Abstract
Objective
To evaluate the effect of wearable device training on improving upper limb motor function in patients who experienced strokes.
Methods
The PubMed, Embase, Cochrane Library, Web of Science, MEDLINE, SCOPUS, China National Knowledge Infrastructure, WanFang, and VIP databases were searched for randomized controlled trials (RCTs) that assessed the effectiveness of wearable device training in improving upper limb motor function in patients with stroke. Two investigators independently screened studies by their titles and abstracts and cross-checked, downloaded, and evaluated the results. Disagreements were resolved by a third highly experienced researcher. Risk of bias was evaluated using the Cochrane risk-of-bias tool. This meta-analysis was registered in PROSPERO (registration No. CRD42023421633).
Results
This study comprised 508 patients from 14 RCTs. The experimental group assessed various wearable devices, including 3D-printed dynamic orthoses, inertial measurement unit (IMU) sensors, electrical stimulation devices, and virtual reality (VR) devices for virtual interactive training. The control group received traditional rehabilitation therapies, including physical and conventional rehabilitation. The experimental group scored better on the Fugl–Meyer Assessment (FMA-UE) scale (standardized mean difference [SMD] 0.26, 95% confidence interval [CI] 0.07, 0.45) and Box and Block Test (BBT) (SMD 0.43, 95% CI 0.17, 0.69) versus controls. No significant intergroup differences were observed in the Action Research Arm Test (SMD 0.20, 95% CI −0.15, 0.55), motor activity log (mean difference [MD] 0.32, 95% CI −0.54, 0.33), and modified Ashworth scale (MD −0.08, 95% CI −0.81, 0.64). The probability rankings of wearable devices that improved FMA-UE scores in patients with stroke were: orthotic devices, with the highest probability ranking of 0.45, followed by sensor devices at 0.23, electrical stimulation devices at 0.21, and VR devices at 0.11.
Conclusions
Wearable device training was found to significantly improve upper limb motor function in patients with stroke, particularly for large-range movements. Improvements in FMA-UE and BBT scores reflected reduced impairment and enhanced manual dexterity, respectively. However, the training had no significant effect on hand movement frequency, fine motor skills, or spasticity. Among the different wearable devices tested, orthoses produced the most effective results.
Keywords: Stroke, wearable devices, upper limbs, motor function, meta-analysis
Introduction
Stroke, also known as cerebral infarction or cerebrovascular accident, is an acute cerebrovascular disorder characterized by the loss of neurological function in specific areas of the brain due to ischemia or hemorrhage. 1 Stroke is distinguished by its high mortality and morbidity rates, affecting approximately 70–80% of patients exhibiting physical impairments. 2 In particular, 55–75% of these patients continue to experience upper limb motor dysfunction 3–6 months post-stroke, with symptoms such as reduced motor function, decreased muscle strength, increased muscle tone, joint contractures, and impaired fine motor coordination. 3 Due to the crucial role of upper limbs in performing basic daily tasks and complex and precise activities, upper limb motor dysfunction affects patients’ self-care abilities, including writing, typing, dressing, toothbrushing, and combing hair.4–6 Moreover, upper limb motor dysfunction extends beyond self-care and affects social interactions, thereby increasing the risk of depression among stroke survivors.4,7 Loss of upper limb function may limit participation in social activities, exacerbating social isolation, which is closely associated with depression. 8 Consequently, improving upper limb motor function in patients who have experienced strokes has become a primary focus in the clinical and rehabilitation field. 9
Modern rehabilitation techniques, particularly those utilizing wearable devices, have gained significant attention due to the portability, real-time monitoring capabilities, and personalization features of the devices.10,11 Various types of wearable devices have been used for poststroke upper limb motor function rehabilitation, such as wearable sensor devices that integrate advanced technological features including physiological monitoring, pressure sensors, and inertial sensors, allowing for real-time capture and analysis of patients’ physiological parameters and movement states.12,13 Wearable orthoses are devices designed to support, enhance, or replace the functions of body parts, such as the arms, legs, and spine.14,15 These devices feature passive driving mechanisms that, for example, offer functional, strengthening, and task-specific hand training, enabling the affected limbs to be used repetitively and extensively in daily activities. 16 Wearable dynamic orthoses are adjustable, offering to manage stiffness and provide assistance levels based on the specific needs of the patient. By assisting with correct upper limb movements, the orthosis receives positive physiological and tactile feedback, which reinforces normal movement patterns and supports the maintenance and enhancement of functional neural pathways in the cerebral cortex. 17
Wearable virtual reality (VR) technology offers patients with stroke a novel form of training by allowing them to interact with virtual or augmented reality environments. This immersive experience can contribute to improving limb function. High-quality, high-frequency feedback from the technology enhances synaptic efficacy, thereby improving the flow of information between the cerebral cortex and subcortical structures and establishing normal sensory feedback. Consequently, this enhances hand coordination and limb function.18,19 Electrical stimulation devices use auditory, visual, and tactile feedback to enhance proprioception in patients, thereby correcting their posture and movement patterns. 20
Wearable functional electrical stimulation (FES) devices are associated with the recovery of gross hand coordination by activating neural circuits and facilitating functional reorganization in healthy neural tissue surrounding damaged areas. 21 By utilizing vibratory tactile stimulation, wearable FES devices can reduce the incidence of spastic hypertonia. 22 Compared with traditional devices, wearable electrical stimulators may be used in daily life, overcoming limitations related to portability and operational complexity. Their compact and integrated design allows patients to receive prolonged repetitive stimulation during daily activities. 23 Additionally, these devices assist rehabilitation specialists in developing personalized training programs to enhance treatment efficacy and safety. Their data-driven incentive mechanisms have been shown to improve patient compliance and effectively promote the recovery of upper limb function. 24
Wearable device-based rehabilitation training has become increasingly common among patients who have experienced strokes. Early studies suggest that wearable devices can positively impact post-stroke upper limb motor function.25,26 However, current research on these devices highlights significant methodological variability, including the types of intervention used, their content, and their duration. These discrepancies result in inconsistent treatment effects, limiting comprehensive evaluation of the overall efficacy of these interventions. Despite the extensive use of various types of wearable devices in clinical practice, such as sensor devices, FES units, VR systems, and wearable orthoses, systematic research comparing the effectiveness of different device types on the improvement of post-stroke upper limb motor function is limited. Consequently, clinicians face challenges in selecting the most effective interventions for enhancing rehabilitation.
Several systematic reviews and meta-analyses have evaluated the effectiveness of wearable device training in improving upper limb motor function impairments following stroke. Through their narrative review, Parker et al. 25 concluded that the impact of wearable device training on limb motor function impairments was not significant, possibly due to small sample sizes and the complexity of the interventions. Conversely, Maceira-Elvira et al. 27 explored the effects of different types of wearable devices on patients with upper limb motor function impairment and concluded that wearable devices have a positive impact on upper limb rehabilitation. Previous studies have primarily provided narrative overviews with limited emphasis on the quantitative integration of data.
The novel aim of the present study was to systematically compare the effects of different types of wearable devices on post-stroke upper limb motor function through a network meta-analysis, to not only integrate and quantify existing research data, but also analyse the relative effectiveness of different device types, providing clearer guidance for clinicians. By quantitatively evaluating the effects of various wearable devices, this study aimed to advance the clinical application of personalized and precise rehabilitation.
Therefore, in the present meta-analysis, the impact of wearable device technology was compared with conventional rehabilitation on limb motor dysfunction, hand dexterity, arm and hand function, and daily arm use. The primary outcome measures were the Fugl–Meyer assessment-upper extremity (FMA-UE) and Box and Block Test (BBT) scores, while the secondary outcome measures included the Action Research Arm Test (ARAT), motor activity log (MAL), and modified Ashworth scale (MAS) scores. Furthermore, the extent of improvement in limb motor function provided by different types of wearable devices was assessed, and the overall effects of various interventions were comprehensively compared.
Materials and methods
This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, 28 and was registered on the PROSPERO platform (https://www.crd.york.ac.uk/prospero/) under the registration No. CRD42023421633. Comprehensive searches in PROSPERO and other relevant databases revealed no ongoing or published systematic reviews or meta-analyses on the same intervention method. Ethical approval and patient consent were not required as the meta-analysis and bioinformatic analyses were based on published research and public databases.
Literature search strategy
The PubMed, Embase, Cochrane Library, Web of Science, MEDLINE, SCOPUS, China National Knowledge Infrastructure, Wanfang Data, and VIP databases were searched for studies published in Chinese and/or English from database inception until 1 July 2024. Search keywords included ‘stroke, hemiplegia, apoplexy, cerebrovascular accident, CVA, brain vascular accident, cerebral vascular accident, wearable electronic devices, wearable technology, wearable devices, skin electronic, upper extremity, upper limb, arm, hand, motion capture devices, activity monitor, and inertial measurement unit.’ The specific search strategy, exemplified by the PubMed database, was as follows:
#1 “stroke”[MeSH] OR hemiplegia OR apoplexy OR cerebrovascular accident OR CVA OR brain vascular accident OR cerebral vascular accident
#2 “Wearable Electronic Devices” [MeSH] OR Wearable Technology OR Wearable Devices OR Skin Electronic OR Motion Capture Devices OR Inertial Measurement Unit OR Wearable orthotic OR Wearable accelerometer OR Wearable activity tracker
#3 “upper extremity” [MeSH] OR upper limb OR arm* OR hand*
#4 #1 AND #2 AND #3
Inclusion and exclusion criteria
The study inclusion criteria were: (1) Study population–studies comprising patients diagnosed with stroke,29,30 confirmed through CT and/or MRI, with no restrictions on age or sex; (2) Intervention measures–studies wherein the experimental group received wearable-device training either alone or in combination with conventional rehabilitation treatments; (3) Control measures–studies that adopted basic treatments, including conventional rehabilitation treatments, such as physical and occupational therapy; (4) Outcome indicators–studies that included at least one of the following outcome measures: FMA-UE, ARAT, BBT, MAL, and MAS; (5) Research method–randomized controlled trials (RCTs); and (6) Languages–studies published in Chinese and/or English.
Exclusion criteria comprised the following: (1) Conference papers, academic theses; (2) original data that was incomplete or could not be extracted, with inadequate outcome data even after unsuccessful attempts to contact the authors; (3) duplicate publications; and/or (4) animal experiments and systematic reviews.
Literature screening and data collection
Following the removal of duplicates, the titles and abstracts of studies identified during the database search were independently screened by two investigators (Song and Liang). The results were cross-checked, downloaded, and the full texts were then independently evaluated. Disagreements were resolved by a third highly experienced researcher (Qin).
The following data were extracted from the remaining articles: (1) basic information, including the first author and year of publication; (2) details of the study design, encompassing the sample size, randomization method, allocation concealment (participant blinding and evaluator blinding), and data analysis; (3) participant data, such as age, disease history, and disease stage; (4) details of interventions used for the experimental group and traditional rehabilitation therapies for the control group, such as physical and conventional rehabilitation therapy; and (5) relevant outcome measurements.
Quality assessment of included studies
The quality of included studies was assessed by two researchers (Song and Liang) using the Cochrane Handbook for Systematic Reviews, version 5.1.0 (https://www.cochrane.org/). Assessments focused on several key areas: selection bias, including the generation of random sequences; performance bias, specifically the adequacy of blinding; attrition bias, such as incomplete outcome data; reporting bias, including the selective reporting of results; and other biases. Each study was reviewed for risk of bias based on these criteria.
Outcome indicators
The primary outcome indicators were the FMA-UE and BBT scores. The FMA-UE is a stroke-specific performance-based impairment index designed to assess motor function, balance, sensation, and joint functioning in patients with post-stroke hemiplegia, 31 and has been widely used in clinical settings and research to determine a patient’s motor capacity and track changes over time. The BBT measures manual dexterity and is used to evaluate motor improvements over the course of rehabilitation. During the test, a patient moves blocks from one compartment of a box to another; this action helps quantify their grasp, transport, and release of the objects, reflecting their hand function.32,33 Secondary outcome indicators were the ARAT, MAL, and MAS scores. The ARAT is used to assess specific motor arm functions and the ability to handle objects of varying sizes, weights, and shapes, providing a detailed measure of upper extremity motor function.34,35 The MAL measures how often and how well a patient uses their affected arm and hand for activities of daily living, reflecting the functional use of the impaired limb in everyday tasks. 36 The MAS is a measure of muscle spasticity in patients with neurological conditions that assesses resistance during passive soft tissue stretching and is used to evaluate changes in muscle tone over time.
Statistical analyses
To determine the overall effect of wearable devices, a quantitative analysis of extracted data from the included studies was performed using Review Manager (RevMan) software, version 5.4 (The Cochrane Collaboration, 2020). Continuous results with the same units were analysed using mean difference (MD), while standardized mean difference (SMD) was used to analyse inconsistent scale scores. Uncertainty was determined using a 95% confidence interval (CI). Effect sizes were categorized as: small (SMD < 0.2), medium (0.2 < SMD < 0.5), or large (SMD > 0.5). Heterogeneity was assessed using I2, where I2 ≤ 50% and P ≥ 0.1 indicated minimal heterogeneity and a fixed-effects model was used. For I2 > 50% and P < 0.1, a random-effects model was employed. The significance level was set at α = 0.05. To compare the effects of different wearable devices, R software was used to plot a network relationship diagram comparing the efficacy of different types of wearable devices. A network meta-analysis with FMA-UE as the outcome indicator was performed using Aggregate Data Drug Information System (ADDIS) software, version 1.16.8 (https://drugis.org/software/addis). In ADDIS, the potential scale reduction factor (PSRF) assessed the model convergence, and a PSRF value between 1 and 1.05 indicated good convergence performance, suggesting that the analysis model was relatively stable. Ranking probability plots were used to determine the intervention method with the highest probability of being the most effective. The probabilities of each intervention were ranked from Rank 1 to Rank 5, with Rank 1 indicating the best effect and Rank 5 indicating the worst effect. Higher probabilities for an intervention indicated better treatment effects. Sensitivity was analysed using one-by-one elimination. If the statistical results did not change, it indicated that a single study was not the main reason for the heterogeneity. Funnel plots were used to assess publication bias; a symmetrical distribution on both sides indicated a low likelihood of publication bias, while an asymmetrical plot suggested potential publication bias.
Results
Literature search and process results
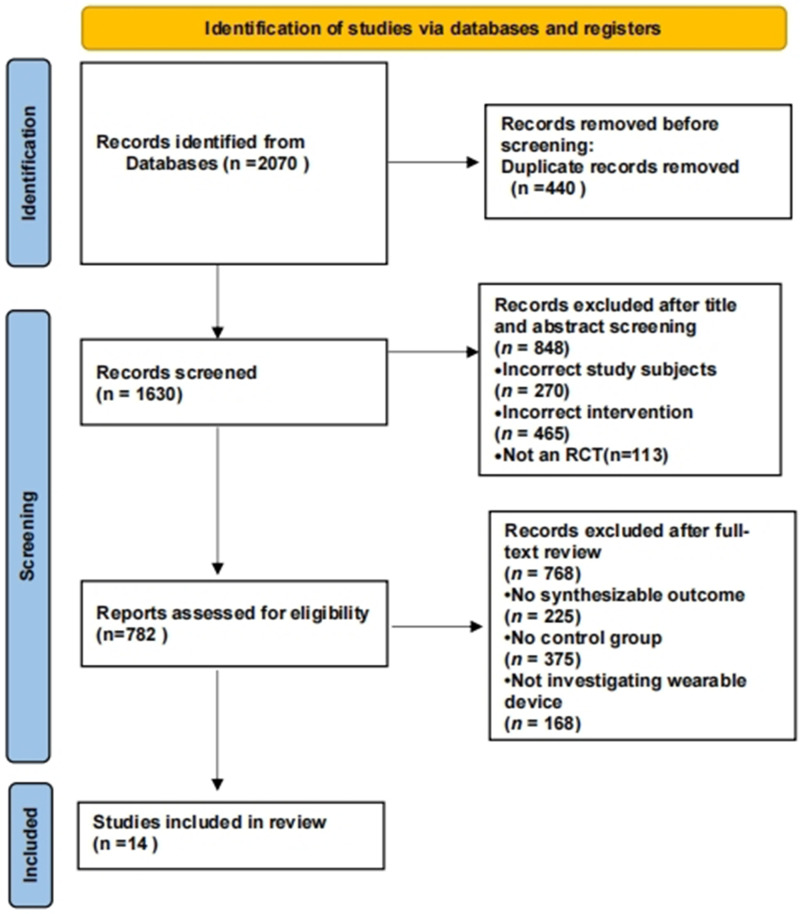
Results of the literature search and screening process are summarised in Figure 1. A total of 14 articles were included,37–50 involving 528 participants with a mean age >50 years.
Figure 1.
PRISMA flowchart diagram of the search process and results.
Study characteristics
Among the included studies, four investigated wearable sensor devices,37–39,41 three investigated wearable VR technology devices,40,42,49 three investigated wearable FES devices,45,48,50 and one investigated a wearable orthotic device. 47 The intervention frequency ranged from 30–60 min per session, 2–5 times a week, for a duration of 3–12 weeks. For the experimental group, interventions included either wearable device training, such as 3D-printed dynamic orthoses, IMU sensors that prompt movement, FES devices, or VR devices, alone or in combination with conventional rehabilitation training. The control group received conventional rehabilitation training, including physical and occupational therapy. Among the included studies, 10 articles used the FMA-UE scale to assess the recovery of upper limb function in patients who experienced strokes.37–42,45,47–49 Eight studies used the BBT to assess hand dexterity.38–40,42–45,48 Four reports used the ARAT to assess fine motor functions of the arm and hand.38,39,42,43 Two studies used the MAL to assess daily arm use.38,39 Two studies used the MAS to assess muscle tone and spasticity of the patient’s hand.46,47 In two studies,40,42 the ‘control group’ received conventional rehabilitation alone, whereas the ‘control group 1’ received mirror therapy combined with conventional rehabilitation in one study, 40 and isometric grip training combined with conventional rehabilitation in the other study. 42 For these two studies, the ‘control group’ and ‘control group 1’ were merged for meta-analysis, according to the Cochrane Handbook for Systematic Reviews, version 5.1.0 formula. One study had an unmatched intervention duration and frequency between the control and intervention groups. 44 The control group received conventional rehabilitation, including group therapy three times a week (45 min per session) and individual therapy at least four times a week (45 min per session). The intervention group received SaeboFlex device training at least once daily (45 min per session), 5 days a week, for 8 weeks. The remaining 13 studies had consistent intervention durations and frequencies, with doses generally matching. Basic information regarding the 14 included studies is summarised in Table 1.
Table 1.
Summary of 14 studies included in the systematic review and meta-analysis of patients who experienced stroke.
| Study | Study sample, total n (exp/ctrl) | Age, years | Clinical course, Y, M, or D | Upper limb impairment degree | Intervention method |
Duration, weeks | Frequency | Outcome measure | |
|---|---|---|---|---|---|---|---|---|---|
| Experimental group | Control group | ||||||||
| Alon, 2008 48 | 26 (13/13) | Exp 63.15 ± 10.91Ctrl 66.46 ± 15.22 | 23.79 ± 8.8 D | FM-UE score (2–10) indicating moderate to severe impairment | Wearable orthotic with functional electrical stimulation (intensity and mode of electrical stimulation controlled by preset program), plus conventional exercise | Conventional exercise | 12 | 30 min, twice daily | FMA-UE, BBT |
| Barry, 2012 43 | 19 (10/9) | Exp 52.7 ± 16.1Ctrl 50.3 ± 7.5 | Exp 4.1 ± 2.3 YCtrl 5.1 ± 5.7 Y | Severe limb impairment | Dynamic wrist-hand orthosis (SaeboFlex) for mechanical assisted treatment | Manual-assisted therapy from therapist | 6 | 1 h/week + home exercise 4 days/week | BBT, ARAT |
| Friedman, 2014 42 | 12 (4/8) | Total 57 ± 30.5 | – | FMA score (34–62) indicating mild to moderate upper limb impairment | Music Glove - wearable musical glove training | Isometric exercises and traditional desktop exercises | 6 | 6 h, 3 times/week | FMA-UE |
| Lannin, 2016 44 | 9 (5/4) | Exp 63 ± 10.4Ctrl 51 ± 21.1 | Exp 2.5 ± 1.7 MCtrl 4.7 ± 6.1 M | Wearable SaeboFlex orthotic device | Conventional therapy | 8 | 45 min/day, 5 times/week | BBT | |
| Knutson, 2016 45 | 72 (40/32) | Exp 55.4 (17.0)Ctrl 56.3 (12.7) | Exp 1.8 (2.5) MCtrl 1.6 (4.9) M | FM-UE: 34 points indicating moderate impairment | Wearable glove functional electrical stimulation (FES) | Cyclic neuromuscular electrical stimulation (cNMES) | 12 | 60 min/time | FMA-UE, BBT |
| Lin, 2018 41 | 18 (9/9) | Exp 52.2 ± 10.2Ctrl 62.6 ± 7.1 | Exp 1115.7 DCtrl 510.9 D | Brunnstrom stages 3–5 | Wearable device based on inertial measurement units (IMUs) and wireless sensor networks (WSNs) + rehabilitation exercise | Conventional therapy | 5 | 15 times/day, 3 days/week | FMA-UE |
| Villafane, 2018 46 | 32 (16/16) | Exp 67 ± 11Ctrl 70 ± 12 | – | Ashworth scale < 3 | Wearable glove device (Gloreha) + PT and OT | Traditional rehabilitation therapy + PT and OT | 3 | 30 min/day, 3 days/week | MAS |
| Wei, 2019 39 | 59 (32/27) | Exp 59.19 ± 11.25Ctrl 63.11 ± 10.27 | Exp 47.75 ± 21.93 DCtrl 53.67 ± 41.16 D | Hemiplegic upper limb functional test (FM-UE) ≥ 3 | Wearable vibration alert watch + regular exercise | Standard care, sports | 4 | 3 h/day | FMA-UE, BBT, ARAT, MAL |
| Yang, 2021 47 | 25 (13/12) | Exp 44.4 ± 10.2Ctrl 47.1 ± 9.2 | Exp 22.5 ± 11.9 MCtrl 20.0 ± 10.4 M | MAS score 1–3 | Wearable dynamic 3D-printed wrist splint + standard rehabilitation | Standard rehabilitation | 6 | 6 h/day | FMA-UE, MAS |
| Park, 2021 49 | 44 (22/22) | Exp 60.59 ± 18.12Ctrl 62.29 ± 13.97 | Exp 17.73 ± 5.98 MCtrl 16.82 ± 7.28 M | Ashworth scale > 3 | Wearable glove device for game-based training equipped with multiple sensors + conventional PT | Conventional PT | 4 | 30 min/time, 5 times/week | FMA-UE |
| Schwerz de Lucena, 2022 38 | 20 (10/10) | Exp 56 ± 17Ctrl 58 ± 12 | Exp 32 ± 14 DCtrl 48 ± 45 D | – | Wearable real-time feedback watch including the ‘Manumeter device’ + regular sports | Standard watch | 3 | 10 h/day | FMA-UE, BBT, ARAT, MAL |
| Kim, 2022 40 | 36 (12/24) | Exp 59.58 ± 5.65Ctrl 62.88 ± 4.71 | Exp 7.00 ± 2.45 MCtrl 6.71 ± 2.44 M | FMA score (26–56) indicating mild to moderate motor impairment | Wearable reflective device mirror therapy +conventional rehabilitation | Traditional mirror +conventional rehabilitation | 4 | 30 min, 5 times/week | FMA-UE, BBT |
| Guo, 2023 37 | 120 (60/60) | Exp 56.25 ± 9.83Ctrl 55.82 ± 9.68 | Exp 46.22 ± 36.63 DCtrl 61.2 ± 46.67 D | Brunnstrom stages 2–6 | Wearable rehabilitation including IMUs and flex sensors, conventional clinical PT training and standard drug therapy | Conventional PT | 3 | 30 min/day, 2 days per week | FMA-UE |
| Maeda, 2024 50 | 20 (10/10) | Exp 71.6 ± 14.1Ctrl 74.1 ± 9.0 | Exp 52.7 ± 21.8 DCtrl 50.9 ± 22.4 D | Classification of finger function test, Class 1 C or above, or Level 2 and above | Standard rehabilitation nursing measures + ‘WIVES’ electrical stimulation device | Standard rehabilitation nursing measures +IVES | 4 | 8 h/day | FMA-UE, ARAT, MAL |
Data presented as n prevalence or mean ± SD, or median (interquartile range).
Exp, experimental group; Ctrl, control group; Y, years; M, months; D, days; PT, physical therapy; OT, occupational therapy; IVES, integrated volitional control electrical stimulation; WIVES, wearable integrated volitional control electrical stimulation.
Bias risk of the included studies
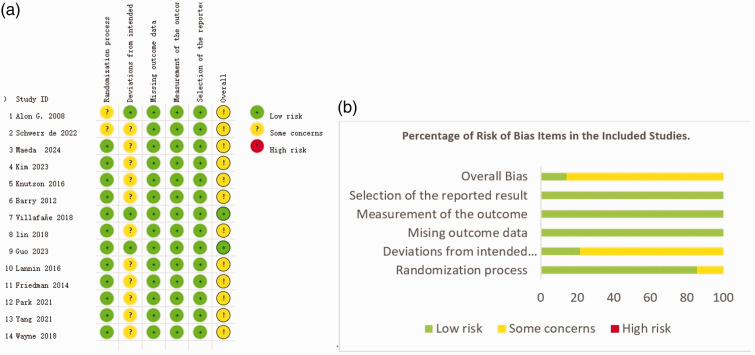
According to the revised Cochrane risk of bias tool (RoB 2) for risk of bias assessment, among the included studies, two did not specify the randomization methods used,38,48 raising concerns. For deviations from intended interventions, only three studies were assessed as low risk,37,45,48 while the remaining 12 studies were evaluated as having some concerns,37–45,47,49,50 primarily due to unclear blinding of participants and intervention providers. Regarding missing outcome data, all 14 studies were assessed as low risk.37–50 To measure the outcome and select the reported results, all studies were assessed as low risk. Notably, two studies were evaluated as having a low risk across all bias categories. The RoB 2 results for each study are shown in Figure 2.
Figure 2.
Risk of bias assessment results in the 14 included studies, showing: (a) risk of bias summary; and (b) percentages of risk of bias items.
Meta-analysis
Upper limb motor impairment score
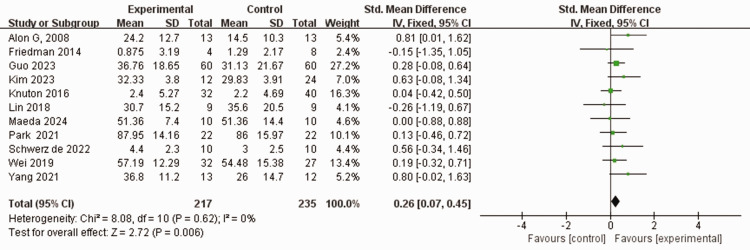
Eleven studies used the FMA-UE scale to assess the severity of upper limb motor impairment in 448 patients who experienced stroke.37–42,45,47–49 No heterogeneity was observed between the studies (I2 = 0%, P = 0.62). Using a fixed-effects model, the FMA-UE score was higher in the experimental group than in the control group who received traditional rehabilitation training including physical and conventional rehabilitation therapy (SMD 0.26, 95% CI 0.07, 0.45, P < 0.05; Figure 3).
Figure 3.
Comparison of Fugl–Meyer assessment-upper extremity (FMA-UE) scores between patients who experienced stroke and received wearable device training (experimental group) and controls who received conventional treatment.
Hand coordination ability score
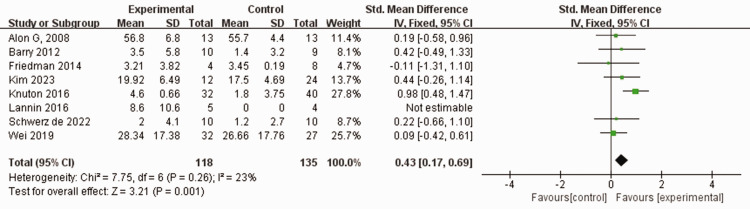
Eight studies assessed hand coordination using the BBT in a combined total of 253 patients who experienced stroke.38–40,42–45,48 No heterogeneity was found among the included studies (I2 = 23%, P = 0.26). Using a fixed-effects model, the BBT score was significantly higher in the experimental group than in the control group that employed traditional rehabilitation training, including physical and conventional rehabilitation therapies (SMD 0.43, 95% CI 0.17, 0.69, P < 0.05; Figure 4).
Figure 4.
Comparison of Box and Block test (BBT) scores between patients who experienced stroke and received wearable device training (experimental group) and controls who received conventional treatment.
Hand fine motor skill score
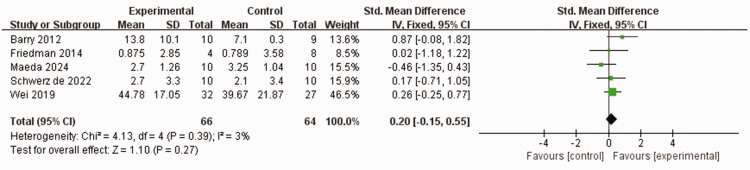
Five studies,38,39,42,43,50 comprising 110 patients who experienced stroke, used the ARAT to assess the ability of patients to handle fine movements of the hand. No heterogeneity was observed between the studies (I2 = 3%, P = 0.39). Furthermore, using a fixed-effects model, no statistically significant differences were observed in ARAT scores between the experimental group and control group (SMD 0.2, 95% CI −0.15, 0.55, P = 0.27; Figure 5).
Figure 5.
Comparison of Action Research Arm Test (ARAT) scores between patients who experienced stroke and received wearable device training (experimental group) and controls who received conventional treatment.
Upper limb activity frequency score
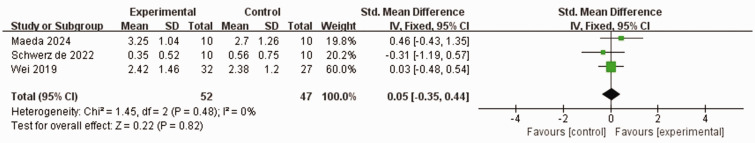
Three studies,37,49,50 comprising 79 patients who experienced stroked, used the MAL to assess the frequency and quality of hand activities. No heterogeneity was observed between the studies (I2 = 0%, P = 0.48). Additionally, using a fixed-effects model, no statistically significant differences in MAL score were observed between the experimental group and control group (SMD 0.05, 95% CI −0.35, 0.44, P = 0.82; Figure 6).
Figure 6.
Comparison of motor activity log (MAL) scores between patients who experienced stroke and received wearable device training (experimental group) and controls who received conventional treatment.
Upper limb muscle spasticity score
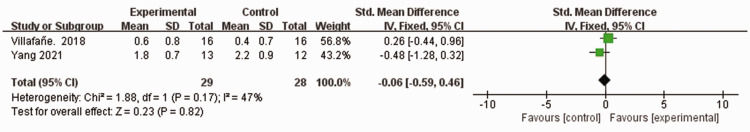
Two studies assessed a combined total of 57 patients who experienced stroke,46,47 using the MAS for muscle tone and spasticity. Moderate heterogeneity was observed among the studies (I2 = 47%, P = 0.17). Using a fixed-effects model, no statistically significant difference was observed in terms of MAS score between the experimental group and control group (MD –0.06, 95% CI −0.59, 0.46, P = 0.82; Figure 7).
Figure 7.
Comparison of modified Ashworth scale (MAS) scores between patients who experienced stroke and received wearable device training (experimental group) and controls who received conventional treatment.
Network meta-analysis
Eleven studies were included in the network meta-analysis,37–42,45,47–50 using the FMA-UE score as an indicator. The network relationship comparing the effects of different wearable devices, with a PSRF value of 1, is shown in Figure 8. The probability ranking is displayed in Table 2.
Figure 8.
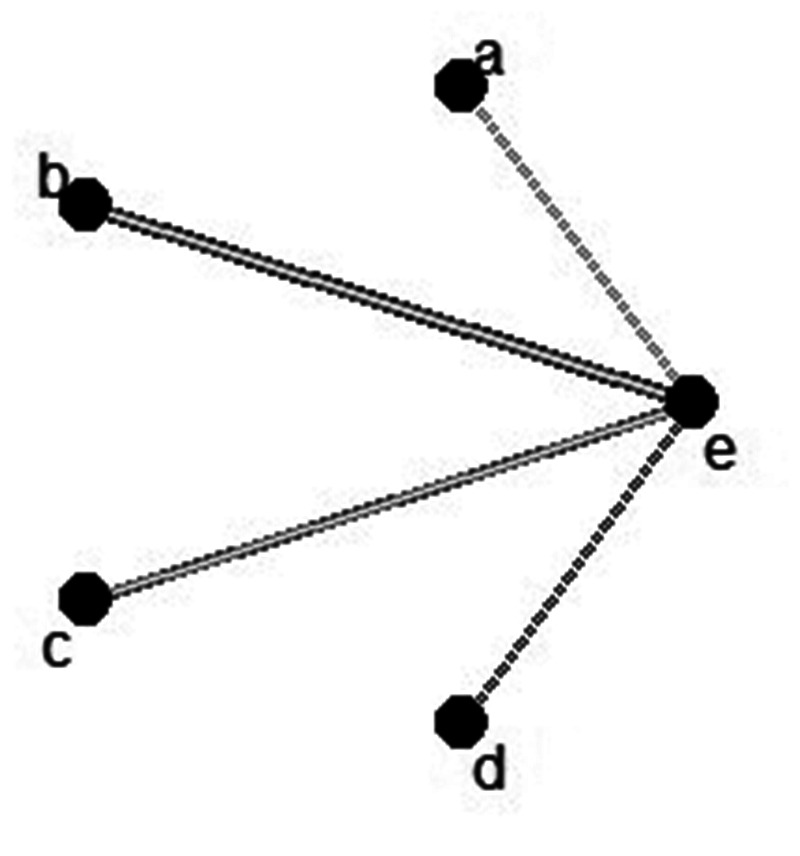
Network relationship diagram comparing the efficacy of different types of wearable devices in patients who experienced stroke and received wearable device training and controls who received conventional treatment: (a) wearable electrical stimulation device; (b) wearable sensor device; (c) wearable sensor technology; (d) wearable upper limb orthosis; and (e) control group.
Table 2.
Probability ranking of the effects of different interventions in 14 studies of patients who experienced stroke.
| Intervention | Rank 1 | Rank 2 | Rank 3 | Rank 4 | Rank 5 |
|---|---|---|---|---|---|
| Wearable sensor device | 0.23 | 0.28 | 0.29 | 0.14 | 0.07 |
| Wearable electrical stimulation device | 0.21 | 0.24 | 0.22 | 0.21 | 0.12 |
| Wearable orthotic device | 0.45 | 0.11 | 0.08 | 0.07 | 0.29 |
| Wearable virtual reality device | 0.11 | 0.35 | 0.25 | 0.18 | 0.12 |
| Control group | 0.00 | 0.03 | 0.16 | 0.40 | 0.41 |
The best probability rankings of different types of wearable devices for FMA-UE scores in stroke patients were as follows: the wearable orthotic device ranked the highest, with a score of 0.39, followed by the FES device with a score of 0.25, the sensor device with a score of 0.2, and lastly the VR device with a score of 0.16.
Assessment of publication bias risk
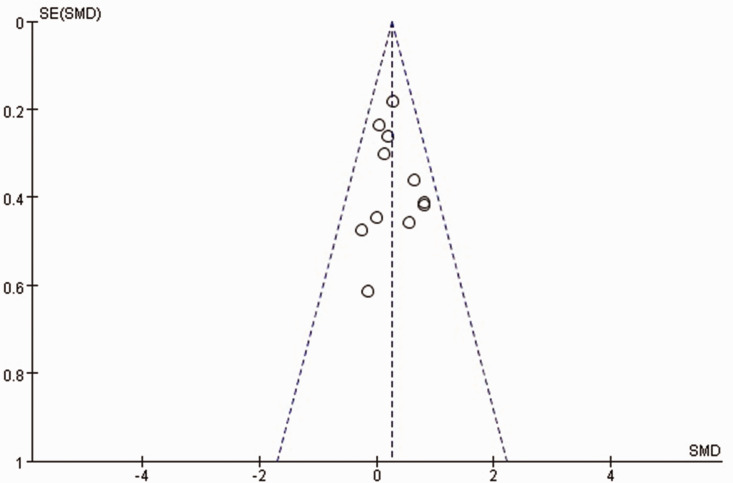
Funnel plots were drawn for outcome indicators with ≥10 articles (all upper limb scores). The funnel plots were not perfectly symmetrical, indicating a potential publication bias (Figure 9).
Figure 9.
Funnel plot for Fugl–Meyer assessment-upper extremity scores in the published studies. SMD, standardised mean difference.
Discussion
The present meta-analysis of 14 studies was conducted to evaluate the impact of wearable devices on upper limb motor rehabilitation in patients who had experienced stroke. Overall, compared with conventional rehabilitation therapy alone, such as physical and occupational therapy, the use of wearable device training combined with conventional rehabilitation therapy improved two outcome measures of upper limb motor function, namely FMA-UE and BBT. Improvement effects measured with the ARAT, MAS, and MAL were not statistically significant. This study revealed that wearable device training is a promising approach for improving upper limb motor function in post-stroke individuals. In particular, the results indicate that wearable device training has a positive effect on enhancing upper limb motor function and gross hand coordination in individuals with upper limb motor impairment following stroke. The probability ranking showed that wearable orthotic devices yielded optimal results. Although improvements in fine motor coordination and the frequency and quality of hand activities were not prominent, this finding presents novel possibilities for stroke rehabilitation.
The present meta-analysis results indicated that wearable device training has a significant positive effect on upper limb motor function. The FMA scores were significantly higher in the experimental group than in the control group. Among the included studies, four utilized sensor-based wearable devices,37–39,41 and three of these studies showed significant improvement in limb motor function. IMUs, components of wearable sensors, integrate accelerometer readings and gyroscope angular rate detection. This integration enables accurate tracking and analysis of complex upper limb movement patterns, thereby providing precise data support for motor rehabilitation.51–53 Among these studies, one showed that the FMA-UE scores of both the experimental and control groups significantly increased over time, suggesting an improvement in upper limb motor function. 38 However, there was no significant between-group difference in FMA-UE score improvement, which was attributed to the 3-week intervention period, suggesting that extending the intervention duration might result in a more pronounced improvement in the experimental group than in the control group. The BBT scores revealed a nonlinear relationship between hand usage intensity and BBT scores, whereby hand usage intensity increased significantly only when BBT scores exceeded 28. 38 Younger participants, with better arm motor abilities, demonstrated a relatively high hand usage intensity even with lower BBT scores. Another study demonstrated a significantly higher improvement in FMA-UE scores in the experimental group versus controls receiving conventional clinical rehabilitation training. 37 The average upper limb score of the experimental group increased by 11.28 points, while that of the control group increased by 7.45 points (P < 0.05). In a study that used a ‘Reminder to Move’ (RTM) wearable device as a treatment intervention for upper limb recovery in patients who experienced a subacute stroke, the experimental group showed significantly higher upper limb motor function scores than the control group. 39 Both groups showed improvement in BBT scores; however, the amount of movement recorded by the RTM device was significantly higher in the experimental group than the control group (12.52% and 9.41%, respectively). Lin et al., 41 demonstrated that wearable device training based on IMU modules (3-axis accelerometers and gyroscopes) resulted in a significant improvement in the experimental group's FMA scores pre- and post-intervention, from 27.2 ± 13.3 to 30.7 ± 15.2 (P < 0.05). Using IMUs for objective functional motor assessment in comprehensive upper limb training post-stroke provides immediate feedback, helping correct maladaptive rehabilitation postures.54,55 Tactile feedback and sensors in wearable devices can correct local abnormal movement patterns and facilitate global neural network reorganization. 55 This process is closely related to ‘motor relearning’, which involves repairing or optimizing damaged neural pathways through repetitive practice and positive feedback, thereby improving upper limb motor function. 56
In the present meta-analysis, three studies used wearable VR devices.40,42,49 In the study by Kim et al. 40 the experimental group received mirror therapy using a video augmented wearable reflection device. Their FMA-UE scores improved significantly from 27.67 ± 4.81 to 32.33 ± 3.80, and the BBT scores increased from 15.83 ± 5.95 to 19.92 ± 6.49 blocks. Both the FMA-UE and BBT scores showed significant improvements versus controls, enhancing patients' visual feedback and engagement. In another study, a MusicGlove device with an engaging game interface was demonstrated to promote functional grasp movements. 42 High levels of engagement and motivation led to significant improvements in upper limb motor function and hand dexterity, and the participants’ FMA-UE scores indicated mild to moderate improvement in upper limb function. Those using MusicGlove experienced a mean increase of 3.21 ± 3.82 blocks in the BBT, which was significantly higher than the control group. 42 Finally, the impact of the RAPAEL smart glove on FMA-UE scores was investigated in patients with acute stroke. 49 In the experimental group, the FMA-UE scores increased significantly from a baseline of 66.50 ± 24.43 to 87.95 ± 14.16, while those of the control group increased from 62.95 ± 28.81 to 86.00 ± 15.97. No significant interaction between group and time was observed (F = 0.123, P = 0.728), indicating that both groups improved over time at similar rates. A longer follow-up period may reveal the potential long-term benefits of wearable devices for rehabilitation. Related studies indicate that wearable VR technology offers a novel training method for patients with stroke, by optimizing neural signal transmission through enhanced multisensory feedback. High-quality, high-frequency feedback may improve synaptic efficacy, facilitating information flow between cortical and subcortical structures. Consequently, this process enhances normal sensory feedback and improves hand coordination and limb function.18,19
Three studies employed wearable FES devices.45,48,50 One study investigated contralaterally controlled functional electrical stimulation (CCFES) and found significant improvements in BBT scores in the CCFES group compared with the cyclic neuromuscular electrical stimulation (cNMES) group after 6 months. 45 The CCFES group showed an average improvement of 4.6 blocks, whereas the cNMES group improved by 1.8 blocks, with an intergroup difference of 2.8 blocks (P = 0.045). For participants with moderate hand impairment and a post-stroke duration of less than 2 years, the CCFES group showed greater improvement (9.6 blocks) than the cNMES group (4.1 blocks), with an intergroup difference of 5.5 blocks (P = 0.023). The greatest improvements in BBT scores were observed in patients who experienced a stroke less than 2 years previously and had moderate hand function impairment. No significant difference in the FMA-UE scores was observed between the groups (P = 0.888), although both improved. 45 Alon et al. 48 studied a Bioness H-200 device (Bioness Inc., Valencia, CA, USA) that features a set of surface electrodes for complete finger and thumb flexion and extension. Using an 11 KHz carrier frequency and 36 Hz time-modulated current, patients underwent 30-minute FES training sessions twice daily for 12 weeks, combined with specific task practice. The FES group showed significantly higher FMA-UE scores (24.2 ± 13.7) than those of the control group (14.5 ± 10.3). Initially, all patients were unable to transfer a block within 60 seconds. However, by the end of the training, the FES group averaged 10.5 ± 12.0 blocks, while the control group averaged 2.5 ± 4.9 blocks. Eight patients in the FES group and three in the control group regained the ability to transfer five or more blocks. Although the BBT scores did not reach statistical significance, FES may aid in recovering functional hand use, with the small sample size potentially contributing to the lack of statistical significance. 48 Another study demonstrated that both integrated volitional control electrical stimulation (IVES) and wearable IVES (WIVES) significantly improved upper limb function in patients who experienced an early subacute stroke. 50 The mean between-group difference in FMA-UE scores was 1.3 points, with the upper limit of the 95% CI not exceeding the non-inferiority margin, indicating that WIVES was not inferior to IVES in terms of improving motor function. The WIVES device, which is smaller and lighter (98 mm × 49 mm × 14.5 mm; 55 g), is designed for ease of use and features electrode pads with magnetic plugs, allowing for optimal electrode positioning without removal. Additionally, wearable FES devices aid in hand coordination recovery by specifically activating neural circuits and reticulospinal pathways, promoting the functional remodelling of healthy neural tissue that surrounds damaged areas. 57 Choudhury et al. 58 observed improvements in gross hand coordination and flexibility, such as grasping ability, with wearable FES devices; however, fine motor enhancements were not observed, possibly because of reticulospinal rather than corticospinal activation.
Among the studies included in the present meta-analysis, one utilized a wearable orthotic device. 42 Although only one study investigated wearable orthotics, a network meta-analysis systematically compared different wearable devices for improving post-stroke upper limb motor function, providing scientific references for clinical decision making. The analysis indicated that all four major types of wearable devices were more effective than interventions used in the control groups, with probability rankings suggesting that wearable orthotics were the most effective. Yang et al. 47 investigated 3D-printed dynamic wrist splints worn for at least 6 hours daily for 6 weeks, and showed significant improvements in FMA-UE scores compared with controls. This wearable orthotic employed a four-bar linkage mechanism to adapt to finger trajectories, achieving customization and low cost through 3D printing, and it was comfortable and easy to wear independently. In another study, 17 wearable orthotics were shown to provide positive physiological and tactile feedback by facilitating correct upper limb movements. This feedback mechanism reinforces normal movement patterns and promotes the maintenance and enhancement of functional neural pathways in the cortex, which are crucial for early-stage upper limb-function recovery. 59 Various factors influence and constrain the efficacy of limb-function rehabilitation. A comparison of the basic information of studies using the FMA-UE score as an outcome measure revealed inconsistent intervention durations, ranging from 3 to 12 weeks. Future research should further verify whether the intervention duration significantly affects improvement of post-stroke upper limb motor function.
Potential reasons for the lack of significant improvement in fine motor coordination, and the frequency and quality of hand activities, in patients who have experienced stroke when using wearable devices after stroke are as follows: In contrast to the present findings, previous studies have reported improvements in fine hand movements and spasticity with wearable device training,58,60 possibly because: (1) The literature reviewed in the present study primarily focused on FES devices designed to improve gross hand coordination. In addition, the mechanical construction and resolution of IMU sensor devices may be insufficient for capturing fine hand movements, rendering them less effective for assessing dexterity, grip exercises, applied forces, and muscle states. 61 Although studies have demonstrated that surface electromyography (sEMG) sensors are effective in improving fine motor skills of the upper limb, 27 the present review did not include studies on sEMG sensors due to their lack of randomization. Instead, this review mainly included studies that used accelerometers (n = 4), which may not offer detailed feedback on fine motor movements. (2) The duration and frequency of rehabilitation interventions are crucial for achieving maximal neuroplasticity. 62 In the present study, the interventions ranged from 3 to 12 weeks, with the timing and frequency tailored to individual patient needs. High-intensity somatosensory stimulation induces changes in motor cortex excitability, thereby affecting limb function. 63 (3) Patient baseline data and compliance: the stroke condition and injury location vary among patients, which may directly affect the effectiveness of rehabilitation. 64 Therefore, individualization of rehabilitation training and patient compliance are factors that need to be considered. (4) Technical limitations of wearable devices: issues with data processing and synchronization may affect real-time feedback and efficacy assessments during training. Slow patient movements may result in underreporting or omission of data by the wearable devices. 20 Therefore, the effectiveness and sensitivity of the device should be evaluated before use. Despite including literature on wearable devices, no statistically significant differences in outcomes were observed in the present study.
Future research directions
Traditional wearable VR devices typically rely on monitors or tablets, which limit user portability and flexibility, 65 however, the portability of smartphones provides users with the flexibility to train at any location. 66 Future research should explore the use of smartphones as visual display devices for VR training. This innovative approach could increase the acceptability and convenience of rehabilitation training and potentially reduce costs and technical barriers. Leveraging smartphones may render VR training more accessible and more easily integrated into daily life, enabling individuals to benefit from personalized rehabilitation programs at home or in other personal spaces. Future research should prioritize dose matching to ensure consistency and comparability of interventions across studies. This includes standardizing the frequency, duration, and intensity of wearable sensor device use. Clear definitions and reporting of these parameters are essential for meaningful comparisons and meta-analyses. Furthermore, continuous advancements in wearable technology should be leveraged to improve the accuracy, usability, and affordability of wearable sensors. Advanced technologies, such as machine learning and artificial intelligence, should be integrated to enhance device functionality and data analysis.
Limitations
The results of the present study may be limited by several factors. First, differences in the intervention duration and conventional rehabilitation measures might limit the ability to judge the results. Secondly, the data were collected post-intervention, and the long-term therapeutic effects remain unclear. Therefore, standardized, high-quality RCTs with large sample sizes are required to verify the effectiveness of wearable device rehabilitation training. Thirdly, the present discussion of the clinical evidence of wearable devices for improving upper limb motor functions after stroke primarily focused on experimental studies, namely RCTs. Future research should include more diverse study designs, including RCTs and observational studies, to explore the research question from different perspectives, thereby providing a more comprehensive understanding.
Conclusion
Meta-analysis and network meta-analysis were conducted to assess the therapeutic efficacy of wearable device-based rehabilitation training for improving upper limb motor function in patients with stroke. The results indicate that wearable device-based rehabilitation training has a positive impact on the enhancement of upper limb motor function and gross hand coordination in these patients. Furthermore, probability ranking revealed that wearable orthotic devices yielded the most optimal outcomes. However, no improvement in fine hand movements, muscle spasticity, or movement frequency was observed in the present study population. Different types of wearable devices vary in convenience and rehabilitation mechanisms. Therefore, individual factors, rehabilitation needs, convenience, and rehabilitation goals should be considered when selecting a suitable wearable device for a patient.
Acknowledgements
We are grateful to all researchers in the enrolled studies. We would like to thank Editage (www.editage.cn) for English language editing.
Author contributions: Qin Qin and Lorna Kwai-Ping Suen conceived the study idea and designed the meta-analysis. Qianqian Song and Guangmei Liang contributed to data acquisition and analysis. Qianqian Song drafted the manuscript and interpreted the data. Haixia Qin and Lingling Zhang critically revised the manuscript for important intellectual content. All authors approved the final manuscript.
The authors declare that there are no conflicts of interest.
Funding: This research received funding from Guangxi University of Chinese Medicine High-Level Talent Cultivation and Innovation Team Project 2022A010.
ORCID iD: Qianqian Song https://orcid.org/0009-0001-3449-8359
Data availability statement
Data are available from the corresponding author upon reasonable request.
References
- 1.Rothwell PM. AVERT: a major milestone in stroke research. Lancet 2015; 386: 7–9. [DOI] [PubMed] [Google Scholar]
- 2.Campbell BCV, Lansberg MG, Broderick JP, STAIR XI Consortium et al. Acute Stroke Imaging Research Roadmap IV: Imaging Selection and Outcomes in Acute Stroke Clinical Trials and Practice. Stroke 2021; 52: 2723–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pundik S, McCabe J, Skelly M, et al. Association of spasticity and motor dysfunction in chronic stroke. Ann Phys Rehabil Med 2019; 62: 397–402. [DOI] [PubMed] [Google Scholar]
- 4.Van der Vliet R, Selles RW, Andrinopoulou ER, et al. Predicting upper limb motor impairment recovery after stroke: a mixture model. Ann Neurol 2020; 87: 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittal N, Majdic BC, Peterson CL. Intermittent theta burst stimulation modulates biceps brachii corticomotor excitability in individuals with tetraplegia. J Neuroeng Rehabil 2022; 19: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irwin ZT, Schroeder KE, Vu PP, et al. Neural control of finger movement via intracortical brain-machine interface. J Neural Eng 2017; 14: 066004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung SE, Bishop AJ, Kim M, et al. Nutritional status of rural older adults is linked to physical and emotional health. J Acad Nutr Diet 2017; 117: 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anstey KJ, Von Sanden C, Sargent-Cox K, et al. Prevalence and risk factors for depression in a longitudinal, population-based study including individuals in the community and residential care. Am J Geriatr Psychiatry 2007; 15: 497–505. [DOI] [PubMed] [Google Scholar]
- 9.Gil-Agudo A, De Los Reyes-Guzmán A, Dimbwadyo-Terrer I, et al. A novel motion tracking system for evaluation of functional rehabilitation of the upper limbs. Neural Regen Res 2013; 8: 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwenk M, Hauer K, Zieschang T, et al. Sensor-derived physical activity parameters can predict future falls in people with dementia. Gerontology 2014; 60: 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domingos C, Costa P, Santos NC, et al. Usability, acceptability, and satisfaction of a wearable activity tracker in older adults: observational study in a real-life context in northern Portugal. J Med Internet Res 2022; 24: e26652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson LA, Menon C, Hodgson AJ, et al. Clinicians' perceptions of a potential wearable device for capturing upper limb activity post-stroke: a qualitative focus group study. J Neuroeng Rehabil 2021; 18: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asada HH, Shaltis P, Reisner A, et al. Mobile monitoring with wearable photoplethysmographic biosensors. IEEE Eng Med Biol Mag 2003; 22: 28–40. [DOI] [PubMed] [Google Scholar]
- 14.Delijorge J, Mendoza-Montoya O, Gordillo JL, et al. Evaluation of a P300-based brain-machine interface for a robotic hand-orthosis control. Front Neurosci 2020; 14: 589659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alibeji NA, Molazadeh V, Dicianno BE, et al. A control scheme that uses dynamic postural synergies to coordinate a hybrid walking neuroprosthesis: theory and experiments. Front Neurosci 2018; 12: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radder B, Prange-Lasonder GB, Kottink AI, et al. A wearable soft-robotic glove enables hand support in ADL and rehabilitation: a feasibility study on the assistive functionality. J Rehabil Assist Technol Eng 2016; 3: 2055668316670553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wickramasuriya DS, Amin MR, Faghih RT. Skin conductance as a viable alternative for closing the deep brain stimulation loop in neuropsychiatric disorders. Front Neurosci 2019; 13: 780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lohre R, Bois AJ, Pollock JW, et al. Effectiveness of immersive virtual reality on orthopedic surgical skills and knowledge acquisition among senior surgical residents: a randomized clinical trial. JAMA Netw Open 2020; 3: e2031217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferris JK, Neva JL, Francisco BA, et al. Bilateral motor cortex plasticity in individuals with chronic stroke, induced by paired associative stimulation. Neurorehabil Neural Repair 2018; 32: 671–681. [DOI] [PubMed] [Google Scholar]
- 20.Schaffer SD, Holzapfel SD, Fulk G, et al. Step count accuracy and reliability of two activity tracking devices in people after stroke. Physiother Theory Pract 2017; 33: 788–796. [DOI] [PubMed] [Google Scholar]
- 21.Biasiucci A, Leeb R, Iturrate I, et al. Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nat Commun 2018; 9: 2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marconi B, Filippi GM, Koch G, et al. Long-term effects on cortical excitability and motor recovery induced by repeated muscle vibration in chronic stroke patients. Neurorehabil Neural Repair 2011; 25: 48–60. [DOI] [PubMed] [Google Scholar]
- 23.Seim CE, Ritter B, Starner TE, et al. Design of a wearable vibrotactile stimulation device for individuals with upper-limb hemiparesis and spasticity. IEEE Trans Neural Syst Rehabil Eng 2022; 30: 1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu TH, Tsai CL, Chi JY, et al. Effect of wearable exoskeleton on post-stroke gait: a systematic review and meta-analysis. Ann Phys Rehabil Med 2023; 66: 101674. [DOI] [PubMed] [Google Scholar]
- 25.Parker J, Powell L, Mawson S. Effectiveness of upper limb wearable technology for improving activity and participation in adult stroke survivors: systematic review. J Med Internet Res 2020; 22: e15981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kortier HG, Sluiter VI, Roetenberg D, et al. Assessment of hand kinematics using inertial and magnetic sensors. J Neuroeng Rehabil 2014; 11: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maceira-Elvira P, Popa T, Schmid AC, et al. Wearable technology in stroke rehabilitation: towards improved diagnosis and treatment of upper-limb motor impairment. J Neuroeng Rehabil 2019; 16: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372: n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo NJ, Ramakrishnan V, Woodbury ML, et al. Concomitant sensory stimulation during therapy to enhance hand functional recovery post stroke. Trials 2022; 23: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pennington A, Ramakrishnan V, Scronce G, et al. Effect of using TheraBracelet on grasping versus reaching in poststroke rehabilitation. OTJR (Thorofare N J) 2023; 43: 702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin GH, Wang I, Lee SC, et al. Development of a 13-item short form for Fugl-Meyer Assessment of upper extremity scale using a machine learning approach. Arch Phys Med Rehabil 2023; 104: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 32.Kontson K, Marcus I, Myklebust B, et al. Targeted box and blocks test: normative data and comparison to standard tests. PLoS One 2017; 12: e0177965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li KY, Lin LJ, Chan AT, et al. Population based norms for the box and blocks test in healthy right-handed Taiwanese adults. Biomed J 2020; 43: 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Da-Silva RH, Moore SA, Rodgers H, et al. Wristband Accelerometers to motiVate arm Exercises after Stroke (WAVES): a pilot randomized controlled trial. Clin Rehabil 2019; 33: 1391–1403. [DOI] [PubMed] [Google Scholar]
- 35.Daghsen L, Fleury L, Bouvier J, et al. Evaluation of a shortened version of the Action Research Arm Test (ARAT) for upper extremity function after stroke: The Mini-ARAT. Clin Rehabil 2022; 36: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 36.Uswatte G, Taub E, Morris D, et al. The Motor Activity Log-28: assessing daily use of the hemiparetic arm after stroke. Neurology 2006; 67: 1189–1194. [DOI] [PubMed] [Google Scholar]
- 37.Guo L, Wang J, Wu Q, et al. Clinical study of a wearable remote rehabilitation training system for patients with stroke: randomized controlled pilot trial. JMIR Mhealth Uhealth 2023; 11: e40416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwerz de Lucena D, Rowe JB, Okita S, et al. Providing real-time wearable feedback to increase hand use after stroke: a randomized, controlled trial. Sensors (Basel) 2022; 22: 6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei WXJ, Fong KNK, Chung RCK, et al. “Remind-to-move” for promoting upper extremity recovery using wearable devices in subacute stroke: a multi-center randomized controlled study. IEEE Trans Neural Syst Rehabil Eng 2019; 27: 51–59. [DOI] [PubMed] [Google Scholar]
- 40.Kim H, Kim J, Jo S, et al. Video augmented mirror therapy for upper extremity rehabilitation after stroke: a randomized controlled trial. J Neurol 2023; 270: 831–842. [DOI] [PubMed] [Google Scholar]
- 41.Lin LF, Lin YJ, Lin ZH, et al. Feasibility and efficacy of wearable devices for upper limb rehabilitation in patients with chronic stroke: a randomized controlled pilot study. Eur J Phys Rehabil Med 2018; 54: 388–396. [DOI] [PubMed] [Google Scholar]
- 42.Friedman N, Chan V, Reinkensmeyer AN, et al. Retraining and assessing hand movement after stroke using the MusicGlove: comparison with conventional hand therapy and isometric grip training. J Neuroeng Rehabil 2014; 11: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barry JG, Ross SA, Woehrle J. Therapy incorporating a dynamic wrist-hand orthosis versus manual assistance in chronic stroke: a pilot study. J Neurol Phys Ther 2012; 36: 17–24. [DOI] [PubMed] [Google Scholar]
- 44.Lannin NA, Cusick A, Hills C, et al. Upper limb motor training using a Saebo orthosis is feasible for increasing task-specific practice in hospital after stroke. Aust Occup Ther J 2016; 63: 364–372. [DOI] [PubMed] [Google Scholar]
- 45.Knutson JS, Gunzler DD, Wilson RD, et al. Contralaterally controlled functional electrical stimulation improves hand dexterity in chronic hemiparesis: a randomized trial. Stroke 2016; 47: 2596–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villafañe JH, Taveggia G, Galeri S, et al. Efficacy of short-term robot-assisted rehabilitation in patients with hand paralysis after stroke: a randomized clinical trial. Hand (N Y) 2018; 13: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang YS, Tseng CH, Fang WC, et al. Effectiveness of a new 3d-printed dynamic hand-wrist splint on hand motor function and spasticity in chronic stroke patients. J Clin Med 2021; 10: 4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alon G, Levitt AF, Mccarthy PA. Functional electrical stimulation (FES) may modify the poor prognosis of stroke survivors with severe motor loss of the upper extremity: a preliminary study. Am J Phys Med Rehabil 2008; 87: 627–636. [DOI] [PubMed] [Google Scholar]
- 49.Park YS, An CS, Lim CG. Effects of a rehabilitation program using a wearable device on the upper limb function, performance of activities of daily living, and rehabilitation participation in patients with acute stroke. Int J Environ Res Public Health 2021; 18: 5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maeda H, Hishikawa N, Sawada K, et al. Wearable integrated volitional control electrical stimulation device as treatment for paresis of the upper extremity in early subacute stroke patients: a randomized controlled non-inferiority trial. Arch Phys Med Rehabil 2024; 105: 227–234. [DOI] [PubMed] [Google Scholar]
- 51.Jones HSR, Stiles VH, Verheul J, et al. Angular velocities and linear accelerations derived from inertial measurement units can be used as proxy measures of knee variables associated with ACL injury. Sensors (Basel) 2022; 22: 9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Meulen FB, Klaassen B, Held J, et al. Objective evaluation of the quality of movement in daily life after stroke. Front Bioeng Biotechnol 2016; 3: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Z, Liparulo L, Panella M, et al. A fuzzy kernel motion classifier for autonomous stroke rehabilitation. IEEE J Biomed Health Inform 2016; 20: 893–901. [DOI] [PubMed] [Google Scholar]
- 54.Whitford M, Schearer E, Rowlett M. Effects of in home high dose accelerometer-based feedback on perceived and actual use in participants chronic post-stroke. Physiother Theory Pract 2020; 36: 799–809. [DOI] [PubMed] [Google Scholar]
- 55.Yang M, Cheng Y, Yue Y, et al. High-performance flexible pressure sensor with a self-healing function for tactile feedback. Adv Sci (Weinh) 2022; 9: e2200507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JY, Sun W, Park E, et al. Day/night difference in extradural cortical stimulation for motor relearning in a subacute stroke rat model. Restor Neurol Neurosci 2016; 34: 379–387. [DOI] [PubMed] [Google Scholar]
- 57.Barsi GI, Popovic DB, Tarkka IM, et al. Cortical excitability changes following grasping exercise augmented with electrical stimulation. Exp Brain Res 2008; 191: 57–66. [DOI] [PubMed] [Google Scholar]
- 58.Choudhury S, Singh R, Shobhana A, et al. A novel wearable device for motor recovery of hand function in chronic stroke survivors. Neurorehabil Neural Repair 2020; 34: 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Del-Ama AJ, Gil-Agudo A, Pons JL, et al. Hybrid FES-robot cooperative control of ambulatory gait rehabilitation exoskeleton. J Neuroeng Rehabil 2014; 11: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore SA, Da Silva R, Balaam M, et al. Wristband Accelerometers to motiVate arm Exercise after Stroke (WAVES): study protocol for a pilot randomized controlled trial. Trials 2016; 17: 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, Zhang X, Gong Y, et al. Motor function evaluation of hemiplegic upper-extremities using data fusion from wearable inertial and surface EMG sensors. Sensors (Basel) 2017; 17: 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rangaraju S, Jovin TG, Frankel M, BASICS Study Group et al. Neurologic examination at 24 to 48 hours predicts functional outcomes in basilar artery occlusion stroke. Stroke 2016; 47: 2534–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Golaszewski SM, Bergmann J, Christova M, et al. Modulation of motor cortex excitability by different levels of whole-hand afferent electrical stimulation. Clin Neurophysiol 2012; 123: 193–199. [DOI] [PubMed] [Google Scholar]
- 64.Kang N, Idica J, Amitoj B, et al. Motor recovery patterns in arm muscles: coupled bilateral training and neuromuscular stimulation. J Neuroeng Rehabil 2014; 11: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim D, Kim B, Shin B, et al. Actuating compact wearable augmented reality devices by multifunctional artificial muscle. Nat Commun 2022; 13: 4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao S, Zhao Z, Huang R, et al. Discovering individual life style from anonymized WiFi scan lists on smartphones. IEEE Access 2019; 7: 22698–22709. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.