Abstract
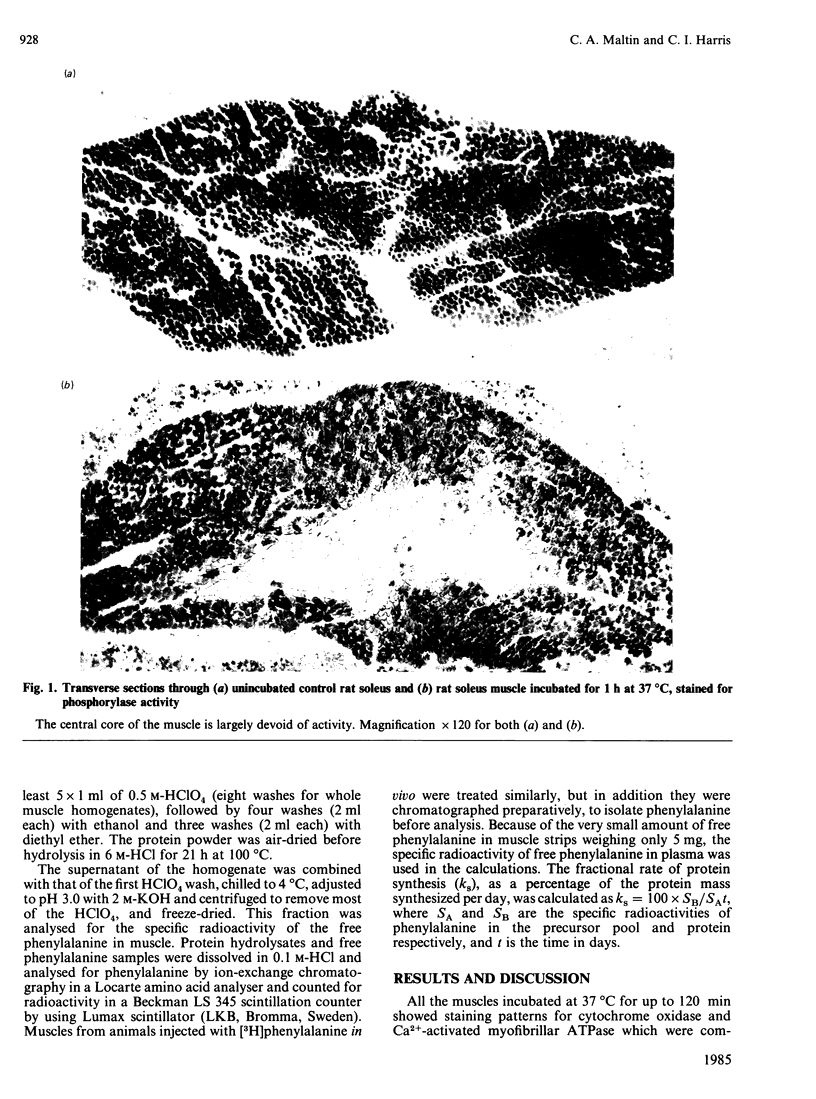
Isolated soleus and extensor digitorum longus muscles from small (40 or 70 g) rats developed a central and substantial (13-57%) loss of glycogen and alpha-glucan phosphorylase activity after incubation for up to 2 h in vitro. The central 'core' of the muscles showed a marked decrease in the rate of protein synthesis. It is suggested that during brief periods of incubation the central core of isolated rat muscles becomes hypoxic, and that consequently the viability of such muscles must be in question.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURSTONE M. S. Histochemical demonstration of cytochrome oxidase with new amine reagents. J Histochem Cytochem. 1960 Jan;8:63–70. doi: 10.1177/8.1.63. [DOI] [PubMed] [Google Scholar]
- Baracos V. E., Wilson E. J., Goldberg A. L. Effects of temperature on protein turnover in isolated rat skeletal muscle. Am J Physiol. 1984 Jan;246(1 Pt 1):C125–C130. doi: 10.1152/ajpcell.1984.246.1.C125. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Preedy V. R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980 Nov 15;192(2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Martel S. B., Kushmerick M. J. In vitro preparations of the diaphragm and other skeletal muscles. Methods Enzymol. 1975;39:82–94. doi: 10.1016/s0076-6879(75)39012-5. [DOI] [PubMed] [Google Scholar]
- Goldspink D. F., Garlick P. J., McNurlan M. A. Protein turnover measured in vivo and in vitro in muscles undergoing compensatory growth and subsequent denervation atrophy. Biochem J. 1983 Jan 15;210(1):89–98. doi: 10.1042/bj2100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. I., Maltin C. A., Palmer R. M., Reeds P. J., Wilson A. B. Biochemical and morphological observations of skeletal muscles incubated in vitro. Prog Clin Biol Res. 1985;180:637–639. [PubMed] [Google Scholar]
- Hayashi M., Freiman D. G. An improved method of fixation for formalin-sensitive enzymes with special reference to myosin adenosine triphosphatase. J Histochem Cytochem. 1966 Aug;14(8):577–581. doi: 10.1177/14.8.577. [DOI] [PubMed] [Google Scholar]
- Kauffman F. C., Albuquerque E. X. Effect of ischemia and denervation on metabolism of fast and slow mammalian skeletal muscle. Exp Neurol. 1970 Jul;28(1):46–63. doi: 10.1016/0014-4886(70)90161-5. [DOI] [PubMed] [Google Scholar]
- Li J. B., Goldberg A. L. Effects of food deprivation on protein synthesis and degradation in rat skeletal muscles. Am J Physiol. 1976 Aug;231(2):441–448. doi: 10.1152/ajplegacy.1976.231.2.441. [DOI] [PubMed] [Google Scholar]
- Libby P., Goldberg A. L. Effects of chymostatin and other proteinase inhibitors on protein breakdown and proteolytic activities in muscle. Biochem J. 1980 Apr 15;188(1):213–220. doi: 10.1042/bj1880213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. L., Engel W. K. Dependency of histochemical phosphorylase staining on amount of cellular glycogen. J Histochem Cytochem. 1972 Jun;20(6):476–479. doi: 10.1177/20.6.476. [DOI] [PubMed] [Google Scholar]
- Meijer A. E. Improved histochemical method for the demonstration of the activity of a-glucan phosphorylase. II. Relation of molecular weight of glucosyl acceptor dextran to activation of phosphorylase. Histochemie. 1968;16(2):134–143. doi: 10.1007/BF00280609. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Reeds P. J., Lobley G. E., Smith R. H. The effect of intermittent changes in tension on protein and collagen synthesis in isolated rabbit muscles. Biochem J. 1981 Sep 15;198(3):491–498. doi: 10.1042/bj1980491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preedy V. R., Pain V. M., Garlick P. J. The metabolic state of muscle in the isolated perfused rat hemicorpus in relation to rates of protein synthesis. Biochem J. 1984 Mar 1;218(2):429–440. doi: 10.1042/bj2180429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeds P. J., Palmer R. M., Smith R. H. Protein and collagen synthesis in rat diaphragm muscle incubated in vitro: the effect of alterations in tension produced by electrical or mechanical means. Int J Biochem. 1980;11(1):7–14. doi: 10.1016/0020-711x(80)90274-8. [DOI] [PubMed] [Google Scholar]
- Segal S. S., Faulkner J. A. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol. 1985 Mar;248(3 Pt 1):C265–C270. doi: 10.1152/ajpcell.1985.248.3.C265. [DOI] [PubMed] [Google Scholar]
- Seider M. J., Kapp R., Chen C. P., Booth F. W. The effects of cutting or of stretching skeletal muscle in vitro on the rates of protein synthesis and degradation. Biochem J. 1980 Apr 15;188(1):247–254. doi: 10.1042/bj1880247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shangraw R. E., Turinsky J. Effect of disuse and thermal injury on protein turnover in skeletal muscle. J Surg Res. 1982 Oct;33(4):345–355. doi: 10.1016/0022-4804(82)90048-8. [DOI] [PubMed] [Google Scholar]
- Tischler M. E., Fagan J. M. Response to trauma of protein, amino acid, and carbohydrate metabolism in injured and uninjured rat skeletal muscles. Metabolism. 1983 Sep;32(9):853–868. doi: 10.1016/0026-0495(83)90198-1. [DOI] [PubMed] [Google Scholar]
- Van Venrooij W. J., Poort C., Kramer M. F., Jansen M. T. Relationship between extracellular amino acids and protein synthesis in vitro in the rat pancreas. Eur J Biochem. 1972 Nov 7;30(3):427–433. doi: 10.1111/j.1432-1033.1972.tb02114.x. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Veerkamp J. H. The effect of starvation on branched-chain 2-oxo acid oxidation in rat muscle. Biochem J. 1984 Apr 1;219(1):253–260. doi: 10.1042/bj2190253. [DOI] [PMC free article] [PubMed] [Google Scholar]