Abstract
Background
Non-small cell lung cancer (NSCLC) is a prevalent form of cancer, often leading to brain metastases (BM) and a significant decline in patient prognosis. Whether immune checkpoint inhibitors (ICIs) combined with brain radiotherapy is superior to conventional chemotherapy combined with brain radiotherapy in those patients remains to be explored.
Materials and methods
Our study enrolled 161 NSCLC patients with BM who underwent either ICIs combined with brain radiotherapy or chemotherapy combined with brain radiotherapy. End points included overall survival (OS), progression-free survival (PFS), intracranial PFS (IPFS), and extracranial PFS (EPFS). Univariate and multivariate Cox regressions were employed to identify prognostic risk variables.
Results
Patients receiving ICIs combined with brain radiotherapy exhibited significantly longer OS compared to those receiving chemotherapy combined with brain radiotherapy (34.80 months vs. 17.17 months, P = 0.005). In the Cox regression analysis, chemotherapy combined with brain radiotherapy (HR, 1.82; 95% CI, 1.09–3.05; P = 0.023), smoking (HR, 1.75; 95% CI, 1.02–2.99; P = 0.043) and squamous cell carcinoma (HR, 2.59; 95% CI, 1.31–5.13; P = 0.006) were associated with a worse prognosis. After propensity score matching (PSM), this finding remained consistent with before PSM (43.73 months vs. 17.17 months, P = 0.018). Squamous cell carcinoma (HR, 2.46; 95% CI, 1.15–5.26; P = 0.021) and CT + RT (HR, 2.11; 95% CI, 1.15–3.88; P = 0.016) were associated with a less favorable prognosis.
Conclusion
The study suggests that the combination of ICIs and brain radiotherapy provides superior OS for NSCLC patients with BM, compared to the chemotherapy combined with brain radiotherapy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-13110-y.
Keywords: Immune checkpoint inhibitors, Brain radiotherapy, Chemotherapy, Non-small cell lung cancer, Brain metastasis
Background
Lung cancer is currently the second most common cancer worldwide, following breast cancer, with 85% of cases being non-small cell lung cancer (NSCLC) [1, 2]. Brain metastasis (BM) is very common in NSCLC, with approximately 10–20% of NSCLC patients already having BM at the initial diagnosis [3], and 25–40% of patients developing BM during the course of the disease [4]. NSCLC patients with BM have a poor prognosis, and those with symptomatic BM experience a rapid deterioration in their quality of life [5].
Surgery can improve the survival outcomes of patients with a single brain metastatic lesion, good Eastern Cooperative Oncology Group Performance Status (ECOG PS) score, and limited extracranial metastases [6, 7]. Whereas brain surgery is costly, carries higher risks, and requires patients to be in better physical condition. Conversely, brain radiation therapy has lower economic and physical requirements for patients. With advancements in radiation therapy techniques, especially, Stereotactic Radiosurgery (SRS) can accurately target tumor lesions, delivering high doses to the tumor while sparing normal tissues, thereby greatly improving patients’ quality of life and prognosis [8, 9]. Therefore, most patients with BM will receive brain radiation therapy.
The emergence of immune checkpoint inhibitors (ICIs) has changed the treatment strategies for NSCLC. ICIs-based regimen has become the first-line treatment for driver gene-negative patients [10–14]. And for locally advanced NSCLC, from maintenance regimen to neoadjuvant therapy, ICIs have also shown promise [15, 16]. In recent years, there has been increasing research on ICIs in NSCLC patients with BM, demonstrating superior efficacy compared to traditional chemotherapy [17], such as the OAK trial showed longer overall survival (OS) in the atezolizumab treatment group compared with the docetaxel chemotherapy group (16.0 months vs. 11.9 months) and longer time to intracranial lesion progression (not reached vs. 9.5 months) [18]. Moreover, numerous preclinical studies have indicated a synergistic effect between radiation therapy and ICIs. Radiation therapy can enhance the release and presentation of tumor antigens [19, 20], promote the activation and initiation of immune cells [21, 22], increase the density of tumor-infiltrating lymphocytes [23, 24], facilitate T cell recognition of tumor cells, and enhance anti-tumor effects [25, 26]. Therefore, the combination of radiation therapy and ICIs is a highly promising treatment approach. Many preclinical and prospective clinical studies are currently underway, yielding promising research outcomes.
However, most clinical trials have excluded patients with BM, limiting the research on the combination of ICIs and brain radiation therapy in NSCLC patients with BM. Currently, data on the combination of radiation therapy and ICIs mainly come from subgroup analyses of clinical trials or retrospective studies with small sample sizes. Many studies also include other tumor types that are prone to BM, such as melanoma and renal cell carcinoma, rather than focusing solely on NSCLC. Even in studies focusing on NSCLC patients with BM, the sample sizes are often small, making it difficult to provide robust evidence. Many questions regarding the combination of brain radiation therapy and ICIs in BM are still unclear. For example, it is unknown whether combination therapy provides additional benefits, whether the side effects of combination therapy are tolerable, and the optimal timing for combination therapy is unclear.
In this study, we aimed to investigate whether the efficacy of ICIs combined with brain radiation therapy is superior to chemotherapy combined with brain radiation therapy in patients with NSCLC and BM. And we explored risk factors associated with survival prognosis.
Methods
Patient cohort and study variables
NSCLC patients with BM who were treated in the Department of Pulmonary Oncology, Zhongnan Hospital of Wuhan University from January 2013 to April 2023 were included in our study, with data censoring on April 16, 2023. The inclusion criteria were as follows: (1) Pathologically confirmed NSCLC; (2) brain metastasis confirmed by enhanced magnetic resonance imaging (MRI) or computed tomography (CT); (3) Patients who received brain radiotherapy after BM and underwent systemic treatment mainly consisting of ICIs and/or chemotherapy; (4) Performance status (PS) score ranging from 0 to 2. The exclusion criteria were: (1) Use of small-molecule inhibitors (SMIs) treatment after BM; (2) Incomplete follow-up information.
Based on the different systemic treatment modalities, the included patients were divided into two groups: the ICIs-based regimen combined with brain radiotherapy group (referred to as the ICI + RT group) and the chemotherapy alone combined with brain radiotherapy group (referred to as the CT + RT group). Detailed patient information, including age, gender, smoking history, PS score, driver gene mutation, number of BM, type of brain radiotherapy, sequence of treatment, extracranial metastases, and symptoms of BM, was collected in our study, as shown in Table 1. The baseline characteristics of the patients were defined at the time of diagnosis of BM.
Table 1.
The characteristics of all patients before PSM
| ALL | ICI + RT | CT + RT | P value | |
|---|---|---|---|---|
| N = 161 | N = 100 | N = 61 | ||
| Age (IQR) | 60 (54–66) | 60 (65–70) | 61(53-67.5) | 0.920 |
| Gender | 0.919 | |||
| Female | 31 (19.3%) | 20 (20.0%) | 11 (18.0%) | |
| Male | 130 (80.7%) | 80 (80.0%) | 50 (82.0%) | |
| PS score | 1.000 | |||
| 1 | 136 (84.5%) | 84 (84.0%) | 52 (85.2%) | |
| 2 | 25 (15.5%) | 16 (16.0%) | 9 (14.8%) | |
| Smoking history | 0.019 | |||
| None | 73 (45.3%) | 53 (53.0%) | 20 (32.8%) | |
| Yes | 88 (54.7%) | 47 (47.0%) | 41 (67.2%) | |
| Primary lesion | 0.026 | |||
| Left lung | 73 (45.3%) | 38 (38.0%) | 35 (57.4%) | |
| Right lung | 88 (54.7%) | 62 (62.0%) | 26 (42.6%) | |
| Surgery of lung | 0.464 | |||
| None | 120 (74.5%) | 77 (77.0%) | 43 (70.5%) | |
| Yes | 41 (25.5%) | 23 (23.0%) | 18 (29.5%) | |
| T stage | 0.653 | |||
| 1 | 22 (13.7%) | 16 (16.0%) | 6 (9.8%) | |
| 2 | 43 (26.7%) | 27 (27.0%) | 16 (26.2%) | |
| 3 | 35 (21.7%) | 20 (20.0%) | 15 (24.6%) | |
| 4 | 56 (34.8%) | 35 (35.0%) | 21 (34.4%) | |
| Unknown | 5 (3.1%) | 2 (2.0%) | 3 (4.9%) | |
| N stage | 0.409 | |||
| 0 | 44 (27.3%) | 25 (25.0%) | 19 (31.1%) | |
| 1 | 11 (6.8%) | 7 (7.0%) | 4 (6.6%) | |
| 2 | 59 (36.6%) | 34 (34.0%) | 25 (41.0%) | |
| 3 | 45 (28.0%) | 33 (33.0%) | 12 (19.7%) | |
| Unknown | 2 (1.2%) | 1 (1.0%) | 1 (1.6%) | |
| Extracranial metastases | 0.395 | |||
| None | 90 (55.9%) | 59 (59.0%) | 31 (50.8%) | |
| Yes | 71 (44.1%) | 41 (41.0%) | 30 (49.2%) | |
| Driver gene | 0.018 | |||
| None | 94 (58.4%) | 54 (54.0%) | 40 (65.6%) | |
| Yes | 28 (17.4%) | 24 (24.0%) | 4 (6.6%) | |
| Unknown | 39 (24.2%) | 22 (22.0%) | 17 (27.9%) | |
| Pathological type | 0.424 | |||
| Adenocarcinoma | 120 (74.5%) | 76 (76.0%) | 44 (72.1%) | |
| Squamous carcinoma | 26 (16.1%) | 17 (17.0%) | 9 (14.8%) | |
| NSCLC(NOS) | 15 (9.3%) | 7 (7.0%) | 8 (13.1%) | |
| Symptoms of BM | 1.000 | |||
| None | 100 (62.1%) | 62 (62.0%) | 38 (62.3%) | |
| Yes | 61 (37.9%) | 38 (38.0%) | 23 (37.7%) | |
| Type of brain radiotherapy | 0.024 | |||
| WBRT | 86 (53.4%) | 46 (46.0%) | 40 (65.6%) | |
| SRS | 75 (46.6%) | 54 (54.0%) | 21 (34.4%) | |
| Sequence of treatment | 0.576 | |||
| Upfront RT | 17(10.6%) | 9(9.0%) | 8(13.1%) | |
| Upfront ICI/CT | 144(89.4%) | 91(91.0%) | 53(86.9%) | |
| Number of BM | 0.857 | |||
| 1 | 70 (43.5%) | 45 (45.0%) | 25 (41.0%) | |
| 2 | 24 (14.9%) | 15 (15.0%) | 9 (14.8%) | |
| >=3 | 67 (41.6%) | 40 (40.0%) | 27 (44.3%) | |
| Size of BM | 0.001 | |||
| < 10 | 37 (23.0%) | 28 (28.0%) | 9 (14.8%) | |
| >=10, < 20 | 57 (35.4%) | 37 (37.0%) | 20 (32.8%) | |
| >=20, < 30 | 29 (18.0%) | 21 (21.0%) | 8 (13.1%) | |
| >=30 | 25 (15.5%) | 12 (12.0%) | 13 (21.3%) | |
| Unknown | 13 (8.1%) | 2 (2.0%) | 11 (18.0%) |
BM, brain metastasis; CT, chemotherapy; ICI, immune checkpoint inhibitor; NSCLC, non-small Cell Lung Cancer; NOS, not otherwise specified; PSM, propensity score matching; RT, radiotherapy; SRS, stereotactic radiosurgery; WBRT, whole-brain radiation therapy
Treatments
About the ICIs-based regimen, the administration of anti-PD-1 or anti-PD-L1 antibodies alone or in combination with chemotherapy is employed. The chemotherapy regimen is decided based on the patient’s pathological type and condition, primarily consisting of platinum-based dual-drug chemotherapy, as well as monotherapy with drugs such as pemetrexed, albumin-bound paclitaxel, docetaxel, and gemcitabine. Types of brain RT include whole-brain radiation therapy (WBRT) and stereotactic radiosurgery (SRS). WBRT is administered at a dose of 30 Gy, divided into 10 fractions, given 5 times a week. SRS is administered at a dose of 18–40 Gy, divided into 3–5 fractions, or as a single dose of 15 to 24 Gy, depending on the volume of the patient’s lesions.
Patients’ follow-up
Regular follow-up is conducted on patients. Radiological examinations include enhanced computed tomography (CT) scans of the chest and upper abdomen, as well as magnetic resonance imaging (MRI) of the brain for disease assessment, which are repeated every 2–3 months. If necessary, bone emission computed tomography (ECT) and positron emission tomography-computed tomography (PET-CT) scans are performed. If there is progression observed during the imaging examinations, the date of the examination is recorded as the date of progression.
Statistics analysis
The primary objective is overall survival (OS), defined as the time from diagnosis of BM to death from any cause. The secondary objective is progression-free survival (PFS), defined as the time from diagnosis of BM to progression of any systemic lesions or death. Intracranial progression-free survival (IPFS), is defined as the time from diagnosis of BM to progression of intracranial lesions. Extracranial progression-free survival (EPFS), is defined as the time from diagnosis of BM to progression of extracranial lesions or death. Chi-square or Fisher’s exact tests are used to compare categorical data, while the Mann-Whitney U test is used to compare continuous data between groups. Kaplan-Meier analysis is used to construct survival curves, and the log-rank test is used to analyze differences between groups. Univariate and multivariate Cox regression analyses are used to determine prognostic risk factors. PSM is used to balance baseline differences between different treatment groups. A P-value of < 0.05 is defined as statistically significant. Data analysis is performed using R 4.2.1 software and SPSS 26.
Result
Characteristics
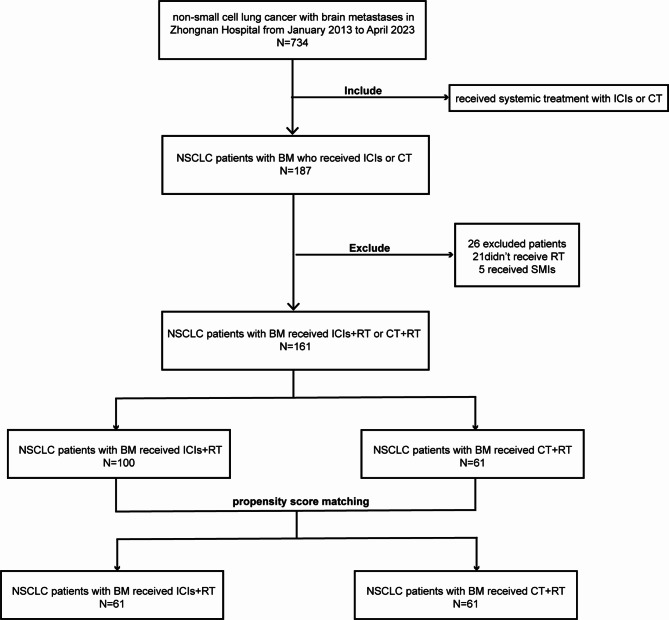
From January 2013 to April 2023, a total of 734 patients with NSCLC and BM were included in the study. After screening, 187 patients who developed BM received systemic treatment primarily consisting of ICIs or chemotherapy. Among them, 21 patients did not receive brain radiotherapy after the occurrence of BM, and 5 patients had previously received TKI treatment after BM, resulting in a final inclusion of 161 patients who met the study’s inclusion and exclusion criteria (Fig. 1). In the ICI + RT group, 86 patients received immunotherapy combined with chemotherapy, while 14 patients received immunotherapy alone.
Fig. 1.
Flow chart of screening patients. BM, brain metastasis; CT, chemotherapy; ICIs, immune checkpoint inhibitors; NSCLC, non-small Cell Lung Cancer; RT, radiotherapy; SMIs, small-molecule inhibitors. (The figure should be placed in the Result section behind Characteristics.)
The median follow-up time for the entire cohort was 18.27 months (95% CI, 13.77–22.76), up until April 16, 2023. Among the patients, 64 (39.75%) died, while 97 (60.25%) either continued to be followed up or were lost to follow-up and marked as censored. In the entire cohort, there was a predominance of male patients (130 cases, 80.7%), with only 31 female patients (19.3%). At the time of BM diagnosis, 71 patients (44.1%) had extracranial metastases. Of the patients, 120 (74.5%) were adenocarcinoma, 26 (16.1%) were squamous cell carcinoma, and 15 (9.3%) were other non-specified types of NSCLC. Among the patients, 28 (17.4%) had driver gene mutations, 94 (58.4%) had no mutations, and 39 (24.2%) did not undergo genetic testing. Other clinical information, such as age, gender, and smoking history, is presented in Table 1.
Before PSM
A total of 161 patients were included in the study, with 100 (62.1%) in the ICI + RT group and 61 (37.9%) in the CT + RT group. The survival and treatment information of the two groups of patients were presented in a swimmer plot (Figure A.1). There were 53 patients (53.0%) in the ICI + RT group while 20 patients (32.8%) in the CT + RT group have smoking history (P = 0.019). In terms of the primary lesion location, 38 patients (38.0%) in the ICI + RT group had lesions in the left lung, while in the CT + RT group, 35 patients (57.4%) had lesions in the left lung (P = 0.026). There were 24 patients (24.0%) in the ICI + RT group with driver gene mutations and 22 patients (22.0%) with unknown status, while in the CT + RT group, only 4 patients (6.6%) had driver gene mutations and 17 patients (27.9%) had unknown status (P = 0.018). In terms of type of brain radiotherapy, 46 patients (46.0%) in the ICI + RT group underwent WBRT, while 54 patients (54.0%) underwent SRS. In the CT + RT group, 40 patients (65.6%) underwent WBRT and 21 patients (34.4%) underwent SRS (P = 0.024). There were also differences between the two groups in terms of BM size (P = 0.001). There were no significant differences in other clinical characteristics between the two groups, as shown in Table 1.
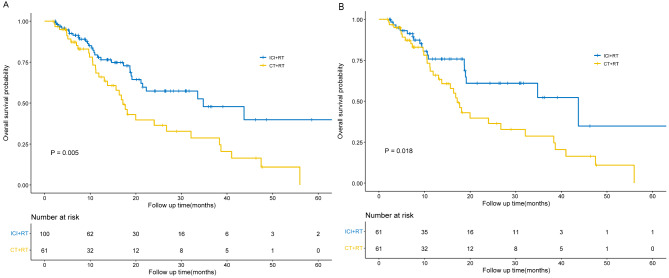
The median OS for the entire cohort was 24.03 months (95% CI, 12.92–35.15). Specifically, the median OS in the ICI + RT group was 34.80 months (95% CI, 14.94–54.66), while in the CT + RT group, it was 17.17 months (95% CI, 14.57–19.76), showing a significant difference (P = 0.005) (Fig. 2A). And our conclusions were further supported by the analysis of prognostic risk factors. The univariate Cox regression analysis was performed on the clinical characteristics of the patients related to OS, and then those significantly different variables were included in the multivariate Cox regression analysis. It was found that treatment modality was significantly associated with prognosis, with CT + RT having a worse prognosis compared to ICI + RT (HR, 1.82; 95% CI, 1.09–3.05; P = 0.023). In addition, smoking (HR, 1.75; 95% CI, 1.02–2.99; P = 0.043) and squamous cell carcinoma pathology type (HR, 2.59; 95% CI, 1.31–5.13; P = 0.006) were also associated with a worse prognosis. The results are presented in Table 2.
Fig. 2.
Kaplan-Meier curves for OS before and after propensity score matching. (A) OS of the 2 cohorts of interest (ICI + RT vs. CT + RT) before propensity score matching; (B) OS of the 2 cohorts of interest (ICI + RT vs. CT + RT) after propensity score matching. CT, chemotherapy; ICI, immune checkpoint inhibitor; OS, overall survival; RT, radiotherapy. (The figure should be placed in the Result section behind Before PSM.)
Table 2.
Univariate and multivariate COX regressions for the factors associated with OS before PSM
| Univariate COX regression analysis | Multivariate COX regression analysis | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P value | Hazard Ratio | 95% CI | P value | |
| Age | 0.99 | 0.96–1.02 | 0.529 | NA | NA | NA |
| Gender | ||||||
| Female | Reference | |||||
| Male | 1.38 | 0.72–2.65 | 0.332 | NA | NA | NA |
| PS score | ||||||
| 1 | Reference | |||||
| 2 | 0.92 | 0.47–1.81 | 0.804 | NA | NA | NA |
| Driver gene | ||||||
| None | Reference | |||||
| Yes | 0.71 | 0.35–1.41 | 0.328 | NA | NA | NA |
| Unknown | 1.30 | 0.78–2.16 | 0.322 | NA | NA | NA |
| Pathological type | ||||||
| Adenocarcinoma | Reference | |||||
| Squamous carcinoma | 2.20 | 1.13–4.28 | 0.021 | 2.59 | 1.31–5.13 | 0.006 |
| NSCLC (NOS) | 0.29 | 0.04–2.09 | 0.217 | 0.29 | 0.04–2.13 | 0.225 |
| Primary lesion | ||||||
| Left lung | Reference | |||||
| Right lung | 0.87 | 0.53–1.43 | 0.593 | NA | NA | NA |
| T stage | ||||||
| T1 | Reference | |||||
| T2 | 0.74 | 0.31–1.76 | 0.493 | NA | NA | NA |
| T3 | 1.05 | 0.44–2.51 | 0.912 | NA | NA | NA |
| T4 | 1.67 | 0.75–3.72 | 0.206 | NA | NA | NA |
| N stage | ||||||
| N0 | Reference | |||||
| N1 | 0.25 | 0.03–1.87 | 0.177 | NA | NA | NA |
| N2 | 1.22 | 0.66–2.27 | 0.528 | NA | NA | NA |
| N3 | 1.05 | 0.53–2.09 | 0.882 | NA | NA | NA |
| Extracranial metastases | ||||||
| None | Reference | |||||
| Yes | 1.12 | 0.68–1.84 | 0.654 | NA | NA | NA |
| Number of BM | ||||||
| >=3 | Reference | |||||
| 1 | 1.23 | 0.72–2.11 | 0.443 | NA | NA | NA |
| 2 | 1.06 | 0.49–2.26 | 0.884 | NA | NA | NA |
| Size of BM | ||||||
| < 10 | Reference | |||||
| >=10, < 20 | 0.97 | 0.48–1.99 | 0.942 | NA | NA | NA |
| >=20,<30 | 1.02 | 0.45–2.33 | 0.958 | NA | NA | NA |
| >=30 | 1.11 | 0.49–2.49 | 0.800 | NA | NA | NA |
| Smoking history | ||||||
| None | Reference | |||||
| Yes | 1.95 | 1.16–3.30 | 0.013 | 1.75 | 1.02–2.99 | 0.043 |
| Treatment modality | ||||||
| ICI + RT | Reference | |||||
| CT + RT | 1.86 | 1.14–3.06 | 0.014 | 1.82 | 1.09–3.05 | 0.023 |
| Type of brain radiotherapy | ||||||
| WBRT | Reference | |||||
| SRS | 1.19 | 0.72–1.95 | 0.497 | NA | NA | NA |
| Sequence of treatment | ||||||
| Upfront brain RT | Reference | |||||
| Upfront ICI/CT | 1.31 | 0.56–3.05 | 0.532 | NA | NA | NA |
| Symptoms of BM | ||||||
| None | Reference | |||||
| Yes | 1.30 | 0.78–2.16 | 0.321 | NA | NA | NA |
| Surgery of lung | ||||||
| None | Reference | |||||
| Yes | 0.98 | 0.56–1.71 | 0.940 | NA | NA | NA |
BM, brain metastasis; CT, chemotherapy; ICI, immune checkpoint inhibitor; NSCLC, non-small Cell Lung Cancer; NOS, not otherwise specified; OS, overall survival; PSM, propensity score matching; RT, radiotherapy; SRS, stereotactic radiosurgery; WBRT, whole-brain radiation therapy
During the course of treatment until the end of follow-up, 75 patients (75.0%) in the ICI + RT group experienced disease progression, including fatalities, with 14 patients (14.0%) only showing the progression of brain lesions, 36 patients (36.0%) only showing extracranial progression and 25 patients (25.0%) developing both intracranial and extracranial disease progression. In the CT + RT group, 54 patients (88.5%) experienced disease progression, including fatalities, with 8 cases (13.1%) having progression of brain lesions, 26 cases (42.6%) developing extracranial disease progression, and 20 cases (32.8%) showing both intracranial and extracranial disease progression. Overall, the recurrence rate was lower in the ICI + RT group. However, there were no statistically significant differences in PFS, IPFS, and EPFS between the two groups. Specifically, the PFS for the ICI + RT and CT + RT groups was 8.17 months (95% CI, 6.67–9.66) and 6.73 months (95% CI, 5.25–8.22), respectively (P = 0.497), as shown in Figure A.3 A. The IPFS was 24.47 months (95% CI, 14.63–34.31) for the ICI + RT group and 21.73 months (95% CI, 6.68–36.79) for the CT + RT group (P = 0.270), as depicted in Figure A.4 A. The EPFS was 10.83 months (95% CI, 9.65–12.02) for the ICI + RT group and 10.50 months (95% CI, 7.37–13.63) for the CT + RT group (P = 0.275), as depicted in Figure A.5 A. We conducted univariate and multivariate COX analyses to explore factors associated with PFS, IPFS, and EPFS. For PFS, squamous carcinoma (HR, 2.64; 95% CI, 1.60–4.38; P < 0.001) and Upfront ICI/CT (HR, 3.39; 95% CI, 1.90–6.04; P < 0.001) were associated with a higher risk of lesion progression, as shown in Table A.1. For IPFS, both univariate and multivariate COX analyses revealed associations with male gender (HR, 3.61; 95% CI, 1.54–8.49; P = 0.003), squamous carcinoma histology (HR, 23.36; 95% CI, 4.34-125.77; P < 0.001) and upfront ICI/CT (HR, 8.62; 95% CI, 4.58–46.23; P < 0.001), resulting in a higher risk of intracranial lesion progression, as presented in Table A.2. We did not obtain variables that were significantly associated with EPFS, as detailed in Table A.3.
Subsequently, we will employ the prognostic-associated factors of smoking history, treatment modality, and pathological type as covariates for conducting PSM to ensure a balanced comparison of patient baseline characteristics between the ICI + RT group and the CT + RT group. Furthermore, our analysis will incorporate additional covariates, including age, PS score, primary location, surgery of lung, T stage, N stage, extracranial metastases, gender, symptoms of BM, type of brain radiotherapy, size and number of BM, as well as driver gene mutations.
After PSM
After the matching process, each group comprised 61 patients, and there were no significant differences in prognostic-related variables between the two groups. Some disparities still persisted in terms of the type of brain radiotherapy (P = 0.068) and the number of BM (P = 0.073), although these differences were less pronounced compared to the pre-matching. A comprehensive presentation of the clinical characteristics of both groups of patients is available in Table 3. The survival and treatment information of the two groups of patients after PSM were presented in a swimmer plot (Figure A.2).
Table 3.
The characteristics of all patients after PSM
| ALL | ICI + RT | CT + RT | P value | |
|---|---|---|---|---|
| N = 122 | N = 61 | N = 61 | ||
| Age (IQR) | 61.5(55–67) | 62 (55.5–66.5) | 61 (53-67.5) | 0.783 |
| Gender | 1.000 | |||
| Female | 23 (18.9%) | 12 (19.7%) | 11 (18.0%) | |
| Male | 99 (81.1%) | 49 (80.3%) | 50 (82.0%) | |
| PS score | 0.807 | |||
| 1 | 102 (83.6%) | 50 (82.0%) | 52 (85.2%) | |
| 2 | 20 (16.4%) | 11 (18.0%) | 9 (14.8%) | |
| Smoking history | 0.572 | |||
| None | 44 (36.1%) | 24 (39.3%) | 20 (32.8%) | |
| Yes | 78 (63.9%) | 37 (60.7%) | 41 (67.2%) | |
| Primary lesion | 0.365 | |||
| Left lung | 64 (52.5%) | 29 (47.5%) | 35 (57.4%) | |
| Right lung | 58 (47.5%) | 32 (52.5%) | 26 (42.6%) | |
| Surgery of lung | 0.132 | |||
| None | 94 (77.0%) | 51 (83.6%) | 43 (70.5%) | |
| Yes | 28 (23.0%) | 10 (16.4%) | 18 (29.5%) | |
| T stage | 0.918 | |||
| 1 | 11 (9.0%) | 5 (8.2%) | 6 (9.8%) | |
| 2 | 31 (25.4%) | 15 (24.6%) | 16 (26.2%) | |
| 3 | 28 (23.0%) | 13 (21.3%) | 15 (24.6%) | |
| 4 | 47 (38.5%) | 26 (42.6%) | 21 (34.4%) | |
| Unknown | 5 (4.1%) | 2 (3.3%) | 3 (4.9%) | |
| N stage | 0.919 | |||
| 0 | 35 (28.7%) | 16 (26.2%) | 19 (31.1%) | |
| 1 | 8 (6.6%) | 4 (6.6%) | 4 (6.6%) | |
| 2 | 49 (40.2%) | 24 (39.3%) | 25 (41.0%) | |
| 3 | 28 (23.0%) | 16 (26.2%) | 12 (19.7%) | |
| Unknown | 2 (1.6%) | 1 (1.6%) | 1 (1.6%) | |
| Extracranial metastases | 0.362 | |||
| None | 68 (55.7%) | 37 (60.7%) | 31 (50.8%) | |
| Yes | 54 (44.3%) | 24 (39.3%) | 30 (49.2%) | |
| Driver gene | 0.627 | |||
| None | 77 (63.1%) | 37 (60.7%) | 40 (65.6%) | |
| Yes | 11 (9.0%) | 7 (11.5%) | 4 (6.6%) | |
| Unknown | 34 (27.9%) | 17 (27.9%) | 17 (27.9%) | |
| Pathological type | 0.784 | |||
| Adenocarcinoma | 88 (72.1%) | 44 (72.1%) | 44 (72.1%) | |
| Squamous carcinoma | 20 (16.4%) | 11 (18.0%) | 9 (14.8%) | |
| NSCLC(NOS) | 14 (11.5%) | 6 (9.8%) | 8 (13.1%) | |
| Symptoms of BM | 1.000 | |||
| None | 75 (61.5%) | 37 (60.7%) | 38 (62.3%) | |
| Yes | 47 (38.5%) | 24 (39.3%) | 23 (37.7%) | |
| Type of brain radiotherapy | 0.068 | |||
| WBRT | 69 (56.6%) | 29 (47.5%) | 40 (65.6%) | |
| SRS | 53 (43.4%) | 32 (52.5%) | 21 (34.4%) | |
| Sequence of treatment | 0.557 | |||
| Upfront RT | 109(89.3%) | 56(91.8%) | 53(86.9%) | |
| Upfront ICI/CT | 13(10.7%) | 5(8.2%) | 8(13.1%) | |
| Number of BM | 0.928 | |||
| 1 | 51 (41.8%) | 26 (42.6%) | 25 (41.0%) | |
| 2 | 19 (15.6%) | 10 (16.4%) | 9 (14.8%) | |
| >=3 | 52 (42.6%) | 25 (41.0%) | 27 (44.3%) | |
| Size of BM | 0.073 | |||
| < 10 | 20 (16.4%) | 11 (18.0%) | 9 (14.8%) | |
| >=10, < 20 | 45 (36.9%) | 25 (41.0%) | 20 (32.8%) | |
| >=20, < 30 | 21 (17.2%) | 13 (21.3%) | 8 (13.1%) | |
| >=30 | 23 (18.9%) | 10 (16.4%) | 13 (21.3%) | |
| Unknown | 13 (10.7%) | 2 (3.3%) | 11 (18.0%) |
BM, brain metastasis; CT, chemotherapy; ICI, immune checkpoint inhibitor; NSCLC, non-small Cell Lung Cancer; NOS, not otherwise specified; PSM, propensity score matching; RT, radiotherapy; SRS, stereotactic radiosurgery; WBRT, whole-brain radiation therapy
Following PSM, the median OS for the entire cohort was calculated as 24.03 months (95% CI, 11.45–36.62). Specifically, the ICI + RT group exhibited a median OS of 43.73 months (95% CI, 14.34–73.13), while the CT + RT group had a median OS of 17.17 months (95% CI, 14.57–19.76) (P = 0.018) (Fig. 2B). Additionally, we conducted univariate and multivariate COX regression analyses related to OS after PSM, and the results resembled those observed before PSM. Squamous cell carcinoma (HR, 2.46; 95% CI, 1.15–5.26; P = 0.021) and CT + RT (HR, 2.11; 95% CI, 1.15–3.88; P = 0.016) were both associated with a less favorable prognosis. Smoking (HR, 1.91; 95% CI, 0.98–3.75; P = 0.059) also demonstrated a connection with inferior OS, although the statistical significance was not achieved. The results are displayed in Table 4.
Table 4.
Univariate and multivariate COX regressions for the factors associated with OS after PSM
| Univariate COX regression analysis | Multivariate COX regression analysis | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P value | Hazard Ratio | 95% CI | P value | |
| Age | 0.99 | 0.96–1.03 | 0.756 | NA | NA | NA |
| Gender | ||||||
| Female | Reference | |||||
| Male | 1.71 | 0.77–3.80 | 0.190 | NA | NA | NA |
| PS score | ||||||
| 1 | Reference | |||||
| 2 | 0.90 | 0.43–1.89 | 0.777 | NA | NA | NA |
| Driver gene | ||||||
| None | Reference | |||||
| Yes | 0.78 | 0.28–2.20 | 0.638 | NA | NA | NA |
| Unknown | 1.28 | 0.72–2.27 | 0.393 | NA | NA | NA |
| Pathological type | ||||||
| Adenocarcinoma | Reference | |||||
| Squamous carcinoma | 2.25 | 1.10–4.60 | 0.026 | 2.46 | 1.15–5.26 | 0.021 |
| NSCLC (NOS) | 0.26 | 0.04–1.94 | 0.191 | 0.34 | 0.05–2.52 | 0.291 |
| Primary lesion | ||||||
| Left lung | Reference | |||||
| Right lung | 0.90 | 0.51–1.57 | 0.703 | NA | NA | NA |
| T stage | ||||||
| T1 | Reference | |||||
| T2 | 0.65 | 0.20–2.09 | 0.468 | NA | NA | NA |
| T3 | 1.06 | 0.34–3.29 | 0.915 | NA | NA | NA |
| T4 | 1.33 | 0.46–3.89 | 0.599 | NA | NA | NA |
| N stage | ||||||
| N0 | Reference | |||||
| N1 | 0.00 | 0-Inf | 0.997 | NA | NA | NA |
| N2 | 0.99 | 0.51–1.93 | 0.974 | NA | NA | NA |
| N3 | 0.74 | 0.33–1.69 | 0.479 | NA | NA | NA |
| Extracranial metastases | ||||||
| None | Reference | |||||
| Yes | 0.98 | 0.56–1.71 | 0.937 | NA | NA | NA |
| Number of BM | ||||||
| >=3 | Reference | |||||
| 1 | 1.31 | 0.73–2.36 | 0.371 | NA | NA | NA |
| 2 | 0.61 | 0.23–1.62 | 0.323 | NA | NA | NA |
| Size of BM | ||||||
| < 10 | Reference | |||||
| >=10, < 20 | 0.90 | 0.36–2.24 | 0.813 | NA | NA | NA |
| >=20,<30 | 1.28 | 0.46–3.55 | 0.633 | NA | NA | NA |
| >=30 | 0.86 | 0.31–2.34 | 0.765 | NA | NA | NA |
| Smoking history | ||||||
| None | Reference | |||||
| Yes | 2.31 | 1.21–4.43 | 0.012 | 1.91 | 0.98–3.75 | 0.059 |
| Treatment modality | ||||||
| ICI + RT | Reference | |||||
| CT + RT | 1.98 | 1.11–3.53 | 0.020 | 2.11 | 1.15–3.88 | 0.016 |
| Type of brain radiotherapy | ||||||
| WBRT | Reference | |||||
| SRS | 1.86 | 1.06–3.29 | 0.031 | 1.64 | 0.91–2.98 | 0.102 |
| Sequence of treatment | ||||||
| Upfront brain RT | Reference | |||||
| Upfront ICI/CT | 1.31 | 0.55–3.15 | 0.540 | NA | NA | NA |
| Symptoms of BM | ||||||
| None | Reference | |||||
| Yes | 1.03 | 0.58–1.85 | 0.915 | NA | NA | NA |
| Surgery of lung | ||||||
| None | Reference | |||||
| Yes | 1.26 | 0.68–2.35 | 0.465 | NA | NA | NA |
BM, brain metastasis; CT, chemotherapy; ICI, immune checkpoint inhibitor; NSCLC, non-small Cell Lung Cancer; NOS, not otherwise specified; OS, overall survival; PSM, propensity score matching; RT, radiotherapy; SRS, stereotactic radiosurgery; WBRT, whole-brain radiation therapy
Following PSM, there were no statistically significant differences observed between the two groups in terms of PFS, IPFS, and EPFS. Specifically, the PFS for the ICI + RT and the CT + RT groups were 9.10 months (95% CI, 6.42–11.78) and 6.73 months (95% CI, 5.25–8.22), respectively (P = 0.229), as illustrated in Figure A.3B. The IPFS for these groups were 18.83 months (95% CI, 9.80-27.87) and 21.73 months (95% CI, 6.68–36.79), respectively (P = 0.156), as shown in Figure A.4B. The EPFS for these groups were 13.47 months (95% CI, 7.11–19.82) and 10.50 months (95% CI, 7.37–13.63), respectively (P = 0.226), as shown in Figure A.5B. Regarding the Cox regression analysis for PFS, similar to before PSM, squamous carcinoma (HR, 2.93; 95% CI, 1.62–5.29; P < 0.001) and upfront ICI/CT (HR, 4.16; 95% CI, 2.16–7.99; P < 0.001) were associated with a higher risk of recurrence. Notably, after PSM, positive driver gene status (HR, 2.21; 95% CI, 1.10–4.44; P = 0.026) was also associated with a higher risk of recurrence, as shown in Table A.4. For IPFS, the results after PSM were largely similar to those before PSM. Male gender (HR, 3.77; 95% CI, 1.27–11.19; P = 0.017), squamous carcinoma histology (HR, 3.18; 95% CI, 1.41–7.19; P = 0.005), and upfront ICI/CT (HR, 8.46; 95% CI, 4.04–17.72; P < 0.001) were associated with a higher risk of intracranial lesion progression. Additionally, positive driver gene status (HR, 3.78; 95% CI, 1.37–10.44; P = 0.010) and SRS (HR, 1.83; 95% CI, 1.01–3.32; P = 0.045) were also associated with a higher risk of intracranial recurrence, as detailed in Table A.5. As before PSM, we did not obtain variables significantly associated with EPFS, as shown in Table A.6.
Discussion
Relative to the ICI + RT group, patients in the CT + RT group were treated at an earlier time. The increase in lung cancer incidence can be attributed to various factors beyond smoking, including environmental pollution, lifestyle changes, and increased stress. This also explains why there are fewer smokers in the ICI + RT group. Patients in the CT + RT group generally had larger BM and received WBRT more frequently. With the advent of the immunotherapy era, advances in treatment and improved medical quality have allowed for earlier detection of BM, especially asymptomatic ones. This has led to an increased use of SRS, which requires greater precision in radiotherapy. We believe these factors contribute to the baseline differences between the two groups. To address this, we utilized PSM to minimize baseline imbalances and derive more objective conclusions.
Although there is an increasing amount of research investigating treatment strategies involving the combination of ICIs and brain radiotherapy in patients with BM, most of these studies suffer from limited sample sizes. Furthermore, these studies often analyze cohorts that encompass a variety of cancer types prone to BM, such as melanoma and renal cancer. Our study, in contrast, incorporates a larger sample, focusing exclusively on patients with NSCLC who developed BM. Our study provides compelling evidence that the effectiveness of ICIs combined with brain radiotherapy is superior to that of traditional chemotherapy combined with brain radiotherapy. Among the 161 patients we examined, those in the ICI + RT group exhibited significantly longer median OS than those in the CT + RT group, with respective median OS of 34.80 months (95% CI, 14.94–54.66) and 17.17 months (95% CI, 14.57–19.76) (P = 0.005). This finding was further corroborated by univariate and multivariate COX regression analysis associated with OS, which consistently demonstrated a poorer prognosis for the CT + RT group (HR, 1.82; 95% CI, 1.09–3.05; P = 0.023). Even after conducting a 1:1 PSM, our study still arrived at the same conclusion. The ICI + RT group maintained a significantly longer median OS compared to the CT + RT group, with respective median OS of 43.73 months (95% CI, 14.34–73.13) and 17.17 months (95% CI, 14.57–19.76) (P = 0.018). Likewise, the univariate and multivariate COX regression analysis results associated with OS continued to emphasize the CT + RT group’s inferior prognosis (HR, 2.11; 95% CI, 1.15–3.88; P = 0.016). It becomes evident that patients with NSCLC and BM who undergo ICIs combined with brain radiotherapy consistently achieve the most favorable survival outcomes. For instance, Julia Onken and her colleagues conducted a study involving 171 patients with NSCLC and BM who underwent adjuvant treatment following the resection of BM. Their findings demonstrated a significantly superior OS in the ICIs combined with brain radiotherapy group compared to the chemotherapy combined with brain radiotherapy group, with median OS of 23.0 months (95% CI, 20.3–53.8) and 10.4 months (95% CI, 7.4–14.7; P < 0.001), respectively [27]. Some studies have also found that ICIs combined with brain radiotherapy is superior to radiotherapy alone, but they did not involve comparisons with the combination of chemotherapy and brain radiotherapy, and the sample sizes were relatively small [28–30]. And there were some small-sample studies that did not yield consistent conclusions. For example, Veronica L. Chiang’s study suggested that the median OS for the ICIs group (N = 39) and chemotherapy group (N = 46) were 10 months (95% CI 8.3–13.2 months) and 11.6 months (95% CI 7.7–15.6 months), respectively (P = 0.23) [31]. In summary, the combination of radiotherapy and ICIs has demonstrated notable efficacy, affording patients extended OS compared to other treatment modalities.
Although the ICI + RT group exhibits a significant advantage in OS, it is important to note the benefits in terms of local lesion control. In our study, PFS, IPFS, and EPFS showed no significant differences between the two treatment modalities before and after PSM. This issue has also been a topic of debate in the existing research literature. Some studies have suggested that ICIs combined with brain radiotherapy can result in improved rates of local control [29, 30, 32, 33]. For instance, the research conducted by Andrew M. Baschnagel found that patients receiving ICIs combined with SRS achieved a higher 2-year local disease control rate compared to those receiving SRS alone (97% vs. 86%, P = 0.046). Additionally, this combination therapy was associated with a lower 2-year rate of distant brain lesion failure (39% vs. 66%, P = 0.016) [30]. However, some studies, like ours, have not found compelling evidence to support the notion that combination therapy yields a superior local control rate. For instance, Jason P. Sheehan’s study did not observe significant advantages of ICIs combined with radiotherapy over radiotherapy alone in terms of IPFS (HR, 2.18; 95% CI, 0.72–6.62; P = 0.11) and the 1-year local tumor control rate (84.9% vs. 76.3%, P = 0.94) [34].
In theory, the combination of ICIs and brain radiotherapy could produce a synergistic effect, leading to an improved local control rate. However, existing studies have not agreed on the impact of ICIs in conjunction with brain radiotherapy on local disease control in NSCLC with BM patients. In our study, the absence of significant differences in PFS, IPFS, and EPFS between the two treatment groups may be attributed to several factors. It is possible that patients with positive driver gene mutations had more active brain metastases, while the higher proportion of patients with driver gene mutations in the ICI + RT group offset the effect of the combination of ICIs and brain radiotherapy, resulting in shorter PFS, IPFS and EPFS [35]. In the Cox regression analysis after PSM, driver gene positivity (HR, 3.78; 95% CI, 1.37–10.44; P = 0.010) was associated with a higher risk of intracranial lesion progression. Additionally, the choice of the type of brain radiotherapy could have affected the result. In the ICI + RT group, 54 patients (54.0%) received SRS, while in the CT + RT group, 21 cases (34.4%) received SRS. And in the Cox regression analysis after PSM, SRS (HR, 1.83; 95% CI, 1.01–3.32; P = 0.045) was also associated with an increased risk of intracranial disease progression. In our study, systemic therapy and brain radiotherapy were not consistently administered concurrently to all patients; rather, they were at times delivered successively or intermittently, thereby exerting a discernible influence on PFS. Whether the combination of ICIs and brain radiotherapy can indeed enhance local control in BM may necessitate further research to provide conclusive evidence. It’s gratifying to note that despite not improving local lesion control, the inclusion of ICIs significantly enhanced patients’ OS.
In our univariate and multivariate Cox regression analyses, we identified several factors that influence OS, including the treatment modality, smoking history, and the pathological type. Importantly, our conclusions remained consistent both before and after PSM. Notably, patients with lung squamous cell carcinoma demonstrated a poorer prognosis, a finding that is consistent with the results from Silvia Scoccianti’s study [32]. Additionally, other researchers have also identified factors such as a Karnofsky Performance Status (KPS) score below 70, the use of hypofractionated stereotactic radiotherapy (HFSRT), and a modified Graded Prognostic Assessment (m-GPA) score of ≥ 1.5 as being associated with a worse prognosis [28, 31, 36, 37]. Furthermore, we conducted analyses related to PFS, IPFS, and EPFS. We found that squamous carcinoma, positive driver gene status, and delayed radiotherapy were associated with a higher risk of intracranial progression. This result remained consistent before and after PSM, also leading to an increased risk of disease progression. Some studies suggest that patients with EGFR mutations are more prone to intracranial metastasis, possibly resulting in a higher risk of intracranial progression for these patients [38–40]. Patients received upfront ICI/CT are more likely to experience intracranial progression due to less effective local control compared to upfront brain RT. Additionally, after PSM, we also found that male patients and those treated with SRS were associated with a higher risk of intracranial progression. We consider that SRS controls only the irradiated area, leaving non-irradiated intracranial regions susceptible to recurrence and metastasis. However, it is important to note that our study is a retrospective analysis, which inherently includes selection bias, and thus the conclusions drawn should be interpreted with caution. We hope that these findings will serve as valuable references for future research.
Due to the incomplete record of central nervous system radionecrosis and systemic toxicity reactions in our database, we regretfully did not provide a detailed analysis of these aspects in the results section. Martin performed a study that included patients with NSCLC, melanoma, and renal cell carcinoma, they conducted a dedicated analysis of the toxicity associated with combination therapy. They found that among 115 patients who received ICIs, 23 cases (20%) experienced symptomatic radionecrosis, while among 365 patients who did not receive ICIs, 25 cases (6.8%) had symptomatic radionecrosis [41]. However, the aforementioned studies, along with several others investigating the combination of ICIs and brain radiotherapy, did not observe a significant increase in adverse reactions associated with this treatment modality [28, 37, 42–44]. It’s important to note that these studies are retrospective, introducing potential selection bias, and the reliability of toxicity outcomes remains subject to debate. Prospective studies are needed to investigate the toxicity profiles and feasibility of combination therapy in a scientific manner. Additionally, the data regarding patients’ PD-L1 expression status was missing in our clinical database, preventing us from further identifying whether the beneficial population is associated with PD-L1 expression.
Currently, ICIs in combination with radiotherapy has achieved promising results, notably with the recent groundbreaking findings from Professor Joy Yang’s Stereotactic ablative radiotherapy with ICIs (I-SABR) study in lung cancer [45]. These developments have fueled our optimism for broader applications of combination therapies. Based on the various studies published to date, combination therapy appears to offer superior treatment outcomes for NSCLC patients with BM and is generally well-tolerated. However, several questions remain to be explored. These include the optimal sequence and interval between radiotherapy and ICIs interventions, the potential impact of different radiotherapy modalities on combination therapy outcomes, and whether combination therapy significantly increases toxicity. It is likely that combination therapy will become the mainstream approach for the treatment of NSCLC patients with BM, particularly in those without driver gene mutations or who have developed resistance to targeted therapies. However, further collaborative research efforts are needed to enhance the effectiveness of combination therapy, maximize patient benefits, and identify which patient subgroups stand to gain the most.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the Department of Radiation and Medical Oncology, Zhongnan Hospital of Wuhan University for their support to this study. And we are grateful for smart servier providing the medical drawing material.
Abbreviations
- BM
Brain metastasis
- NSCLC
Non-Small Cell Lung Cancer
- ICIs
Immune Checkpoint Inhibitors
- OS
Overall Survival
- IPFS
Intracranial Progression-Free Survival
- EPFS
Extracranial Progression-Free Survival
- PFS
Progression-Free Survival
- SMIs
Small-Molecule Inhibitors
- SRS
Stereotactic Radiosurgery
- WBRT
Whole-Brain Radiation Therapy
- MRI
Magnetic Resonance Imaging
- CT
Computed Tomography
- PSM
Propensity Score Matching
- PS
Performance Status
- ECOG
Eastern Cooperative Oncology Group
- ECT
Emission Computed Tomography
- PET-CT
Positron Emission Tomography-Computed Tomography
Author contributions
Tengfei Wang and Wen Ouyang proposed the study design. Tengfei Wang, Rumeng Li, and Wen Ouyang performed data analysis, data interpretation, and manuscript writing. Tengfei Wang, Rumeng Li, and Shuyan Liu performed data collection. Wen Ouyang, Qiuji Wu, and Conghua Xie performed manuscript revision. All authors reviewed the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [81972852], Key Research & Development Project of Hubei Province [2020BCA069], Health Commission of Hubei Province Medical Leading Talent Project, and Chinese Society of Clinical Oncology TopAlliance Tumor Immune Research Fund [Y-JS2019-036], and Health Commission of Hubei Province scientific research project [WJ2021M171]. Chen Xiao-ping Foundation for the development of science and technology of Hubei province (CXPJJH124001-2420). Beijing Bethune Charitable Foundation(2023-YJ-152-J-008).
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to the data concerning patient privacy but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University. The Ethics Committee approved oral informed consent, as the data were reviewed and analyzed anonymously. Informed consent was obtained orally from the included patients by telephone. All the methods included in this study are in accordance with the declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tengfei Wang, Rumeng Li and Shuyan Liu contributed equally to this work.
Contributor Information
Qiuji Wu, Email: wuqiuji@126.com.
Wen Ouyang, Email: wen19860213@163.com.
Conghua Xie, Email: chxie_65@whu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians. 2021;71:209–49. [DOI] [PubMed]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. [DOI] [PubMed]
- 3.Waqar SN, Samson PP, Robinson CG, Bradley J, Devarakonda S, Du L, et al. Non-small-cell lung cancer with brain metastasis at presentation. Clin Lung Cancer. 2018;19:e373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Page S, Milner-Watts C, Perna M, Janzic U, Vidal N, Kaudeer N, et al. Systemic treatment of brain metastases in non-small cell lung cancer. Eur J Cancer. 2020;132:187–98. [DOI] [PubMed] [Google Scholar]
- 5.Matsui JK, Perlow HK, Baiyee C, Ritter AR, Mishra MV, Bovi JA, et al. Quality of Life and Cognitive Function Evaluations and Interventions for Patients with Brain Metastases in the Radiation Oncology Clinic. Cancers (Basel). 2022;14:4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuentes R, Osorio D, Expósito Hernandez J, Simancas-Racines D, Martinez-Zapata MJ, Bonfill Cosp X. Surgery versus stereotactic radiotherapy for people with single or solitary brain metastasis. Cochrane Database Syst Rev. 2018;8:CD012086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bindal AK, Bindal RK, Hess KR, Shiu A, Hassenbusch SJ, Shi WM, et al. Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg. 1996;84:748–54. [DOI] [PubMed] [Google Scholar]
- 8.Suh JH, Kotecha R, Chao ST, Ahluwalia MS, Sahgal A, Chang EL. Current approaches to the management of brain metastases. Nat Rev Clin Oncol. 2020;17:279–99. [DOI] [PubMed] [Google Scholar]
- 9.Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J Clin Oncol. 2022;40:492–516. [DOI] [PubMed] [Google Scholar]
- 10.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919–29. [DOI] [PubMed] [Google Scholar]
- 11.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379:2342–50. [DOI] [PubMed] [Google Scholar]
- 12.Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–30. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y-L, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J Thorac Oncol. 2019;14:867–75. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078–92. [DOI] [PubMed] [Google Scholar]
- 15.Wakelee H, Liberman M, Kato T, Tsuboi M, Lee S-H, Gao S, et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J Med. 2023;389:491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Provencio M, Nadal E, González-Larriba JL, Martínez-Martí A, Bernabé R, Bosch-Barrera J, et al. Perioperative Nivolumab and Chemotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2023;389:504–13. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gadgeel SM, Lukas RV, Goldschmidt J, Conkling P, Park K, Cortinovis D, et al. Atezolizumab in patients with advanced non-small cell lung cancer and history of asymptomatic, treated brain metastases: Exploratory analyses of the phase III OAK study. Lung Cancer. 2019;128:105–12. [DOI] [PubMed] [Google Scholar]
- 19.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, et al. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res. 2015;3:345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin W, Xu Y, Chen X, Liu J, Weng Y, Zhuang Q, Lin F, Huang Z, Wu S, Ding J, Chen L, Qiu X, Zhang L, Wu J, Lin D, Qiu S. Radiation-induced small extracellular vesicles as “carriages” promote tumor antigen release and trigger antitumor immunity. Theranostics. 2020;10:4871–84. [DOI] [PMC free article] [PubMed]
- 21.Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor-specific effector CD8 + T cells via dendritic cell activation. J Immunol. 2012;189:558–66. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Z, Zhao J, Hu K, Hou X, Sun X, Pan X, Wang X, Li N, Yang Z, Zhang F, Zhou Q, Zhan L. Single high-dose radiation enhances dendritic cell homing and T cell priming by promoting reactive oxygen species-induced cytoskeletal reorganization. Int J Radiat Oncol Biol Phys. 2021;109:95–108. [DOI] [PubMed]
- 23.Kovács A, Stenmark Tullberg A, Werner Rönnerman E, Holmberg E, Hartman L, Sjöström M, et al. Effect of Radiotherapy After Breast-Conserving Surgery Depending on the Presence of Tumor-Infiltrating Lymphocytes: A Long-Term Follow-Up of the SweBCG91RT Randomized Trial. J Clin Oncol. 2019;37:1179–87. [DOI] [PubMed] [Google Scholar]
- 24.Anitei M-G, Zeitoun G, Mlecnik B, Marliot F, Haicheur N, Todosi A-M, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20:1891–9. [DOI] [PubMed] [Google Scholar]
- 25.Garnett CT, Palena C, Chakraborty M, Tsang K-Y, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–94. [DOI] [PubMed] [Google Scholar]
- 26.Kwilas AR, Donahue RN, Bernstein MB, Hodge JW. In the field: exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Front Oncol. 2012;2:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasilewski D, Radke J, Xu R, Raspe M, Trelinska-Finger A, Rosenstock T, et al. Effectiveness of Immune Checkpoint Inhibition vs Chemotherapy in Combination With Radiation Therapy Among Patients With Non-Small Cell Lung Cancer and Brain Metastasis Undergoing Neurosurgical Resection. JAMA Netw Open. 2022;5:e229553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Douglass J, Kleinberg L, Ye X, Marciscano AE, Forde PM, et al. Concurrent Immune Checkpoint Inhibitors and Stereotactic Radiosurgery for Brain Metastases in Non-Small Cell Lung Cancer, Melanoma, and Renal Cell Carcinoma. Int J Radiat Oncol Biol Phys. 2018;100:916–25. [DOI] [PubMed] [Google Scholar]
- 29.Abdulhaleem M, Johnston H, D’Agostino R, Lanier C, LeCompte M, Cramer CK, et al. Local control outcomes for combination of stereotactic radiosurgery and immunotherapy for non-small cell lung cancer brain metastases. J Neurooncol. 2022;157:101–7. [DOI] [PubMed] [Google Scholar]
- 30.Enright TL, Witt JS, Burr AR, Yadav P, Leal T, Baschnagel AM. Combined Immunotherapy and Stereotactic Radiotherapy Improves Neurologic Outcomes in Patients with Non-small-cell Lung Cancer Brain Metastases. Clin Lung Cancer. 2021;22:110–9. [DOI] [PubMed] [Google Scholar]
- 31.Singh C, Qian JM, Yu JB, Chiang VL. Local tumor response and survival outcomes after combined stereotactic radiosurgery and immunotherapy in non-small cell lung cancer with brain metastases. J Neurosurg. 2019;132:512–7. [DOI] [PubMed] [Google Scholar]
- 32.Scoccianti S, Olmetto E, Pinzi V, Osti MF, Di Franco R, Caini S, et al. Immunotherapy in association with stereotactic radiotherapy for non-small cell lung cancer brain metastases: results from a multicentric retrospective study on behalf of AIRO. Neuro Oncol. 2021;23:1750–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le A, Mohammadi H, Mohammed T, Burney H, Zang Y, Frye D, et al. Local and distant brain control in melanoma and NSCLC brain metastases with concurrent radiosurgery and immune checkpoint inhibition. J Neurooncol. 2022;158:481–8. [DOI] [PubMed] [Google Scholar]
- 34.Shepard MJ, Xu Z, Donahue J, Eluvathingal Muttikkal TJ, Cordeiro D, Hansen L et al. Stereotactic radiosurgery with and without checkpoint inhibition for patients with metastatic non-small cell lung cancer to the brain: a matched cohort study. J Neurosurg. 2019;133:685–92. [DOI] [PubMed]
- 35.Kang Y, Jin Y, Li Q, Yuan X. Advances in Lung Cancer Driver Genes Associated With Brain Metastasis. Front Oncol. 2020;10:606300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanier CM, Hughes R, Ahmed T, LeCompte M, Masters AH, Petty WJ, et al. Immunotherapy is associated with improved survival and decreased neurologic death after SRS for brain metastases from lung and melanoma primaries. Neurooncol Pract. 2019;6:402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed KA, Kim S, Arrington J, Naghavi AO, Dilling TJ, Creelan BC, et al. Outcomes targeting the PD-1/PD-L1 axis in conjunction with stereotactic radiation for patients with non-small cell lung cancer brain metastases. J Neurooncol. 2017;133:331–8. [DOI] [PubMed] [Google Scholar]
- 38.Kim M, Suh CH, Lee SM, Park JE, Kim HC, Kim S-O, et al. Development of Brain Metastases in Patients With Non-Small Cell Lung Cancer and No Brain Metastases at Initial Staging Evaluation: Cumulative Incidence and Risk Factor Analysis. AJR Am J Roentgenol. 2021;217:1184–93. [DOI] [PubMed] [Google Scholar]
- 39.Yang B, Lee H, Um S-W, Kim K, Zo JI, Shim YM, et al. Incidence of brain metastasis in lung adenocarcinoma at initial diagnosis on the basis of stage and genetic alterations. Lung Cancer. 2019;129:28–34. [DOI] [PubMed] [Google Scholar]
- 40.Chen S, Hua X, Jia J, Wu Y, Wei S, Xu L, et al. Risk factors for brain metastases in patients with non-small cell lung cancer: a meta-analysis of 43 studies. Ann Palliat Med. 2021;10:3657–72. [DOI] [PubMed] [Google Scholar]
- 41.Martin AM, Cagney DN, Catalano PJ, Alexander BM, Redig AJ, Schoenfeld JD, et al. Immunotherapy and Symptomatic Radiation Necrosis in Patients With Brain Metastases Treated With Stereotactic Radiation. JAMA Oncol. 2018;4:1123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowalski ES, Remick JS, Sun K, Alexander GS, Khairnar R, Morse E, et al. Immune checkpoint inhibition in patients treated with stereotactic radiation for brain metastases. Radiat Oncol. 2020;15:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weingarten N, Kruser TJ, Bloch O. Symptomatic radiation necrosis in brain metastasis patients treated with stereotactic radiosurgery and immunotherapy. Clin Neurol Neurosurg. 2019;179:14–8. [DOI] [PubMed] [Google Scholar]
- 44.Colaco RJ, Martin P, Kluger HM, Yu JB, Chiang VL. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg. 2016;125:17–23. [DOI] [PubMed] [Google Scholar]
- 45.Chang JY, Lin SH, Dong W, Liao Z, Gandhi SJ, Gay CM, et al. Stereotactic ablative radiotherapy with or without immunotherapy for early-stage or isolated lung parenchymal recurrent node-negative non-small-cell lung cancer: an open-label, randomised, phase 2 trial. Lancet. 2023;402:871–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to the data concerning patient privacy but are available from the corresponding author on reasonable request.