3K27me3 in aging links glycolysis to healthy longevity in Drosophila. eLife 7, e35368. 10.7554/eLife.35368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meireles-Filho ACA, Kyriacou CP, 2013. Circadian rhythms in insect disease vectors. Mem. Inst. Oswaldo Cruz 108, 48–58. 10.1590/0074-0276130438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuti ME, Stone M, Ikeno T, Denlinger DL, 2015. Functional circadian clock genes are essential for the overwintering diapause of the Northern house mosquito, Culex pipiens. J. Exp. Biol 218, 412–422. 10.1242/jeb.113233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CJ, 1981. Diapause Termination, Gonoactivity, and Differentiation of Host-Seeking Behavior from Blood-Feeding Behavior in Hibernating Culex Tarsalis (Diptera: Culicidae). J. Med. Entomol 18, 386–394. 10.1093/jmedent/18.5.386 [DOI] [Google Scholar]
- Mitchell CJ, Briegel H, 1989. Inability of Diapausing Culex pipiens (Diptera: Culicidae) to Use Blood for Producing Lipid Reserves for Overwinter Survival. J. Med. Entomol 26, 318–326. 10.1093/jmedent/26.4.318 [DOI] [PubMed] [Google Scholar]
- Noguchi H, Hayakawa Y, 2001. Dopamine is a key factor for the induction of egg diapause of the silkworm, Bombyx mori. Eur. J. Biochem 268, 774–780. 10.1046/j.1432-1327.2001.01933.x [DOI] [PubMed] [Google Scholar]
- Noguchi H, Hayakawa Y, 1997. Role of dopamine at the onset of pupal diapause in the cabbage armyworm Mamestra brassicae. FEBS Lett. 413, 157–161. 10.1016/S0014-5793(97)00848-X [DOI] [PubMed] [Google Scholar]
- Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, Partridge L, 2002. Genome-Wide Transcript Profiles in Aging and Calorically Restricted Drosophila melanogaster. Curr. Biol 12, 712–723. 10.1016/S0960-9822(02)00808-4 [DOI] [PubMed] [Google Scholar]
- Poelchau MF, Reynolds JA, Elsik CG, Denlinger DL, Armbruster PA, 2013. Deep sequencing reveals complex mechanisms of diapause preparation in the invasive mosquito, Aedes albopictus. Proc. R. Soc. B Biol. Sci 280, 20130143. 10.1098/rspb.2013.0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poupardin R, Schöttner K, Korbelová J, Provazník J, Doležel D, Pavlinic D, Beneš V, Koštál V, 2015. Early transcriptional events linked to induction of diapause revealed by RNAseq in larvae of drosophilid fly, Chymomyza costata. BMC Genomics 16, 720. 10.1186/s12864-015-1907-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruisscher P, Lehmann P, Nylin S, Gotthard K, Wheat CW, 2022. Extensive transcriptomic profiling of pupal diapause in a butterfly reveals a dynamic phenotype. Molecular Ecology 31, 1269–1280. 10.1111/mec.16304 [DOI] [PubMed] [Google Scholar]
- Riddell CE, Lobaton Garces JD, Adams S, Barribeau SM, Twell D, Mallon EB, 2014. Differential gene expression and alternative splicing in insect immune specificity. BMC Genomics 15, 1031. 10.1186/1471-2164-15-1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart JP, Robich RM, Denlinger DL, 2006. Enhanced Cold and Desiccation Tolerance in Diapausing Adults of Culex pipiens, and a Role for Hsp70 in Response to Cold Shock but Not as a Component of the Diapause Program. J. Med. Entomol 43, 713–722. 10.1093/jmedent/43.4.713 [DOI] [PubMed] [Google Scholar]
- Robich RM, Denlinger DL, 2005. Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony. Proc. Natl. Acad. Sci 102, 15912–15917. 10.1073/pnas.0507958102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Smykal V, Johnson L, Saha TT, Zou Z, Raikhel AS, 2016. Regulation of Reproductive Processes in Female Mosquitoes, in: Advances in Insect Physiology. Elsevier, pp. 115–144. 10.1016/bs.aiip.2016.05.004 [DOI] [Google Scholar]
- Santos PKF, de Souza Araujo N, Françoso E, Zuntini AR, Arias MC, 2018. Diapause in a tropical oil-collecting bee: molecular basis unveiled by RNA-Seq. BMC Genomics 19, 305. 10.1186/s12864-018-4694-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PS, Matzkin L, Ippolito M, Eanes WF, 2005. Geographic Variation in Diapause Incidence, Life-History Traits, and Climatic Adaptation in Drosophila Melanogaster. Evolution 59, 1721–1732. 10.1111/j.0014-3820.2005.tb01821.x [DOI] [PubMed] [Google Scholar]
- Shah D, Mital K, 2018. The Role of Trypsin:Chymotrypsin in Tissue Repair. Adv. Ther 35, 31–42. 10.1007/s12325-017-0648-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebold AP, Banerjee R, Tie F, Kiss DL, Moskowitz J, Harte PJ, 2010. Polycomb Repressive Complex 2 and Trithorax modulate Drosophila longevity and stress resistance. Proc. Natl. Acad. Sci 107, 169–174. 10.1073/pnas.0907739107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, Denlinger D, 2013. Insulin signaling and the regulation of insect diapause. Front. Physiol 4, 189. 10.3389/fphys.2013.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, Denlinger DL, 2008a. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl. Acad. Sci 105, 6777. 10.1073/pnas.0802067105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, Denlinger DL, 2008b. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl. Acad. Sci 105, 6777–6781. 10.1073/pnas.0802067105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, Kang DS, Kim S, Bai X, Denlinger DL, 2015. Identification of FOXO targets that generate diverse features of the diapause phenotype in the mosquito Culex pipiens. Proc. Natl. Acad. Sci 112, 3811–3816. 10.1073/pnas.1502751112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacht DE, Teets NM, Denlinger DL, 2018. Two isoforms of Pepck in Sarcophaga bullata and their distinct expression profiles through development, diapause, and in response to stresses of cold and starvation. J. Insect Physiol 111, 41–46. 10.1016/j.jinsphys.2018.10.008 [DOI] [PubMed] [Google Scholar]
- Sriram G, Rahib L, He J-S, Campos AE, Parr LS, Liao JC, Dipple KM, 2008. Global Metabolic Effects of Glycerol Kinase Overexpression in Rat Hepatoma Cells. Mol. Genet. Metab 93, 145–159. 10.1016/j.ymgme.2007.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swigoňová Z, Mohsen A-W, Vockley J, 2009. Acyl-CoA Dehydrogenases: Dynamic History of Protein Family Evolution. J. Mol. Evol 69, 176–193. 10.1007/s00239-009-9263-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tain LS, Jain C, Nespital T, Froehlich J, Hinze Y, Grönke S, Partridge L, 2020. Longevity in response to lowered insulin signaling requires glycine N-methyltransferase-dependent spermidine production. Aging Cell 19, e13043. 10.1111/acel.13043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torriglia A, Martin E, Jaadane I, 2017. The Hidden side of SERPINB1/Leukocyte Elastase Inhibitor. Semin. Cell Dev. Biol 62, 178–186. 10.1016/j.semcdb.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Dhungana P, Sim C, n.d. The diapausing mosquito Culex pipiens exhibits reduced levels of H3K27me2 in the fat body. Insect Mol. Biol n/a. 10.1111/imb.12871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y-L, Dong S-J, Zou M-M, Qin Y-D, Liu L-L, Cao M-H, Huang M-Q, Vasseur L, You M-S, Peng L, 2022. Vitelline Membrane Protein 26 Mutagenesis, Using CRISPR/Cas9, Results in Egg Collapse in Plutella xylostella. Int. J. Mol. Sci 23, 9538. 10.3390/ijms23179538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H-Z, Li Y-Y, An T, Huang F-X, Wang M-Q, Liu C-X, Mao J-J, Zhang L-S, 2018. Comparative Transcriptome and iTRAQ Proteome Analyses Reveal the Mechanisms of Diapause in Aphidius gifuensis Ashmead (Hymenoptera: Aphidiidae). Front. Physiol 9. 10.3389/fphys.2018.01697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Lu Y-X, Xu W-H, 2013. Proteomic and metabolomic profiles of larval hemolymph associated with diapause in the cotton bollworm, Helicoverpa armigera. BMC Genomics 14, 751. 10.1186/1471-2164-14-751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Miesfeld RL, 2009. Energy metabolism during diapause in Culex pipiens mosquitoes. J. Insect Physiol 55, 40–46. 10.1016/j.jinsphys.2008.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Noriega FG, 2016. The Role of Juvenile Hormone in Mosquito Development and Reproduction, in: Advances in Insect Physiology. Elsevier, pp. 93–113. 10.1016/bs.aiip.2016.04.005 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Functional enrichment analysis shows significant clusters in molecular functions consisting of annotation clustering of downregulated genes in diapausing mosquito fat bodies. Complete GO analysis results are summarized in Table S10.
Figure S2. Functional enrichment analysis shows significant clusters in biological processes consisting of annotation clustering of commonly upregulated genes in both fat bodies and ovaries. Complete GO analysis results are summarized in Table S7.
Figure S3. Metabolic pathways significantly enriched based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database of genes upregulated in both fat bodies and ovaries of diapausing mosquitoes 7-10 days post-eclosion. Diamonds symbolize the quantity of genes present in each pathway.


