Abstract
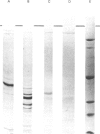
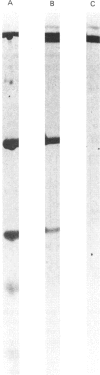
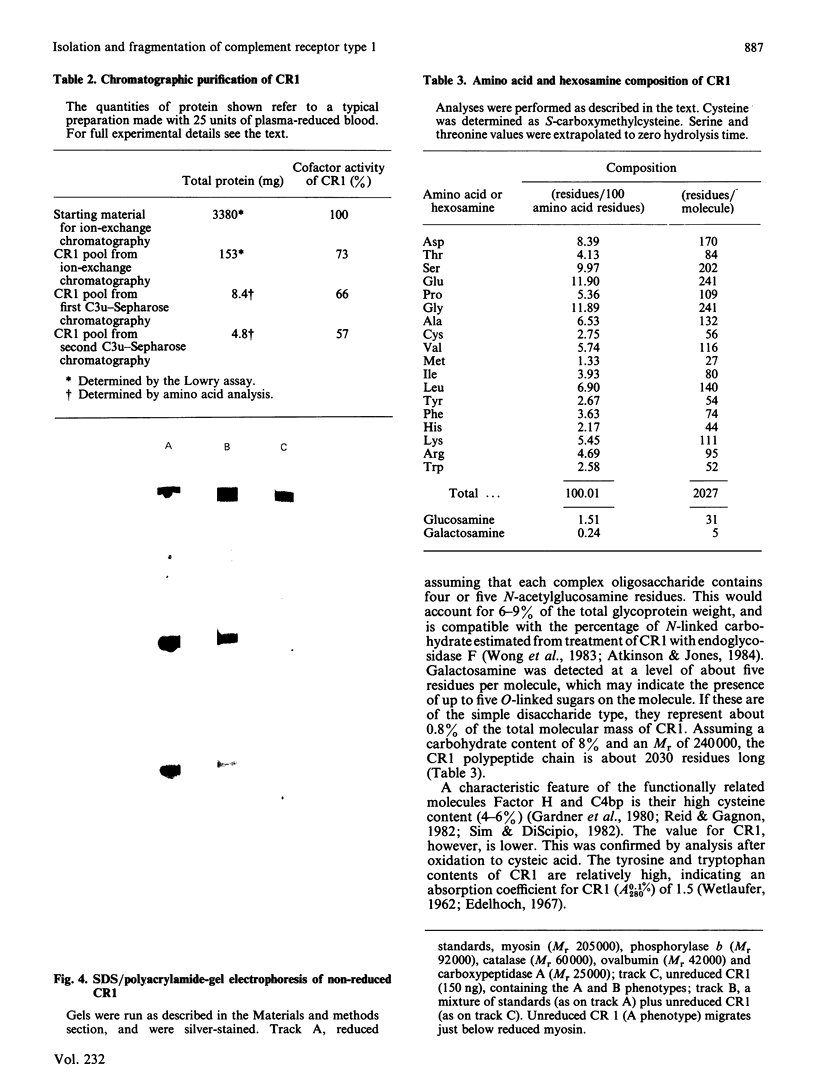
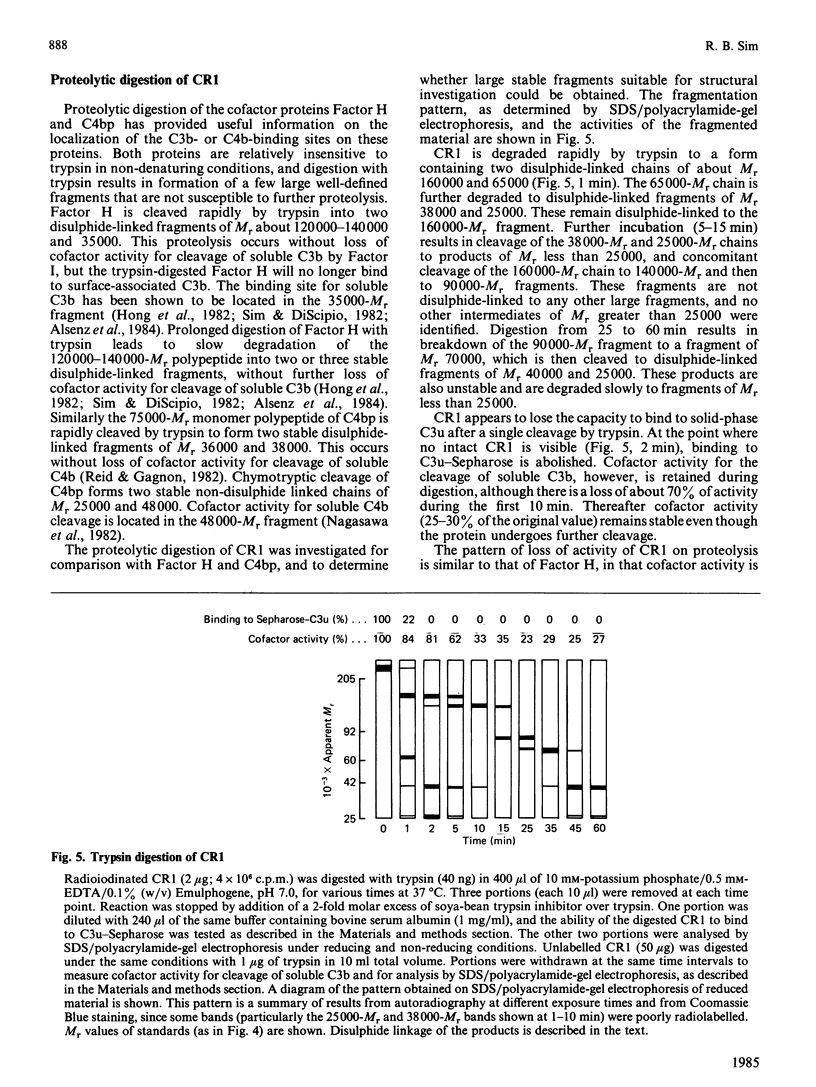
A large-scale procedure for the isolation of complement receptor type 1 (CR1, the C3b receptor) from human erythrocytes is described. Two of the four known phenotypes of CR1 are detectable in the isolated material. Amino acid and hexosamine analysis of the A phenotype (Mr 240 000) indicates a polypeptide chain length of about 2030 amino acids and a carbohydrate content of 8%. Both N- and O-linked sugars appear to be present. Trypsin digestion of isolated CR1 shows that it is degraded rapidly and extensively, and no stable products of Mr greater than 25000 are found. The ability of the receptor to bind to solid-phase ligand is destroyed after a single cleavage by trypsin. The capacity of the receptor to act as a cofactor for Factor I-mediated cleavage of soluble C3b is, however, only gradually decreased by proteolysis, and 30% of this activity remains after extensive degradation. The same pattern of loss of binding to solid-phase ligand, with partial retention of interaction with soluble ligand, is also characteristic of the complement proteins Factor H and C4bp, which are functionally related to CR1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsenz J., Lambris J. D., Schulz T. F., Dierich M. P. Localization of the complement-component-C3b-binding site and the cofactor activity for factor I in the 38kDa tryptic fragment of factor H. Biochem J. 1984 Dec 1;224(2):389–398. doi: 10.1042/bj2240389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout M. A., Dana N., Melamed J., Medicus R., Colten H. R. Low ionic strength or chemical cross-linking of monomeric C3b increases its binding affinity to the human complement C3b receptor. Immunology. 1983 Feb;48(2):229–237. [PMC free article] [PubMed] [Google Scholar]
- Atkinson J. P., Jones E. A. Biosynthesis of the human C3b/C4b receptor during differentiation of the HL-60 cell line. Identification and characterization of a precursor molecule. J Clin Invest. 1984 Nov;74(5):1649–1657. doi: 10.1172/JCI111581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daha M. R., Bloem A. C., Ballieux R. E. Immunoglobulin production by human peripheral lymphocytes induced by anti-C3 receptor antibodies. J Immunol. 1984 Mar;132(3):1197–1201. [PubMed] [Google Scholar]
- Dobson N. J., Lambris J. D., Ross G. D. Characteristics of isolated erythrocyte complement receptor type one (CR1, C4b-C3b receptor) and CR1-specific antibodies. J Immunol. 1981 Feb;126(2):693–698. [PubMed] [Google Scholar]
- Dykman T. R., Cole J. L., Iida K., Atkinson J. P. Polymorphism of human erythrocyte C3b/C4b receptor. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1698–1702. doi: 10.1073/pnas.80.6.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykman T. R., Hatch J. A., Atkinson J. P. Polymorphism of the human C3b/C4b receptor. Identification of a third allele and analysis of receptor phenotypes in families and patients with systemic lupus erythematosus. J Exp Med. 1984 Mar 1;159(3):691–703. doi: 10.1084/jem.159.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Wong W. W. Complement ligand-receptor interactions that mediate biological responses. Annu Rev Immunol. 1983;1:243–271. doi: 10.1146/annurev.iy.01.040183.001331. [DOI] [PubMed] [Google Scholar]
- Gardner W. D., White P. J., Hoch S. O. Identification of a major human serum DNA-binding protein as beta 1H of the alternative pathway of complement activation. Biochem Biophys Res Commun. 1980 May 14;94(1):61–67. doi: 10.1016/s0006-291x(80)80187-2. [DOI] [PubMed] [Google Scholar]
- Hong K., Kinoshita T., Dohi Y., Inoue K. Effect of trypsinization on the activity of human factor H. J Immunol. 1982 Aug;129(2):647–652. [PubMed] [Google Scholar]
- Hsiung L., Barclay A. N., Brandon M. R., Sim E., Porter R. R. Purification of human C3b inactivator by monoclonal-antibody affinity chromatography. Biochem J. 1982 Apr 1;203(1):293–298. doi: 10.1042/bj2030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Mornaghi R., Nussenzweig V. Complement receptor (CR1) deficiency in erythrocytes from patients with systemic lupus erythematosus. J Exp Med. 1982 May 1;155(5):1427–1438. doi: 10.1084/jem.155.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Nussenzweig V. Complement receptor is an inhibitor of the complement cascade. J Exp Med. 1981 May 1;153(5):1138–1150. doi: 10.1084/jem.153.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazatchkine M. D., Fearon D. T., Appay M. D., Mandet C., Bariety J. Immunohistochemical study of the human glomerular C3b receptor in normal kidney and in seventy-five cases of renal diseases: loss of C3b receptor antigen in focal hyalinosis and in proliferative nephritis of systemic lupus erythematosus. J Clin Invest. 1982 Apr;69(4):900–912. doi: 10.1172/JCI110529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maddy A. H., Spooner R. L. Ox erythrocyte agglutinability. 1. Variation in the membrane protein. Vox Sang. 1970 Jan;18(1):34–41. doi: 10.1111/j.1423-0410.1970.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Micklem K. J., Sim R. B., Sim E. Analysis of C3-receptor activity on human B-lymphocytes and isolation of the complement receptor type 2 (CR2). Biochem J. 1984 Nov 15;224(1):75–86. doi: 10.1042/bj2240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa S., Mizuguchi K., Ichihara C., Koyama J. Limited chymotryptic cleavage of human C4-binding protein: isolation of a carbohydrate-containing core domain and an active fragment. J Biochem. 1982 Oct;92(4):1329–1332. doi: 10.1093/oxfordjournals.jbchem.a134052. [DOI] [PubMed] [Google Scholar]
- Nyland H., Matre R., Tönder O. Complement receptors in human peripheral nerve tissue. Acta Pathol Microbiol Scand C. 1979 Feb;87C(1):7–10. [PubMed] [Google Scholar]
- Parkes C., DiScipio R. G., Kerr M. A., Prohaska R. The separation of functionally distinct forms of the third component of human complement (C3). Biochem J. 1981 Mar 1;193(3):963–970. doi: 10.1042/bj1930963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Gagnon J. Human C4-binding protein: N-terminal amino acid sequence analysis and limited proteolysis by trypsin. FEBS Lett. 1982 Jan 11;137(1):75–79. doi: 10.1016/0014-5793(82)80318-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Cordoba S., Dykman T. R., Ginsberg-Fellner F., Ercilla G., Aqua M., Atkinson J. P., Rubinstein P. Evidence for linkage between the loci coding for the binding protein for the fourth component of human complement (C4BP) and for the C3b/C4b receptor. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7890–7892. doi: 10.1073/pnas.81.24.7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim E., Palmer M. S., Puklavec M., Sim R. B. Monoclonal antibodies against the complement control protein factor H (beta 1 H). Biosci Rep. 1983 Dec;3(12):1119–1131. doi: 10.1007/BF01120205. [DOI] [PubMed] [Google Scholar]
- Sim E., Sim R. B. Enzymic assay of C3b receptor on intact cells and solubilized cells. Biochem J. 1983 Feb 15;210(2):567–576. doi: 10.1042/bj2100567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., DiScipio R. G. Purification and structural studies on the complement-system control protein beta 1H (Factor H). Biochem J. 1982 Aug 1;205(2):285–293. doi: 10.1042/bj2050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh P. V., Bahl O. P. Sugar residues on proteins. CRC Crit Rev Biochem. 1981;10(4):307–377. doi: 10.3109/10409238109113602. [DOI] [PubMed] [Google Scholar]
- Walport M. J., Ross G. D., Mackworth-Young C., Watson J. V., Hogg N., Lachmann P. J. Family studies of erythrocyte complement receptor type 1 levels: reduced levels in patients with SLE are acquired, not inherited. Clin Exp Immunol. 1985 Mar;59(3):547–554. [PMC free article] [PubMed] [Google Scholar]
- Wong W. W., Wilson J. G., Fearon D. T. Genetic regulation of a structural polymorphism of human C3b receptor. J Clin Invest. 1983 Aug;72(2):685–693. doi: 10.1172/JCI111018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]