Abstract
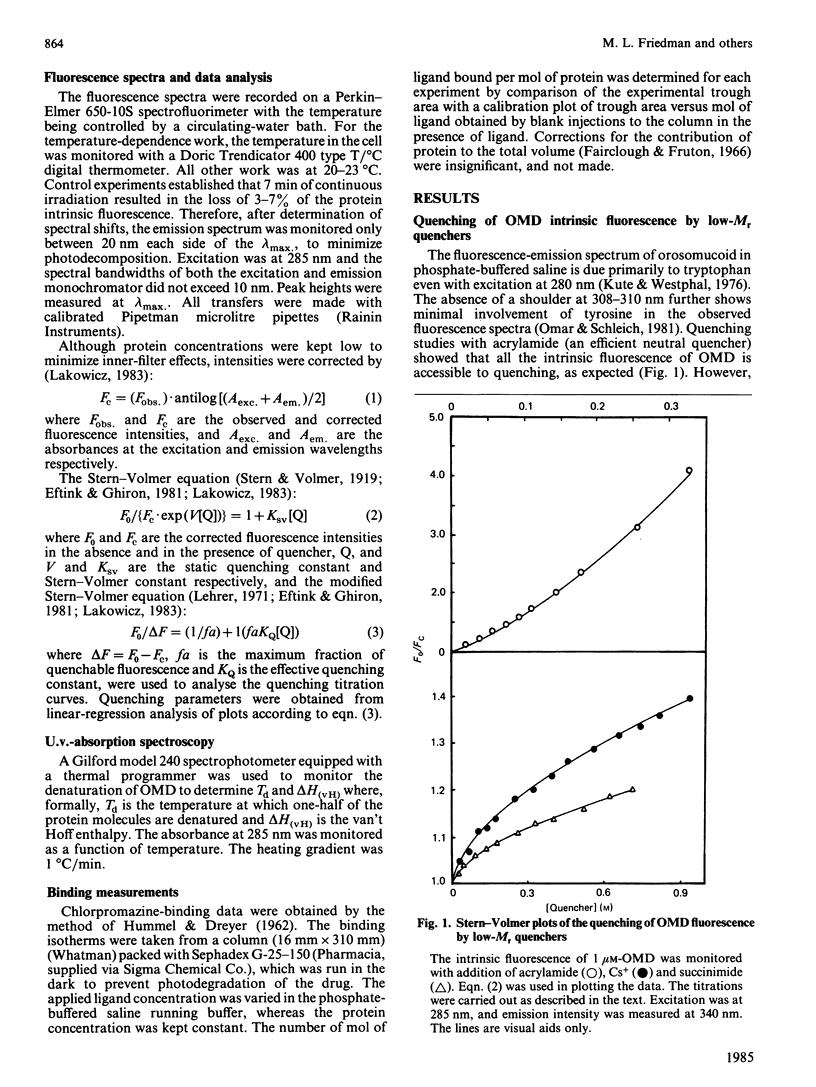
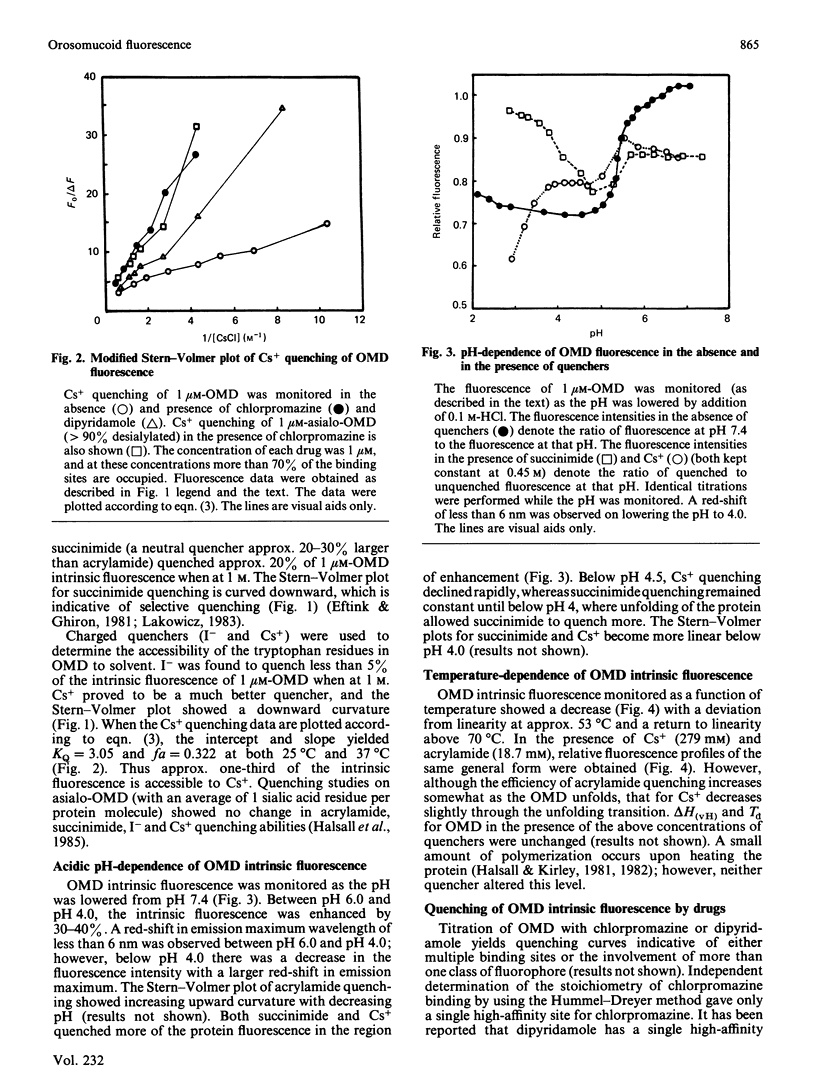
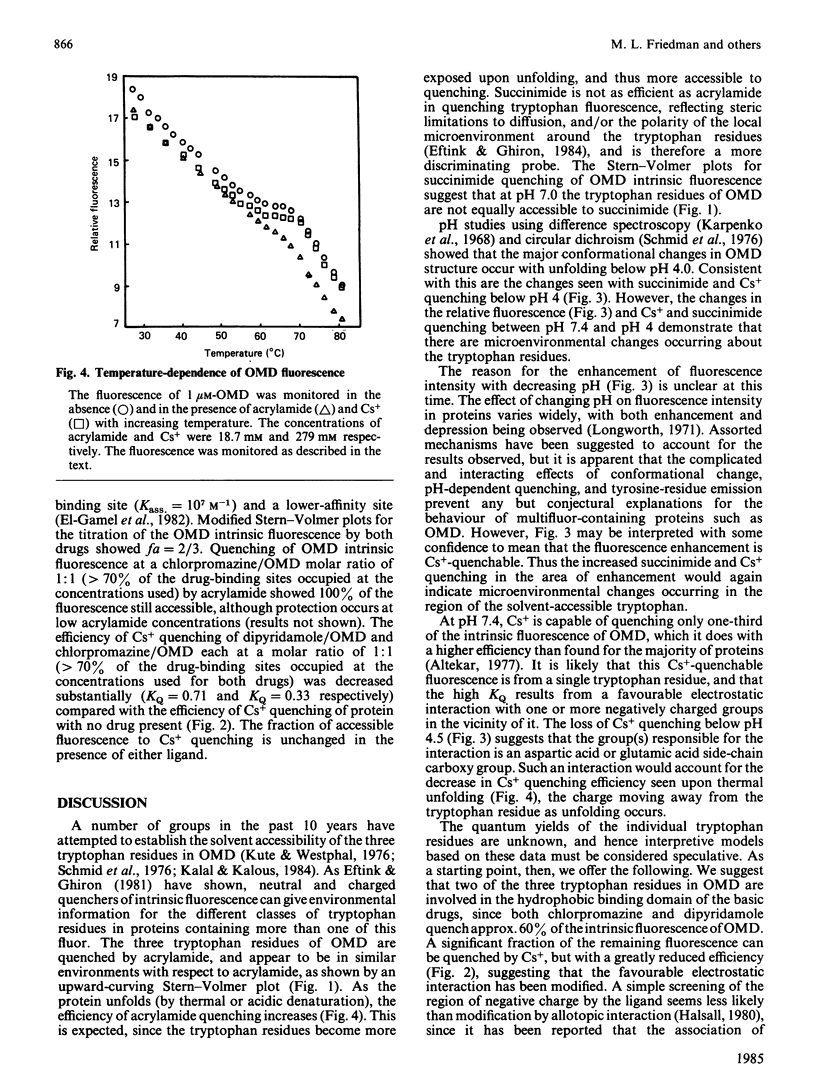
The fluorescence behaviour of human orosomucoid was investigated. The intrinsic fluorescence was more accessible to acrylamide than to the slightly larger succinimide, indicating limited accessibility to part of the tryptophan population. Although I- showed almost no quenching, that of Cs+ was enhanced, and suggested a region of negative charge proximal to an emitting tryptophan residue. Removal of more than 90% of sialic acid from the glycan chains led to no change in the Cs+, I-, succinimide or acrylamide quenching, indicating that the negatively charged region originates with the protein core. Quenching as a function of pH and temperature supported this view. The binding of chlorpromazine monitored by fluorescence quenching, in the presence and in the absence of the small quenching probes (above), led to a model of its binding domain on orosomucoid that includes two tryptophan residues relatively shielded from the bulk solvent, with the third tryptophan residue being on the periphery of the domain, or affected allotopically and near the negatively charged field.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altekar W. Fluorescence of proteins in aqueous neutral salt solutions. II. Influence of monovalent cation chlorides, particularly cesium chloride. Biopolymers. 1977 Feb;16(2):369–386. doi: 10.1002/bip.1977.360160211. [DOI] [PubMed] [Google Scholar]
- Andersen P., Eika C. Inhibition of thrombin-induced platelet aggregation by crude and highly purified alpha 1-acid glycoprotein. Scand J Haematol. 1980 Sep;25(3):202–204. doi: 10.1111/j.1600-0609.1981.tb01389.x. [DOI] [PubMed] [Google Scholar]
- Bennett M., Schmid K. Immunosuppression by human plasma alpha 1-acid glycoprotein: importance of the carbohydrate moiety. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6109–6113. doi: 10.1073/pnas.77.10.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftink M. R., Ghiron C. A. Fluorescence quenching studies with proteins. Anal Biochem. 1981 Jul 1;114(2):199–227. doi: 10.1016/0003-2697(81)90474-7. [DOI] [PubMed] [Google Scholar]
- El-Gamal S., Wollert U., Müller W. E. Binding of several phenothiazine neuroleptics to a common binding site of alpha 1-acid glycoprotein, orosomucoid. J Pharm Sci. 1983 Feb;72(2):202–205. doi: 10.1002/jps.2600720229. [DOI] [PubMed] [Google Scholar]
- El-Gamel S., Wollert U., Müller W. E. Optical studies on the specific interaction of dipyridamole with alpha 1-acid glycoprotein (orosomucoid). J Pharm Pharmacol. 1982 Mar;34(3):152–157. doi: 10.1111/j.2042-7158.1982.tb04212.x. [DOI] [PubMed] [Google Scholar]
- Fairclough G. F., Jr, Fruton J. S. Peptide-protein interaction as studied by gel filtration. Biochemistry. 1966 Feb;5(2):673–683. doi: 10.1021/bi00866a038. [DOI] [PubMed] [Google Scholar]
- Glasson S., Zini R., d'Athis P., Tillement J. P., Boissier J. R. The distribution of bound propranolol between the different human serum proteins. Mol Pharmacol. 1980 Mar;17(2):187–191. [PubMed] [Google Scholar]
- HUMMEL J. P., DREYER W. J. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- Halsall H. B., Kirley T. L. Observations on the thermostability of orosomucoid. Biochem Biophys Res Commun. 1981 Sep 30;102(2):761–765. doi: 10.1016/s0006-291x(81)80197-0. [DOI] [PubMed] [Google Scholar]
- Halsall H. B., Kirley T. L. The denaturation of orosomucoid. Arch Biochem Biophys. 1982 Jul;216(2):392–399. doi: 10.1016/0003-9861(82)90227-2. [DOI] [PubMed] [Google Scholar]
- Karpenko V., Pavlícek Z., Kalous V. Behavior of human orosomucoid in acid media. Biochim Biophys Acta. 1968 Jan 22;154(1):245–247. doi: 10.1016/0005-2795(68)90283-3. [DOI] [PubMed] [Google Scholar]
- Kirley T. L., Sprague E. D., Halsall H. B. The binding of spin-labeled propranolol and spin-labeled progesterone by orosomucoid. Biophys Chem. 1982 Jun;15(3):209–216. doi: 10.1016/0301-4622(82)80004-5. [DOI] [PubMed] [Google Scholar]
- Kute T., Westphal U. Steroid-protein interactions. XXXIV. Chemical modification of alpha1-acid glycoprotein for characterization of the progesterone binding site. Biochim Biophys Acta. 1976 Jan 20;420(1):195–213. doi: 10.1016/0005-2795(76)90358-5. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S. Solute perturbation of protein fluorescence. The quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion. Biochemistry. 1971 Aug 17;10(17):3254–3263. doi: 10.1021/bi00793a015. [DOI] [PubMed] [Google Scholar]
- Omar S. B., Schleich T. Assessment of the exposure and environments of tryptophanyl residues in ribosomal protein S1 by fluorescence quenching. Biochemistry. 1981 Oct 27;20(22):6371–6378. doi: 10.1021/bi00525a014. [DOI] [PubMed] [Google Scholar]
- Piafsky K. M., Borgá O., Odar-Cederlöf I., Johansson C., Sjöqvist F. Increased plasma protein binding of propranolol and chlorpromazine mediated by disease-induced elevations of plasma alpha1 acid glycoprotein. N Engl J Med. 1978 Dec 28;299(26):1435–1439. doi: 10.1056/NEJM197812282992604. [DOI] [PubMed] [Google Scholar]
- Schmid K., Chen L. H., Occhino J. C., Foster J. A., Sperandio K. Topography of human plasma alpha1-acid glycoprotein. Biochemistry. 1976 Jun 1;15(11):2245–2254. doi: 10.1021/bi00656a001. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]


