Abstract
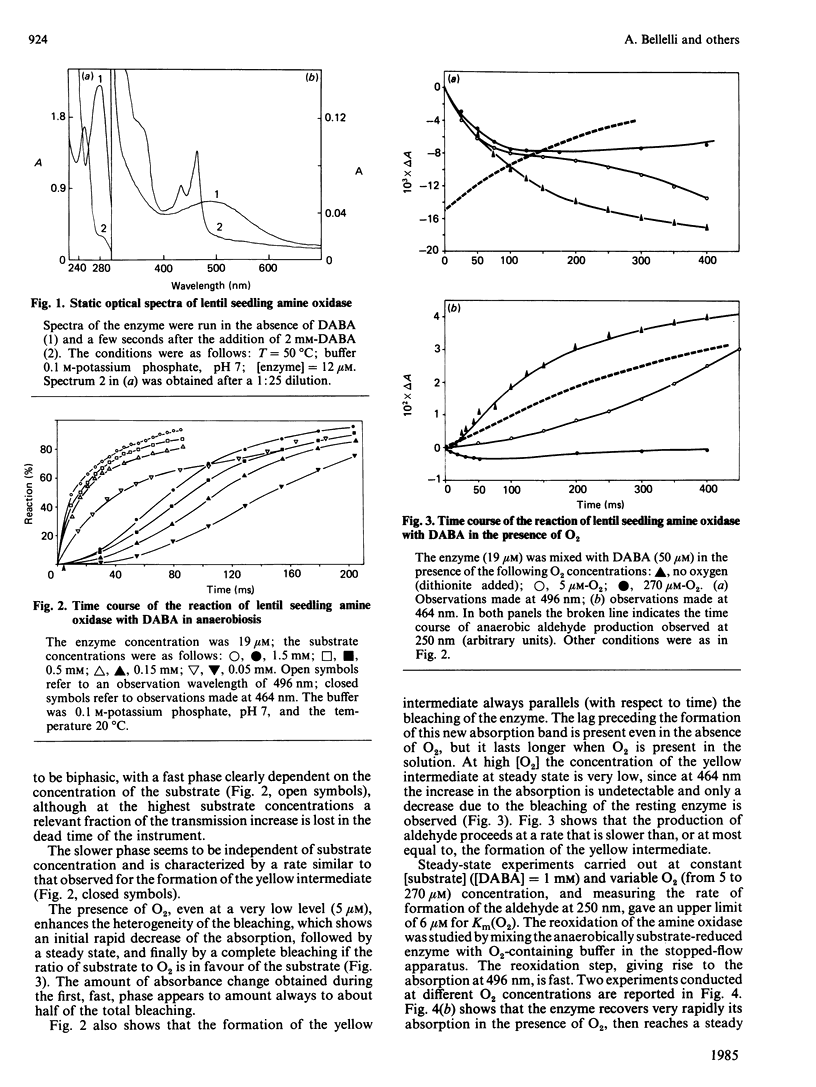
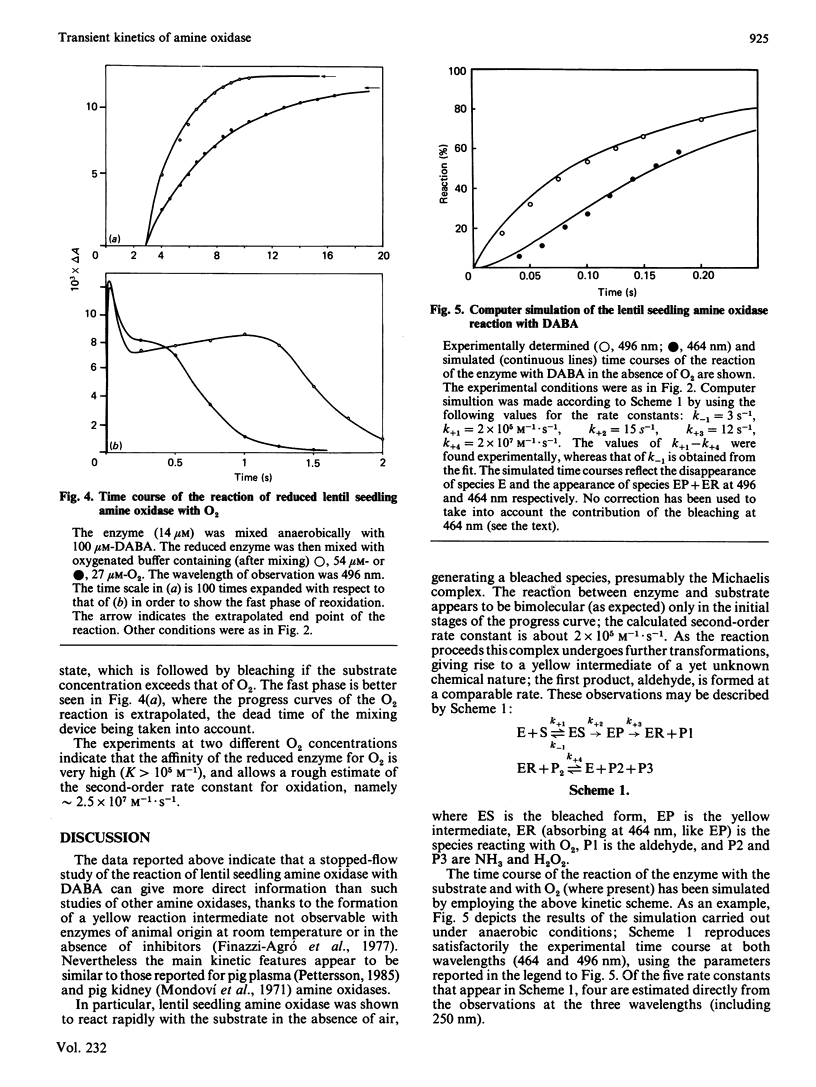
The reaction between lentil (Lens culinaris) seedling amine oxidase and its chromogenic substrate, p-dimethylaminomethylbenzylamine, has been studied by the stopped-flow technique. Upon being mixed with substrate in the absence of oxygen, the enzyme is bleached in a complex kinetic process. A yellow intermediate absorbing at 464 nm and the first product (aldehyde) are formed in subsequent steps. When oxygenated buffer is mixed with substrate-reduced amine oxidase, the 496 nm absorption of the oxidized enzyme is very rapidly restored in a second-order process (k = 2.5 X 10(7) M-1 X S-1). This reaction is appreciable even at very low oxygen concentration, in keeping with the fairly low Km for O2 measured by steady-state kinetics.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrò A. F., Guerrieri P., Costa M. T., Mondovì B. On the nature of chromophore in pig kidney diamine oxidase. Eur J Biochem. 1977 Apr 15;74(3):435–440. doi: 10.1111/j.1432-1033.1977.tb11409.x. [DOI] [PubMed] [Google Scholar]
- Bardsley W. G., Crabbe M. J., Shindler J. S., Ashford J. S. Oxidation of p-dimethylaminomethylbenzylamine by pig kidney diamine oxidase. A new method for spectrophotometric assay. Biochem J. 1972 May;127(5):875–879. doi: 10.1042/bj1270875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q. H., Milnes L. Apparatus for rapid and sensitive spectrophotometry. Biochem J. 1964 Apr;91(1):161–171. doi: 10.1042/bj0910161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobenstein-Verbeek C. L., Jongejan J. A., Frank J., Duine J. A. Bovine serum amine oxidase: a mammalian enzyme having covalently bound PQQ as prosthetic group. FEBS Lett. 1984 May 21;170(2):305–309. doi: 10.1016/0014-5793(84)81333-2. [DOI] [PubMed] [Google Scholar]
- Rinaldi A., Giartosio A., Floris G., Medda R., Finazzi Agrò A. Lentil seedlings amine oxidase: preparation and properties of the copper-free enzyme. Biochem Biophys Res Commun. 1984 Apr 16;120(1):242–249. doi: 10.1016/0006-291x(84)91440-2. [DOI] [PubMed] [Google Scholar]



