Abstract
Objective
To conduct a systematic review and meta-analysis to assess the impact of transcranial electrical stimulation (TES), proposed as a potential therapy for post-stroke dysphagia, on swallowing function in stroke survivors.
Methods
The PubMed, Embase, Web of Science, and Cochrane Library databases were searched for relevant studies on TES for post-stroke dysphagia. Search results were reviewed following PRISMA guidelines, and the following data were extracted from included studies: study characteristics, demographics, and outcomes. Bias was assessed using the Cochrane tool. Heterogeneity and effect sizes were analysed using I2 statistics and appropriate effects models. The study protocol was registered with PROSPERO (registration No. CRD42024578243)
Results
Six randomized controlled trials met the inclusion criteria (I2 = 0.0%). The meta-analysis indicated a significant improvement in dysphagia with TES (standardized mean difference [SMD] 0.43, 95% confidence interval [CI] 0.13, 0.73). Subgroup analysis suggested that low-intensity TES was effective (SMD 0.46, 95% CI 0.09, 0.82), whereas high-intensity TES showed no significant improvement (SMD 0.37, 95% CI –0.17, 0.91). No publication bias was detected.
Conclusion
TES may improve swallowing in stroke patients, with potential benefits from low-intensity protocols.
Keywords: Transcranial electrical stimulation, post-stroke dysphagia, swallowing function, rehabilitation, meta-analysis
Introduction
Stroke, a prominent contributor to disability on a global scale, results in a range of consequences after the event. One of the most serious sequelae is post-stroke dysphagia, which directly affects the patient's well-being and likelihood of survival.1,2 The incidence of post-stroke dysphagia in individuals who have experienced a stroke varies significantly in the available literature, but it has been reported to impact as many as 80% of patients during the acute phase, with a considerable number experiencing chronic symptoms that require long-term care strategies. 3 The pathophysiology of post-stroke dysphagia is multifaceted, involving a complex interplay of neurological deficits resulting from damage not only to the cerebral cortex, but also critically involving the brainstem. 4 Damage to the brainstem may disrupt the central pattern generator networks essential for coordinating the pharyngeal and oesophageal phases of swallowing, leading to severe oropharyngeal dysphagia. 5 This impairment distresses affected individuals and poses significant challenges for healthcare systems. 6 Profound complications such as malnutrition, dehydration, and aspiration pneumonia can significantly prolong hospital stays, increase the need for medical interventions, and elevate healthcare costs, underscoring an urgent need for effective treatment modalities.7,8
Transcranial electrical stimulation (TES) represents a frontier in non-invasive brain stimulation techniques for exploring therapeutic options for post-stroke dysphagia.9,10 Transitioning from transcranial magnetic stimulation, which uses magnetic fields to modulate neural activity, TES involves direct electrical stimulation to enhance synaptic plasticity and aid neurological recovery. Recent evaluations, such as the umbrella review by Georgiou et al. 11 indicate that the evidence for transcranial magnetic stimulation is currently limited and inconclusive, whereas transcranial direct current stimulation, a form of TES, has shown more promising results according to recent high-quality meta-analyses. 12 TES, by targeting specific cortical areas involved in swallowing, aims to re-establish neural connections disrupted by stroke, thereby improving swallow function. Despite its potential, the effectiveness of TES varies, possibly due to differences in protocols, patient heterogeneity, and timing of intervention relative to stroke events.13,14
Despite the availability of systematic reviews and meta-analyses on TES for post-stroke dysphagia, there remains a critical need for the current meta-analysis that rigorously addresses gaps in parameter definition, evidence quality, and subgroup responsiveness. The present focused approach not only refines these aspects but also systematically evaluates the methodological quality of existing studies, providing definitive insights that may significantly enhance clinical protocols for treating post-stroke dysphagia.15–17 In light of these considerations, the aim of the present systematic review and meta-analysis was to comprehensively assess the available literature on TES for post-stroke dysphagia, striving to provide clarity on its therapeutic potential. By meticulously analysing the collective evidence, this study sought to delineate the conditions under which TES may be most beneficial, with the hypothesis that TES, particularly at low intensity, significantly improves swallowing function in stroke survivors versus high-intensity TES or standard care. The present review aimed to identify specific TES parameters that yield the most favourable outcomes and to clarify the role TES in managing post-stroke dysphagia. Through rigorous evaluation, the authors hope to inform clinical decisions and support the potential integration of TES into standard care protocols for post-stroke dysphagia.
Materials and methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for the present systematic review and meta-analysis, to ensure a structured and transparent approach. 18 The search strategy was initiated on 19 September 2023, utilizing four prominent electronic databases: PubMed, Embase, Web of Science, and the Cochrane Library, with no restrictions on the publication period or limitation to articles published in English only, thereby expanding the scope to include potentially valuable research reported in other languages. Searches were conducted using a combination of key terms, including ‘Stroke’, ‘Dysphagia’, and ‘transcranial direct current stimulation’, aligning with the ‘Patient, Intervention, Comparison, Outcome’ (PICO) framework, to guarantee a thorough and inclusive literature search. The search keywords were selected to capture a broad spectrum of relevant studies, thereby enhancing the depth and breadth of the meta-analysis. To complement the database search, the reference lists of pertinent articles were manually examined to identify additional studies that might contribute to the analysis. The study protocol was registered on the PROSPERO database, registration No. CRD42024578243 (https://www.crd.york.ac.uk/prospero/).
Inclusion and exclusion criteria
Inclusion criteria
Study design: Randomized controlled trials (RCTs) that investigated the effects of TES on post-stroke dysphagia and provided comparative data between intervention groups receiving TES and control groups not receiving such treatment.
Participants: Patients who had experienced a stroke and were diagnosed with post-stroke dysphagia, including patients with either acute or chronic phases of stroke, to encompass a broad patient population.
Interventions: TES, including transcranial direct current stimulation and its various modalities. Studies were included if they provided clear details on TES parameters, such as intensity, frequency, duration, and electrode placement.
Outcomes: Parameters relating to swallowing function, assessed through objective measures, such as video fluoroscopic swallow studies, clinical swallow assessments, or validated dysphagia scales.
Exclusion criteria
Non-original research: Reviews, meta-analyses, editorials, commentaries, and opinion pieces.
Non-human studies: Animal studies, in vitro studies, and/or other non-human research.
Incomplete data: Studies lacking sufficient data on the outcomes of interest or missing critical information on TES intervention parameters.
Duplicate publications: In instances of multiple publications reporting on the same study population and outcomes, only the most comprehensive or recent report was included to avoid data duplication.
Screening and data extraction
Literature screening and data extraction were performed by two independent evaluators (Y Zhao and Z Zhang) to ensure accuracy and objectivity. Any discrepancies were resolved through discussion or, if necessary, by consulting a third reviewer (C Wang). The extracted data included study author, sample size, participants' mean age, and sex, stroke type, TES intervention details (including stimulation site, treatment duration, dosage, electrode size, and current density), main outcome measures, time from stroke to treatment, and any concurrent therapies. For studies lacking detailed data, original investigators were contacted to obtain the necessary unpublished information, ensuring a comprehensive analysis.
Quality assessment
The methodological integrity of the selected studies was appraised using the Cochrane Collaboration's risk of bias assessment framework. 19 Two independent reviewers (Y Zhao and Z Zhang) scrutinized several key aspects, including the generation of allocation sequences, concealment of allocation details, implementation of blinding for both participants and study personnel, handling of incomplete outcome data, presence of selective outcome reporting, and identification of any other potential biases. Each of these critical domains was evaluated and categorized based on the risk of bias it presented, with possible judgments being ‘low risk’, ‘unclear risk’, or ‘high risk’. In instances where the two reviewers held differing opinions, a resolution was sought through mutual discussion, or by involving a third reviewer (C Wang) when needed.
Statistical analyses
Data are presented as n prevalence or mean. Initially, the heterogeneity across studies was evaluated by applying χ2 statistics and determining the I2 value to quantify the extent of variance attributable to study heterogeneity rather than chance. A fixed-effects model was utilized to calculate the pooled effect size when the I2 value fell below 50% and the corresponding P-value was ≥ 0.10, indicating negligible heterogeneity among the studies. Conversely, significant heterogeneity was inferred when the I2 value was ≥ 50%, or the associated P-value was < 0.10, prompting the use of a random-effects model to derive the combined effect size. Sensitivity analysis was conducted to explore the sources of heterogeneity and assess the stability of the present findings. This process involved the systematic exclusion of individual studies from the analysis to observe the impact on the overall effect size, thereby identifying studies that might disproportionately influence the meta-analysis results. To assess the potential for publication bias, the symmetry of a funnel plot was examined, where an equal distribution of studies on both sides of the apex would indicate minimal risk of bias in the meta-analysis outcomes. Further, Egger's linear regression test provided a quantitative method for detecting publication bias. Statistical significance was established at a P-value < 0.05, with all tests being two-sided to ensure comprehensive analysis. The statistical procedures and analyses were executed using Stata software, version 17 (StataCorp, College Station, TX, USA), ensuring rigorous and standardized evaluation of the collected data.
Results
Search results and study selection
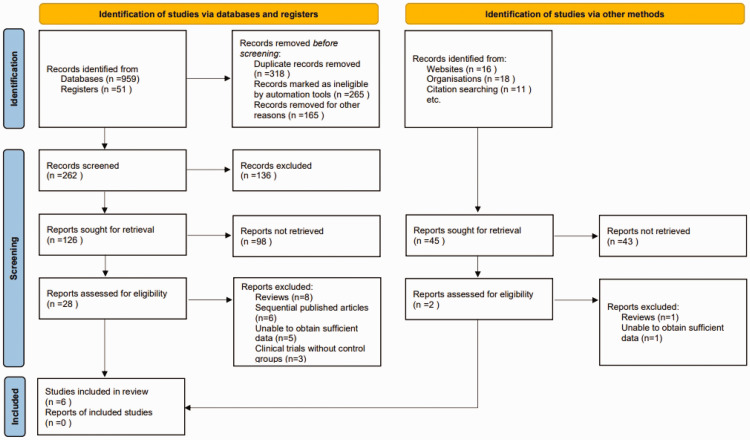
An initial search of electronic databases, websites, and citation searching yielded 1055 potentially relevant articles. A deduplication algorithm was then applied to eliminate duplicates, leaving each unique study to be considered once, and automation tools were used to remove ineligible articles. Following this, titles and abstracts of 262 articles were screened against the predefined inclusion and exclusion criteria, which took into account factors such as study design, participant demographics, measured clinical outcomes, and research quality, resulting in the exclusion of 136 records. A remaining 171 articles were sought for retrieval of full texts (126 following title and abstract screening and 45 from websites, organisations and citation searching). Of these, 141 reports were not retrieved due to the following: full text not accessible, publication archive missing, and/or other access issues. This process narrowed the pool to 30 articles for full-text review. Independent evaluations of these full texts led to the exclusion of 24 articles for the following reasons: review articles (n = 9), overlapping publications (n = 6), lack of sufficient data (n = 6), and absence of control groups in clinical trials (n = 3). Consequently, a total of six articles satisfied the selection criteria and were included in the final analysis (Figure 1).20–25
Figure 1.
Flowchart for inclusion of studies on transcranial electrical stimulation for post-stroke dysphagia.
Study characteristics
The meta-analysis encompassed six studies, published between 2011 and 2018, focusing on the application of TES for treating dysphagia in stroke patients. The studies varied in terms of methodology, with sample sizes ranging from 14 to 59 participants, and mean participant ages spanning from 64 to 71 years. Patients with ischemic stroke and intracerebral haemorrhage were included, with a balanced representation of sexes across studies. The treatment duration varied from 4 to 10 days, with daily sessions lasting between 20 to 30 minutes. Interventions predominantly involved anodal stimulation, targeting either the affected or unaffected pharyngeal motor cortex, with one study applying both anodal and cathodal stimulation. Dosage levels were consistent at 1 or 2 mA, while electrode sizes and current densities varied, reflecting differences in stimulation protocols. The time from stroke onset to the initiation of treatment varied significantly among studies, ranging from as few as 4 days to as long as 357 days post-stroke, indicating a wide spectrum of intervention timing. This diverse collection of studies illustrates the breadth of research into TES as a rehabilitative intervention for post-stroke dysphagia, highlighting variations in treatment parameters and patient demographics. The meta-analysis aimed to synthesize these findings to elucidate the efficacy of TES in enhancing swallowing function in stroke survivors (Table 1).
Table 1.
Characteristics of studies on transcranial electrical stimulation for post-stroke dysphagia included in the meta-analysis.
| Author, year | Stroke Type | Sex, F/M | Sample Size | Mean age, years | Treatment duration, days | Session duration, min | Dosage, mA | Electrode size, cm² | Current density, mA/cm² | Intervention | Mean time post-stroke to treatment, days | Outcome measures |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahn et al., 2017 | Ischemic stroke | 11/15 | 26 | 64 | 10 | 20 | 1 | 25 | 0.04 | Anodal to both hemispheres’ pharyngeal motor cortex | 357 | VFSS, clinical swallow assessments, dysphagia scales |
| Kumar et al., 2011 | Ischemic stroke | 7/7 | 14 | 70 | 5 | 30 | 2 | 15 | 0.133 | Anodal to unaffected inferior sensorimotor cortex and premotor regions | 4.03 | VFSS, clinical swallow assessments, dysphagia scales |
| Pingue et al., 2018 | Ischemic stroke and intracerebral haemorrhage | 20/20 | 40 | 65.25 | 10 | 30 | 2 | 25 | 0.08 | Anodal to affected, cathodal to unaffected pharyngeal motor cortex | 29.5 | VFSS, clinical swallow assessments, dysphagia scales |
| Shigematsu et al., 2013 | Ischemic stroke and intracerebral haemorrhage | 7/13 | 20 | 65.8 | 10 | 20 | 1 | 35 | 0.029 | Anodal to affected pharyngeal motor cortex | 87.5 | VFSS, clinical swallow assessments, dysphagia scales |
| Suntrup et al., 2018 | Ischemic stroke | 25/34 | 59 | 68.05 | 4 | 20 | 1 | 35 | 0.029 | Anodal to unaffected pharyngeal motor cortex | 4.85 | FEES, clinical swallow assessments, dysphagia scales |
| Yang et al., 2012 | Ischemic stroke | 6/10 | 16 | 71 | 10 | 20 | 1 | 25 | 0.04 | Anodal to affected pharyngeal motor cortex | 25.9 | VFSS, clinical swallow assessments, dysphagia scales |
F, female; M, male; VFSS, video fluoroscopic swallow studies; FEES, fibreoptic endoscopic evaluation of swallowing.
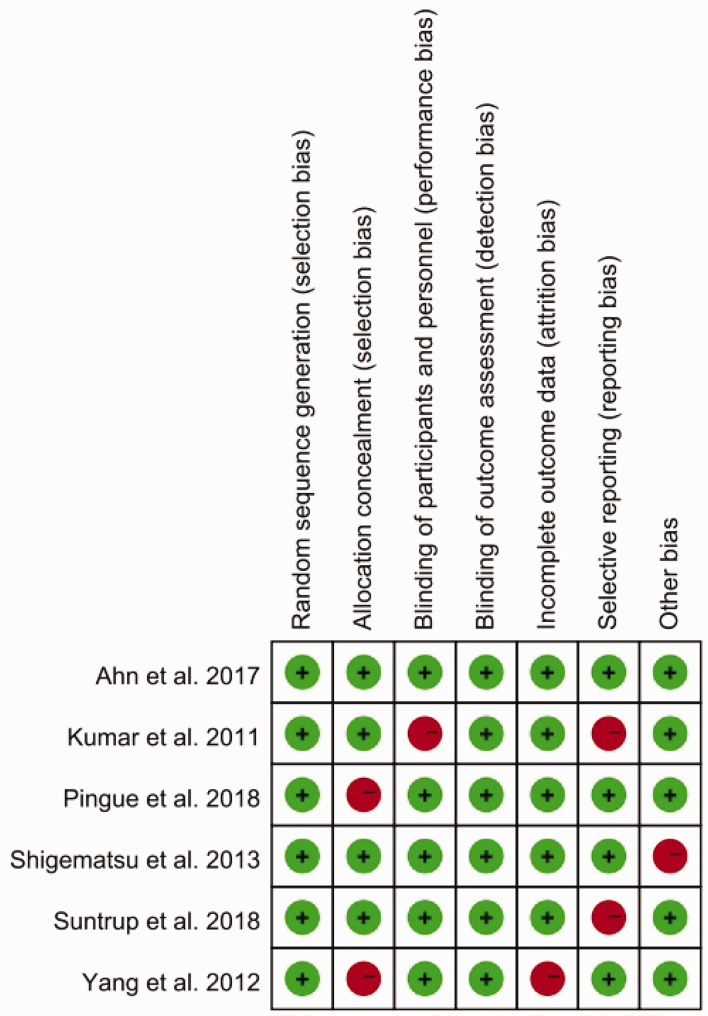
Results of quality assessment
The overall quality assessment of the included studies, using the Cochrane Collaboration’s risk of bias tool, showed a relatively high standard of methodological rigor. Most studies exhibited a low risk of bias in random sequence generation and blinding of outcome assessment, indicating strong internal validity. However, concerns were noted in allocation concealment and selective reporting, with several studies presenting unclear or high risk of bias. These issues may reflect challenges in implementing double-blind protocols in clinical settings. Despite these concerns, blinding of outcome assessment was generally well-executed across the studies, ensuring reliable results (Figure 2).
Figure 2.
Risk of bias evaluation in six studies on transcranial electrical stimulation for post-stroke dysphagia, according to Cochrane criteria (red, high risk; green, low risk).
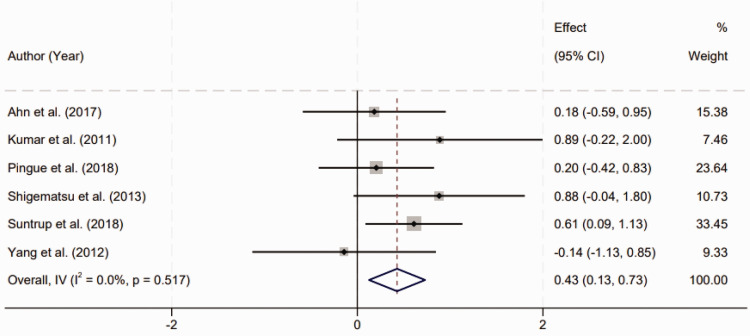
Meta-analysis on the efficacy of TES for post-stroke dysphagia
Six RCTs were reviewed to assess the efficacy of TES in the treatment of dysphagia following stroke. A homogenous effect size was observed across the included studies, as indicated by the lack of significant heterogeneity (I2 = 0.0%, P = 0.517). This suggests a consistent treatment effect of TES across different study designs and patient populations. The pooled results of the meta-analysis revealed a statistically significant improvement in post-stroke dysphagia among patients who received TES therapy. The standardized mean difference (SMD) was calculated to be 0.43 with a 95% confidence interval (CI) ranging from 0.13 to 0.73 (Figure 3). This effect size is indicative of a moderate treatment benefit, and the statistical significance (P < 0.01) underscores the reliability of these findings. The consistency of the results across various studies reinforces the potential of TES as a therapeutic intervention for enhancing swallowing function in stroke survivors. The analysis demonstrates not only the effectiveness of TES but also its applicability as a standard treatment modality in post-stroke rehabilitation protocols to address dysphagia, a prevalent and debilitating condition among this patient cohort.
Figure 3.
Forest plot depicting the effectiveness of transcranial electrical stimulation in treating post-stroke dysphagia.
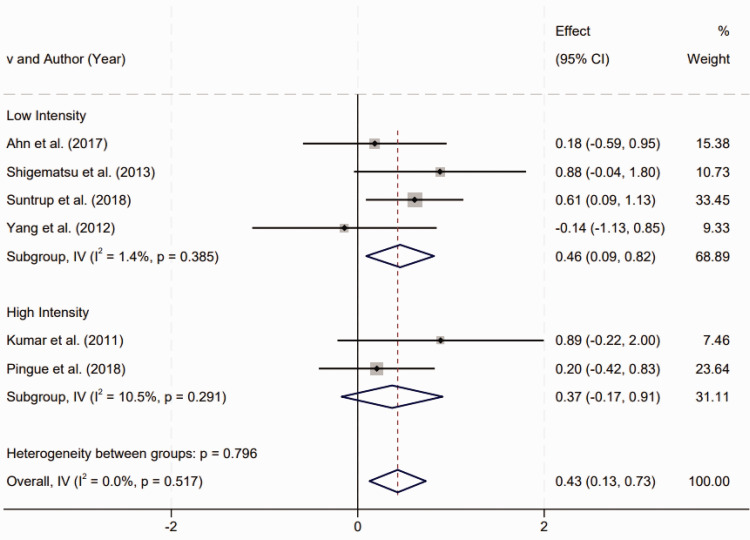
Subgroup analysis of TES intensity on post-stroke dysphagia outcomes
Subgroup analysis based on treatment intensity was conducted to evaluate the effect of TES on post-stroke dysphagia. Studies were categorized into two groups: low-intensity TES (1 mA electrical current) and high-intensity TES (2 mA electrical current). Homogeneity tests within each subgroup revealed no statistically significant heterogeneity (P > 0.1), indicating uniformity in treatment effects across the studies within each intensity category. The results of the subgroup analysis showed that low-intensity TES had a statistically significant positive effect on the improvement of dysphagia symptoms in stroke survivors. The SMD for low-intensity TES was 0.46 (95% CI 0.09, 0.82), with a P-value < 0.01, supporting the efficacy of low-intensity TES in this context. Conversely, high-intensity TES did not show a statistically significant improvement in post-stroke dysphagia, with an SMD of 0.37 (95% CI –0.17, 0.91) and P > 0.05, suggesting that the treatment effect of high-intensity TES was not statistically significant (Figure 4). These findings suggest that while low-intensity TES may be an effective intervention for mitigating dysphagia symptoms following a stroke, high-intensity TES does not demonstrate a statistically significant benefit. This distinction is critical for clinical practice, as it guides the optimization of TES protocols to maximize therapeutic outcomes for stroke survivors with dysphagia.
Figure 4.
Subgroup analysis of transcranial electrical stimulation intensity on post-stroke dysphagia outcomes (low intensity, 1 mA current; high intensity, 2 mA current).
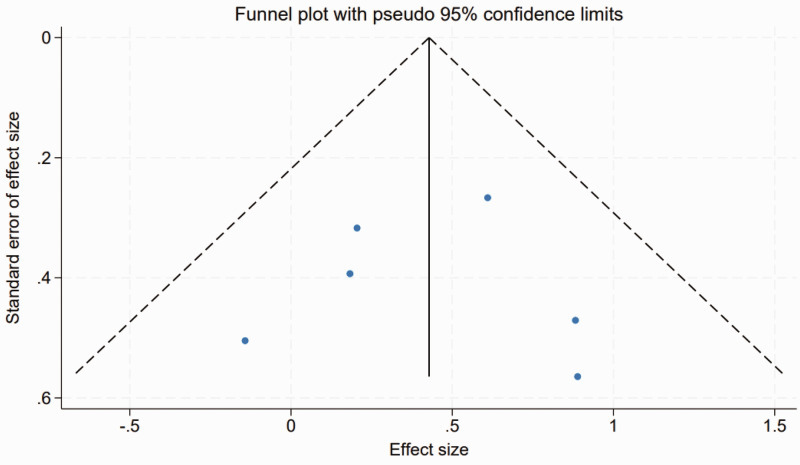
Publication bias
Publication bias was evaluated using funnel plot analysis, which graphically assesses the relationship between study size and effect size, and Egger's linear regression test, to provide a statistical measure of publication bias. The funnel plot demonstrated symmetry across the included studies (Figure 5), suggesting an absence of publication bias, as both smaller and larger studies reported similar effect sizes. Complementing the visual assessment, Egger's linear regression test results confirmed the funnel plot assessment, with no significant evidence of publication bias found across different variables (P > 0.05 for all). This statistical verification reinforces the reliability of the results presented in the meta-analysis.
Figure 5.
Funnel plot of assessment of publication bias across six included studies on transcranial electrical stimulation for post-stroke dysphagia.
Discussion
Transcranial electrical stimulation aims to restore disrupted neural pathways and improve the coordination of muscles essential for a functional swallow. The relevance of the present systematic review and meta-analysis lies in its critical evaluation of the existing evidence to determine the efficacy of TES for improving swallowing function in patients who have experienced stroke.26,27 By analysing data across six RCTs, the present study provides an overview of the impact of TES, considering variations in treatment intensity and patient outcomes. The findings provide robust evidence that low-intensity TES significantly improves swallowing function in stroke survivors, offering a promising intervention for a condition with limited treatment options. This research not only clarifies the optimal parameters for TES application but also underscores its potential integration into standard care protocols. By demonstrating the specific conditions under which TES is most effective, the present study contributes valuable insights that could guide clinical decisions and potentially redefine rehabilitation strategies for stroke-related dysphagia.28,29 The present meta-analysis ensures that these conclusions are both reliable and relevant to clinical practice, providing a significant step forward in post-stroke dysphagia treatment.
The findings of the present meta-analysis support the efficacy of TES as a therapeutic intervention for dysphagia in post-stroke patients. The analysis showed a moderate, yet statistically significant, improvement in swallowing function with an SMD of 0.43. The lack of heterogeneity suggests that TES is beneficial across various clinical settings and stroke populations. The positive effects of low-intensity TES on dysphagia may potentially be attributed to its role in modulating cortical excitability and promoting neural plasticity. 30 After a stroke, the disruption of neural networks impairs communication between the brain and muscles involved in swallowing. 1 TES may facilitate the reorganization of these networks by enhancing synaptic efficacy and promoting the formation of new neural pathways. This, in turn, could lead to the recovery of swallowing function. 31 The discovery that low-intensity TES yields significant benefits, whilst high-intensity TES does not, prompts inquiries on the most effective dosage of electrical stimulation. Lower intensities of electrical stimulation may plausibly be adequate to elicit neuroplastic changes without excessively burdening the neural circuits, which may occur with larger intensities.14,32
The results of the subgroup analysis emphasized the importance of tailoring the TES intervention based on specific patient variables, such as the severity of dysphagia and the time interval between stroke onset and the initiation of TES treatment. In the present subgroup analysis, patients with less severe dysphagia and those who received earlier intervention post-stroke were observed to exhibit greater improvements in swallowing function when treated with low-intensity TES (1 mA), as indicated by a statistically significant standardized mean difference of 0.46 (95% CI 0.09, 0.82, P < 0.01). Conversely, high-intensity TES (2 mA) did not show a statistically significant improvement (SMD 0.37 [95% CI –0.17, 0.91], P > 0.05), suggesting that higher intensities may be less effective or may induce maladaptive plasticity. These findings underscore the need to customize TES intensity based on individual patient characteristics, such as dysphagia severity and timing of intervention, to optimize therapeutic outcomes. The differential effects observed between low- and high-intensity TES also support a closer examination of the dose-response relationship in TES therapy. 33 It is conceivable that higher intensities may induce a ceiling effect, where beyond a certain threshold, additional stimulation does not translate to greater therapeutic benefit. 34 Alternatively, higher intensities may lead to maladaptive plasticity or could be less well-tolerated, resulting in reduced efficacy. 35 The absence of publication bias, as indicated by symmetrical funnel plots and non-significant Egger's test results, strengthens the validity of the present findings, 36 and suggests that the meta-analysis results are likely to be a true reflection of the effects of TES on post-stroke dysphagia and not an artifact of selective reporting or other biases.
The consistent improvement in dysphagia with TES observed in this meta-analysis aligns with current understandings of stroke recovery, where non-invasive brain stimulation techniques have been shown to aid in the rehabilitation of motor functions. 37 Considering the severe effects of dysphagia and its influence on both quality of life and health outcomes, the implementation of TES might be a valuable enhancement to post-stroke rehabilitative therapy. Nevertheless, it is crucial to take into account the customization of treatment regimens, as the timing of intervention, severity of stroke, and unique neurophysiological responses of each patient can impact the efficacy of TES. Additional research is required to investigate the prolonged consequences of TES and to determine the most effective parameters for its implementation in clinical settings. This involves examining the length of time that TES effects last after therapy and comprehending the mechanisms by which TES brings about advantageous alterations in swallowing function. 38 Furthermore, forthcoming clinical trials should strive to incorporate more extensive sample sizes and extended follow-up periods in order to more accurately evaluate the durability of the enhancements in dysphagia.
The results of the present study may be limited by several factors. First, the inclusion of only six RCTs limits the generalizability of the findings, as a broader selection of studies might provide a more comprehensive analysis. The sample sizes of these studies vary, which might impact the statistical power to detect differences. There is also notable diversity in TES protocols, including stimulation parameters and treatment durations, contributing to variability in outcomes. Furthermore, intervention timing across studies varied, with only one study focusing on chronic dysphagia, two on acute, and three on subacute, affecting the interpretation of TES effectiveness. Although a subgroup analysis to assess TES efficacy at different stages of dysphagia was considered, the small number of studies in each subgroup limits the statistical power and reliability of these analyses. Moreover, the majority of studies featured short follow-up periods, constraining the ability to assess the long-term efficacy of TES. Finally, the lack of detailed patient-specific information, such as stroke severity and the timing since stroke onset, restricts the ability to generalize the findings across the stroke population.
Addressing the limitations identified in the present meta-analysis sets a clear direction for future research on TES in post-stroke dysphagia. Future studies should aim to include larger and more diverse populations to enhance the generalizability of findings. Expanding the number of included RCTs and encompassing a wider range of stroke phases, particularly increasing the focus on chronic dysphagia, will allow for a more comprehensive assessment of TES effectiveness. It is also critical to standardize TES protocols regarding stimulation parameters and treatment durations to reduce outcome variability. Longitudinal studies with extended follow-up periods are necessary to evaluate the long-term efficacy and sustainability of TES benefits. Additionally, detailed collection of patient-specific data, such as stroke severity, exact time since stroke onset, and individual patient characteristics will enable more precise subgroup analyses. Such detailed studies will help refine treatment protocols and identify which patient subgroups are most likely to benefit from TES, ultimately guiding clinical decisions and integrating TES into standardized care protocols for stroke rehabilitation more effectively.
Conclusions
In conclusion, the present systematic review and meta-analysis indicated that TES may be an effective intervention for improving swallowing function in post-stroke patients. Low-intensity TES may be of particular benefit, as evidenced by the observed improvements in dysphagia symptoms across the included studies. The consistent treatment effects, despite variability in TES protocols and patient characteristics, suggest that TES could be adapted to diverse clinical settings, enhancing its applicability. Future research should focus on standardizing protocols, extending follow-up periods, and conducting detailed subgroup analyses to refine TES guidelines and more effectively integrate TES into stroke rehabilitation.
Author contributions: Conceptualization: Yanan Zhao; Data curation: Yanan Zhao and Zhicheng Zhang; Formal analysis: Yanan Zhao and Cuicui Wang; Methodology: Yanan Zhao and Hui Zhang; Resources: Ying Wang; Software: Yanan Zhao; Writing – original draft: Yanan Zhao; Writing – review and editing: Jing Bian.
The authors declare that there are no conflicts of interest.
Funding: This research received funding from Jilin Province Science and Technology Development Plan Project (202512JC010478154) and Jilin Provincial Department of Education Project (JJKH20241067KJ).
ORCID iD: Jing Bian https://orcid.org/0009-0003-4451-621X
Data availability statement
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.
References
- 1.Jones CA, Colletti CM, Ding MC. Post-stroke dysphagia: recent insights and unanswered questions. Curr Neurol Neurosci Rep 2020; 20: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dziewas R, Michou E, Trapl-Grundschober M, et al. European Stroke Organisation and European Society for Swallowing Disorders guideline for the diagnosis and treatment of post-stroke dysphagia. Eur Stroke J 2021; 6: LXXXIX–CXV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Xu M, Marshall IJ, et al. Prevalence and natural history of depression after stroke: a systematic review and meta-analysis of observational studies. PLoS Med 2023; 20: e1004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin Y, Tang Y, Liu X, et al. Neural basis of dysphagia in stroke: a systematic review and meta-analysis. Front Hum Neurosci 2023; 17: 1077234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajipour M, Sobhani-Rad D, Zainaee S, et al. Dysphagia following cerebellar stroke: analyzing the contribution of the cerebellum to swallowing function. Front Neurol 2023; 14: 1276243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarty EB, Chao TN. Dysphagia and swallowing disorders. Med Clin North Am 2021; 105: 939–954. [DOI] [PubMed] [Google Scholar]
- 7.Banda KJ, Chu H, Kang XL, et al. Prevalence of dysphagia and risk of pneumonia and mortality in acute stroke patients: a meta-analysis. BMC Geriatr 2022; 22: 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold M, Liesirova K, Broeg-Morvay A, et al. Dysphagia in acute stroke: incidence, burden and impact on clinical outcome. PLoS One 2016; 11: e0148424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Chen K, Wang J, et al. Research progress on transcranial magnetic stimulation for post-stroke dysphagia. Front Behav Neurosci 2022; 16: 995614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Zheng X, Lu R, et al. Repetitive transcranial magnetic stimulation in combination with neuromuscular electrical stimulation for treatment of post-stroke dysphagia. J Int Med Res 2019; 47: 662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Georgiou AM, Phylactou P, Kambanaros M. The effectiveness of transcranial magnetic stimulation for dysphagia in stroke patients: an umbrella review of systematic reviews and meta-analyses. Front Hum Neurosci 2024; 18: 1355407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alabdulaali L, Hickman L, Punt TD, et al. Effectiveness of transcranial direct current stimulation on hand dexterity in stroke patients: a protocol for a systematic review and meta-analysis. BMJ Open 2022; 12: e056064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammad AB, Elhamrawy EA, Abdel-Tawab H, et al. Transcranial magnetic stimulation versus transcutaneous neuromuscular electrical stimulation in post stroke dysphagia: a clinical randomized controlled trial. J Stroke Cerebrovasc Dis 2022; 31: 106554. [DOI] [PubMed] [Google Scholar]
- 14.Bengisu S, Demir N, Krespi Y. Effectiveness of conventional dysphagia therapy (CDT), neuromuscular electrical stimulation (NMES), and transcranial direct current stimulation (tDCS) in acute post-stroke dysphagia: a comparative evaluation. Dysphagia 2024; 39: 77–91. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-García N, Álvarez-Barrio L, Leirós-Rodríguez R, et al. Transcranial direct current stimulation for post-stroke dysphagia: a meta-analysis. J Neuroeng Rehabil 2023; 20: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Q, Lin SF, Ke XH, et al. A systematic review and meta-analysis on the effectiveness of transcranial direct current stimulation on swallowing function of poststroke patients. Am J Phys Med Rehabil 2022; 101: 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao N, Sun W, Xiao Z, et al. Effects of transcranial direct current stimulation on poststroke dysphagia: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil 2022; 103: 1436–1447. [DOI] [PubMed] [Google Scholar]
- 18.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372: n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn YH, Sohn HJ, Park JS, et al. Effect of bihemispheric anodal transcranial direct current stimulation for dysphagia in chronic stroke patients: A randomized clinical trial. J Rehabil Med 2017; 49: 30–35. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Wagner CW, Frayne C, et al. Noninvasive brain stimulation may improve stroke-related dysphagia: a pilot study. Stroke 2011; 42: 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pingue V, Priori A, Malovini A, et al. Dual transcranial direct current stimulation for poststroke dysphagia: a randomized controlled trial. Neurorehabil Neural Repair 2018; 32: 635–644. [DOI] [PubMed] [Google Scholar]
- 23.Shigematsu T, Fujishima I, Ohno K. Transcranial direct current stimulation improves swallowing function in stroke patients. Neurorehabil Neural Repair 2013; 27: 363–369. [DOI] [PubMed] [Google Scholar]
- 24.Suntrup-Krueger S, Ringmaier C, Muhle P, et al. Randomized trial of transcranial direct current stimulation for poststroke dysphagia. Ann Neurol 2018; 83: 328–340. [DOI] [PubMed] [Google Scholar]
- 25.Yang EJ, Baek SR, Shin J, et al. Effects of transcranial direct current stimulation (tDCS) on post-stroke dysphagia. Restor Neurol Neurosci 2012; 30: 303–311. [DOI] [PubMed] [Google Scholar]
- 26.Labeit B, Muhle P, Dziewas R, et al. Diagnostics and treatment of post-stroke dysphagia. Nervenarzt 2023; 94: 676–683 [In German, English abstract]. [DOI] [PubMed] [Google Scholar]
- 27.Marin S, Serra-Prat M, Ortega O, et al. Healthcare costs of post-stroke oropharyngeal dysphagia and its complications: malnutrition and respiratory infections. Eur J Neurol 2021; 28: 3670–3681. [DOI] [PubMed] [Google Scholar]
- 28.Farpour S, Asadi-Shekaari M, Borhani Haghighi A, et al. Improving swallowing function and ability in post stroke dysphagia: a randomized clinical trial. Dysphagia 2023; 38: 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Song WQ, Wang L. Application of noninvasive brain stimulation for post-stroke dysphagia rehabilitation. Kaohsiung J Med Sci 2017; 33: 55–61. [DOI] [PubMed] [Google Scholar]
- 30.Cheng I, Sasegbon A, Hamdy S. Evaluating the therapeutic application of neuromodulation in the human swallowing system. Dysphagia 2023; 38: 1005–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li K, Fu C, Xie Z, et al. The impact of physical therapy on dysphagia in neurological diseases: a review. Front Hum Neurosci 2024; 18: 1404398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Shi A, Xue H, et al. Efficacy of transcranial direct current stimulation combined with conventional swallowing rehabilitation training on post-stroke dysphagia. Dysphagia 2023; 38: 1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders LMJ, Hortobágyi T, Karssemeijer EGA, et al. Effects of low- and high-intensity physical exercise on physical and cognitive function in older persons with dementia: a randomized controlled trial. Alzheimers Res Ther 2020; 12: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fehér KD, Wunderlin M, Maier JG, et al. Shaping the slow waves of sleep: a systematic and integrative review of sleep slow wave modulation in humans using non-invasive brain stimulation. Sleep Med Rev 2021; 58: 101438. [DOI] [PubMed] [Google Scholar]
- 35.Su F, Xu W. Enhancing brain plasticity to promote stroke recovery. Front Neurol 2020; 11: 554089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pisegna JM, Kaneoka A, Pearson WG, Jr, et al. Effects of non-invasive brain stimulation on post-stroke dysphagia: a systematic review and meta-analysis of randomized controlled trials. Clin Neurophysiol 2016; 127: 956–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao S, Wang X, Sun J, et al. Efficacy of non-invasive brain stimulation for post-stroke dysphagia: a meta-analysis. Psychogeriatrics 2024; 24: 433–442. [DOI] [PubMed] [Google Scholar]
- 38.Cheng I, Hamdy S. Current perspectives on the benefits, risks, and limitations of noninvasive brain stimulation (NIBS) for post-stroke dysphagia. Expert Rev Neurother 2021; 21: 1135–1146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.