Abstract
Background
Hyperbaric oxygen therapy (HBOT) is used as a treatment for acute wounds (such as those arising from surgery and trauma). However, the effects of HBOT on wound healing are unclear.
Objectives
To determine the effects of HBOT on the healing of acute surgical and traumatic wounds.
Search methods
We searched the Cochrane Wounds Group Specialised Register (searched 9 August 2013); the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 12); Ovid MEDLINE (2010 to July Week 5 2013); Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, August 08, 2013); Ovid EMBASE (2010 to 2013 Week 31); EBSCO CINAHL (2010 to 8 August 2013).
Selection criteria
Randomised controlled trials (RCTs) comparing HBOT with other interventions such as dressings, steroids, or sham HOBT or comparisons between alternative HBOT regimens.
Data collection and analysis
Two review authors conducted selection of trials, risk of bias assessment, data extraction and data synthesis independently. Any disagreements were referred to a third review author.
Main results
Four trials involving 229 participants were included. The studies were clinically heterogeneous, which precluded a meta‐analysis.
One trial (48 participants with burn wounds undergoing split skin grafts) compared HBOT with usual care and reported a significantly higher complete graft survival associated with HBOT (95% healthy graft area risk ratio (RR) 3.50; 95% confidence interval (CI) 1.35 to 9.11). A second trial (10 participants in free flap surgery) reported no significant difference between graft survival (no data available). A third trial (36 participants with crush injuries) reported significantly more wounds healed (RR 1.70; 95% CI 1.11 to 2.61), and significantly less tissue necrosis (RR 0.13; 95% CI 0.02 to 0.90) with HBOT compared to sham HBOT. The fourth trial (135 people undergoing flap grafting) reported no significant differences in complete graft survival with HBOT compared with dexamethasone (RR 1.14; 95% CI 0.95 to 1.38) or heparin (RR 1.21; 95% CI 0.99 to 1.49).
Many of the predefined secondary outcomes of the review were not reported. All four trials were at unclear or high risk of bias.
Authors' conclusions
There is a lack of high quality, valid research evidence regarding the effects of HBOT on wound healing. Whilst two small trials suggested that HBOT may improve the outcomes of skin grafting and trauma, these trials were at risk of bias. Further evaluation by means of high quality RCTs is needed.
Keywords: Humans, Hyperbaric Oxygenation, Wound Healing, Acute Disease, Burns, Burns/therapy, Graft Survival, Graft Survival/drug effects, Randomized Controlled Trials as Topic, Skin Transplantation, Wounds and Injuries, Wounds and Injuries/therapy
Plain language summary
Hyperbaric oxygen therapy for acute surgical and traumatic wounds
Acute surgical and traumatic wounds occur as a result of a trauma or surgical procedures and whilst many heal uneventfully, sometimes poor local blood supply, infection, damage to the blood vessels, or a combination of factors result in these acute wounds taking longer to heal. Hyperbaric oxygen therapy (HBOT), which involves placing the person in an airtight chamber and administering 100% oxygen at a pressure greater than 1 atmosphere, is sometimes used with the aim of speeding wound healing. The aim is to bathe all fluids, tissues and cells of the body in a high concentration of oxygen.
This review did not find any high quality research evidence showing that HBOT is beneficial for wound healing. Two poor quality studies suggested benefits associated with HBOT. The first, in patients with crush injuries, showed improved wound healing and fewer adverse outcomes. The second reported improved survival of split skin grafts in people with burn wounds. Two trials reported no benefits associated with HBOT for either skin grafting or free flap surgery.
Further, better quality research is needed to determine the effects of HBOT on wound healing.
Background
Local and systemic treatment of wounds is characterised by a wide range of therapeutic strategies and large variations in clinical opinion and practice. One of those systemic strategies is hyperbaric oxygen therapy (HBOT).
The first documented application of hyperbaric oxygen can be traced back to 1662, when Henshaw used compressed air to treat multiple diseases (Kindwall 2002). In more recent times Boerema began to study the possible applications of HBOT to surgery, in particular as an aid in open heart surgery (Boerema 1964; Boerema 1965). Since then, interest in this therapy has increased with the result that several centres throughout the world have built high‐pressure chambers (Boerema 1965).
Description of the condition
‘Acute’ wounds are defined as skin injuries that occur as a result of a trauma or surgery and healing usually proceeds normally without complications (Lazarus 1994). Lazarus suggested that acute wounds proceed through to healing “in an orderly and timely reparative process”. Orderliness refers to the healing sequence of inflammation, angiogenesis (the forming of new blood vessels), matrix deposition, wound contraction, epithelialisation and scar remodelling. Timeliness is subjective, but refers to a healing time that could be reasonably expected (Jull 2008).
Acute wounds are more common than chronic wounds and costs increase when complications occur (Franz 2007). Surgical incisions usually heal by primary intention, assisted by sutures, clips etc. Healing by secondary intention occurs when an open wound heals from the base upwards by deposition of new tissue (Vermeulen 2004). This kind of wound healing may occur if there is a tissue deficit and the edges of the wound cannot be brought together, and where wounds are compromised by a poor local blood supply (e.g. ischaemic wounds, skin grafts and flaps), infection (e.g. gas gangrene), and/or damage to the blood vessels (e.g. lacerations, crush, stab and biopsy wounds) (MacFarlane 2001). In such conditions these wounds may prove difficult to heal and additional treatments, including HBOT, have been promoted as assisting the wound healing process.
Description of the intervention
HBOT is defined as the use of 100% oxygen at pressures above 1 atmosphere in order to improve or correct conditions (Bennett 2008). According to the International System of Units (SI), pressure is expressed in KiloPascal (kPa). However, in order to be consistent with other studies which use HBOT we also consider ATA (ATmosphere Absolute) as a measurement for pressure. One ATA is equal to 101,325 kPa.
There are two kinds of HBOT chamber:
a monoplace chamber which is filled with pressurised oxygen and accommodates a single person who directly breathes pure oxygen (Niinikoski 2003);
a multiplace chamber which is filled with pressurised air and can accommodate several people and/or healthcare personnel. The person being treated breathes oxygen through a mask or head tent (Boerema 1964; Villanueva 2004; Wang 2003).
The application of HBOT involves bathing all fluids, tissues and cells of the body in a high concentration of oxygen. HBOT enhances the transportation of oxygen by increasing the oxygen saturation in the blood (mainly as oxyhaemoglobin).
People being treated with HBOT are placed in an airtight chamber where the oxygen pressure is increased and 100% oxygen is administered for respiration. Thus, it is possible to deliver a significantly increased partial pressure of oxygen to the tissues. Treatment involves increasing the pressure up to 1.5 to 3.0 ATA, for a time frame of between 60 to 120 minutes at least once a day, but sometimes there are multiple treatment periods. However, oxygen in high doses is toxic to normally perfused tissue, particularly in the brain and lungs and therefore regular HBOT sessions should not last longer than one to two hours (Kranke 2012). The period of treatment may range from less than one week to several months, the average being two to four weeks. A typical course might involve 15 to 30 such treatments (Kranke 2012).
Using clinical assessment and investigations (e.g. transcutaneous oxygen measurements) designed to confirm significant peri‐wound hypoxia, hyperbaric practitioners attempt to select wounds in which a response to HBOT is considered likely (Hess 2003; Kranke 2012).
Adverse effects
Oxygen in high doses can be toxic, particularly in richly perfused tissue such as the brain (acute cerebral oxygen toxicity) and lungs (chronic pulmonary oxygen toxicity). Acute cerebral toxicity occurs in approximately 1 in 2000 exposures and does not appear to result in any permanent injury, whilst pulmonary changes are dose‐related and reversible at doses used therapeutically. Other potential risks associated with HBOT include damage to the ears, sinuses and lungs from the effect of pressure (barotrauma) and the psychological effect of confinement (Roeckl‐Wiedmann 2005). Whilst serious adverse events are rare, HBOT cannot be regarded as benign (Bennett 2005a).
Indications for hyperbaric oxygen therapy
HBOT is used mainly as a treatment for gas gangrene, Fournier's gangrene, chronic wounds, crush injuries, decompression sickness and carbon monoxide (CO) poisoning. There are several systematic reviews describing the effectiveness of HBOT. HBOT has been recommended as a treatment for poorly healing wounds, anaemia due to blood loss, necrotising soft‐tissue infections, refractory osteomyelitis, radio‐necrosis and intracranial abscesses (MacFarlane 2001). HBOT has been used for treating acute and chronic wounds, including surgical wounds, penetrating wounds, lacerations, burns, skin transplantation, open fractures, gas gangrene and diabetic foot ulcers (Bouachour 1996; Kranke 2012; Villanueva 2004).
The potential value of HBOT for diabetic foot ulcers was investigated in a systematic review by Kranke 2012 which found a significantly reduced risk of major amputation and improved healing at one year associated with HBOT. Another systematic review concluded that people with chronic wounds receiving HBOT are one‐third as likely to require such an amputation in comparison to controls and it is estimated that only four patients need to be treated with HBOT to avoid one major amputation (Roeckl‐Wiedmann 2005).
However, the evidence for the effectiveness of HBOT for acute surgical and traumatic wounds has never been subjected to systematic review.
How the intervention might work
HBOT has been proposed as a useful adjunct in the treatment of problematic wounds and has been shown to cause hyper‐oxygenation of normal tissue and of tissue with poor blood perfusion. Partial arterial oxygen pressures greater than 1000 mmHg are routinely achieved during HBOT. Such oxygen tensions in plasma have been suggested to cause up‐regulation of growth factors, down‐regulation of inflammatory cytokines, increased fibroblast activation, angiogenesis, antibacterial effects and enhanced antibiotic action (Roeckl‐Wiedmann 2005).
Why it is important to do this review
Many claims have been made regarding the effectiveness of HBOT as an adjunctive or primary treatment in wound healing and it is important to test these claims by summarising the evidence systematically.
Objectives
To summarise the evidence for the effects of HBOT as a treatment for acute surgical and traumatic wounds.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) evaluating the effects of hyperbaric oxygen therapy for acute surgical and traumatic wounds.
Types of participants
Trials recruiting people with acute wounds (e.g. surgical wounds, penetrating wounds, lacerations, skin transplantations (surgical procedure), animal bites and traumatic wounds). The review excludes trials involving patients with open fractures and burns since these patient groups are the focus of separate Cochrane Reviews (Villanueva 2004; Bennett 2005b).
Types of interventions
Trials evaluating HBOT delivered in a single, or multiplace chamber, at a greater than atmospheric pressure, as a treatment for acute wounds.
Eligible comparisons were:
HBOT compared with any other intervention, such as dressings, steroids, or sham HBOT;
Comparisons between different HBOT regimens.
Types of outcome measures
Primary outcomes
Wound healing, measured objectively, e.g.: time to complete healing (days), number of wounds completely healed at a specified time point (proportion).
Adverse effects, e.g.: visual disturbance (such as reversible myopia), barotrauma, oxygen toxicity, infection, re‐operations.
Secondary outcomes
Survival of flap, graft or split skin graft (percentage).
Mortality (HBOT‐related; within one month after trauma or surgical procedure)
Pain scores (e.g. Visual Analogue Scale (VAS)).
Quality of life (QoL).
Patient satisfaction (using any questionnaire).
Activities of daily living (ADL) (e.g. BAI, Barthel's Index of Activities of Daily Living).
Transcutaneous oxygen pressure (TcpO2) measurements (Ubbink 1997).
Major and minor amputations (within one month after trauma or surgical procedure).
Length of hospital stay (days).
Costs (medical and non‐medical costs).
Search methods for identification of studies
Electronic searches
The search strategy for the original review is shown in Appendix 1.
For this first update we searched the following databases for reports of eligible trials:
The Cochrane Wounds Group Specialised Register (searched 9 August 2013);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 12);
Ovid MEDLINE (2010 to July Week 5 2013);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, August 08, 2013);
Ovid EMBASE (2010 to 2013 Week 31);
EBSCO CINAHL (2010 to 8 August 2013).
The following search string was used to search the Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor: [Acute Disease] explode all trees 8343 #2 MeSH descriptor: [Wounds and Injuries] explode all trees 13364 #3 (#1 and #2) 312 #4 MeSH descriptor: [Surgical Wound Infection] explode all trees 2521 #5 MeSH descriptor: [Surgical Wound Dehiscence] explode all trees 330 #6 MeSH descriptor: [Wounds, Penetrating] explode all trees 309 #7 MeSH descriptor: [Lacerations] explode all trees 79 #8 MeSH descriptor: [Burns] explode all trees 1078 #9 MeSH descriptor: [Bites and Stings] explode all trees 189 #10 MeSH descriptor: [Skin Transplantation] explode all trees 339 #11 MeSH descriptor: [Fractures, Open] explode all trees 77 #12 MeSH descriptor: [Gas Gangrene] explode all trees 5 #13 ((surgical next wound*) or (incised next wound*)):ti,ab,kw 3138 #14 (laceration* or gunshot or (gun next shot) or stab or stabbing or stabbed or bite* or bitten):ti,ab,kw 1501 #15 ((traumatic next wound*) or (acute next wound*)):ti,ab,kw 75 #16 ((mechanical next trauma) or polytrauma):ti,ab,kw 73 #17 ((thermal or blast or crush or avulsion) next injur*):ti,ab,kw 152 #18 ("burn" or "burns" or burned or scald*):ti,ab,kw 3205 #19 acute next wound*:ti,ab,kw 64 #20 acute next ulcer*:ti,ab,kw 90 #21 ((donor next site*) or (skin next graft*)):ti,ab,kw 639 #22 ((open next fracture*) or (compound next fracture*)):ti,ab,kw 96 #23 "gas gangrene":ti,ab,kw 7 #24 experimental next wound*:ti,ab,kw 12 #25 "skin infarction":ti,ab,kw 0 #26 skin next flap*:ti,ab,kw 60 #27 #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 9293 #28 MeSH descriptor: [Hyperbaric Oxygenation] explode all trees333 #29 (hyperbaric* next oxygen*):ti,ab,kw 598 #30 (HBO or HBOT):ti,ab,kw 166 #31 (high next pressure next oxygen*):ti,ab,kw 11 #32 (#28 or #29 or #30 or #31) 616 #33 #27 and #32 28
This strategy was adapted where necessary to search Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL. These search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL are shown in Appendix 2; Appendix 3; Appendix 4 respectively. The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). The Ovid EMBASE and EBSCO CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (Sign 2012). There were no restrictions on the basis of date or language of publication. We contacted the Trials Search Co‐ordinator of the Cochrane Wounds Group to assist with the development of the various search strategies.
Searching other resources
We searched the reference lists of relevant studies identified to find other potentially relevant studies. We contacted a translation institute to translate a study from Chinese to Dutch. For the original review and the update we contacted local experts in the field (Dr. Lubbers and Dr. van den Brink) for any information about unpublished studies.
Data collection and analysis
Selection of studies
Two review authors (AE and HV) independently selected potentially relevant studies based on the titles and abstracts of the articles retrieved by the search. We obtained full‐text versions of articles after this initial assessment if they matched the inclusion criteria or if further scrutiny was needed to make a decision with regards to inclusion/exclusion. The same review authors made the final selection of studies to be included independently. A third review author (DU) arbitrated any discrepancies.
Data extraction and management
Two review authors (AE and HV) independently extracted data from the included studies using a data extraction sheet. If data were missing from reports, or clarification was needed, we contacted the study authors in an effort to obtain missing information. Data from studies published in duplicate were included only once.
We extracted the following data:
characteristics of the trial (setting, location of care, country, source of funding);
participants (number, age, sex, type of wound, wound size, duration of wound, length of follow‐up, concurrent illnesses);
intervention (including intensities and frequency);
comparison intervention;
results of all relevant outcomes in all groups (intervention and control).
Assessment of risk of bias in included studies
For the update of this review as well as the first version of this review, two review authors (AE and HV) assessed the included studies for risk of bias using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (e.g. baseline imbalance, financial support) (see Appendix 5 for details of criteria on which the judgement was based). We assessed blinding and completeness of outcome data for each outcome separately. We completed a 'Risk of bias' table for each eligible study. Any disagreement was discussed amongst all authors to achieve a consensus.
We presented assessment of risk of bias using a 'Risk of bias' summary figure, which presents all of the judgements in a cross‐tabulation of study by entry. This display of internal validity indicates the weight the reader may give the results of each study. We classified studies as being at high risk of bias if at least one of the criteria was judged to be at high risk (e.g. blinding of outcome assessor and intention‐to‐treat (ITT) analysis), with the exception of blinding participants and caregivers. Although it is possible to blind patients and caregivers, the feasibility of sham‐HBOT is debatable.
Measures of treatment effect
We calculated summary estimates of treatment effect (with 95% confidence interval (CI)) for every comparison. For continuous outcomes, we presented the mean difference (MD) when appropriate. For dichotomous outcomes, we presented the risk ratio (RR). To present numbers needed to treat to benefit (NNT) and number needed to treat to harm (NNH) in case of a significant difference in RR, we calculated risk differences (RD), which is an absolute effect measure that expresses the difference between the experimental and the control event rates. We planned to compare time to event data (e.g. time to complete healing) using hazard ratios (HR) or by dichotomising the data (e.g. comparing healed wounds with non‐healed wounds).
Assessment of heterogeneity
We used a logical model based on the PICO framework to assess the clinical heterogeneity (Ioannidis 2008). Two review authors independently assessed the (dis)similarity of the interventions, outcomes, designs, participants characteristics and settings.
Data synthesis
Data were entered into and analysed using Cochrane RevMan software (Review Manager 5) (RevMan 2011) by one review author (AE), checked by another review author (HV). Methods of synthesising the studies depended on the quality, design and heterogeneity of the studies identified. If the studies were clinically homogeneous, we identified statistical heterogeneity by eyeballing and assessing the value of the I² statistic. If the I² statistic was 30% or less, we used a fixed‐effect model, if the I² was between 31% and 60% we used a random‐effects model, and if the I² was greater than 60% we deemed pooling inappropriate due to high statistical heterogeneity (Deeks 2011). If pooling was appropriate we presented dichotomous outcomes as risk ratios (RR) with 95% CIs.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses for each wound type were planned, but were not conducted because of the small number of studies and the clinical heterogeneity of wound types.
Results
Description of studies
Results of the search
For this first update of the original review article (Eskes 2010) one additional RCT was identified and included (Vishwanath 2011) (Figure 1). One additional study was excluded (Jian 2011).
1.
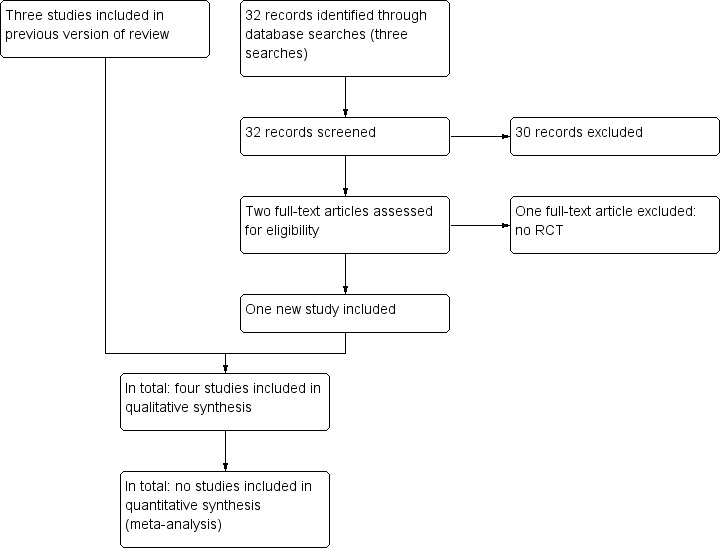
Study flow diagram.
The initial search (n=141) and the searches for the update (n=32) identified four relevant articles (Figure 1). Screening the references of relevant papers did not yield any additional hits. One full‐text article (Xie 2007) was translated from Chinese to Dutch by a translation institute.
Included studies
Four studies met the inclusion criteria for this review, which are described in in the Characteristics of included studies table and summarised below.
The trials ranged in size from 10 to 135 participants, with a total of 229 participants (see Characteristics of included studies).
Perrins 1967 recruited 48 participants with split skin grafts (SSG) who were randomised to either HBOT or to usual care. All participants received closed dressings of paraffin gauze, cotton wool and bandages applied to the operation and donor sites. HBOT treatment consisted of 100% oxygen at 2 ATA for two hours, twice during the first day and once during the next three days. The outcomes measured were wound healing by graft survival (defined as at least 95% take) at day seven.
Bouachour 1996 included 36 participants with crush injuries who were randomised either to HBOT or to sham HBOT. HBOT consisted of sessions of 100% oxygen at 2.5 ATA for 90 minutes, twice daily for six days. Sham HBOT consisted of sessions of 21% oxygen at 1.1 ATA for 90 minutes, twice daily for six days. The outcomes measured were complete wound healing without tissue necrosis requiring surgical excision, time to complete healing (days), length of hospital stay (days), TcpO2 measurements (at the first, fourth, eight and twelfth session) and the number of amputations (exact time point unclear).
Xie 2007 included 135 participants who underwent flap grafting for limb skin defects (not further specified) and were randomised to one of three study arms: HBOT, dexamethasone or a local injection of heparin. The aim of each of the three treatments was to improve the arterial inflow following flap grafting. HBOT consisted of two sessions a day for 3 days and thereafter once a day (a total of 6 to 12 treatments). The intravenous dexamethasone infusion began on the day of operation and stopped at day 6. Participants in the third study arm received subcutaneous local heparin (200 U/ml NS) for 6 days. The outcome measured was wound healing in terms of flap survival at seven days. The definition of wound healing was unclear, but seemed to be based on clinical assessment of colour of the skin, temperature of the skin, capillary refill test, arterial pulsations and bleeding of the flap.
Vishwanath 2011 included 10 participants who underwent flap grafting and were randomised to either HBOT or to usual care. HBOT consisted of one session a day at 2.5 ATA, for seven days, starting on the first postoperative day. The outcomes measured were flap loss, flap oedema, venous congestion, and duration of postoperative recovery. Patients were evaluated up to 14 days postoperatively.
Excluded studies
Five studies are excluded from the review (see Characteristics of excluded studies). Four studies (Hart 1974; Brannen 1997; Niezgoda 1997; Xu N 1999) studied HBOT as a treatment for burn wounds, which is the subject of a separate Cochrane Review (Villanueva 2004). One study was not an RCT (Jian 2011) (see Characteristics of excluded studies).
Risk of bias in included studies
A description of the assessment of random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues of potential bias is reported in the 'Risk of bias' tables. We summarised the results of the methodological quality assessment (Figure 2; Figure 3).
2.
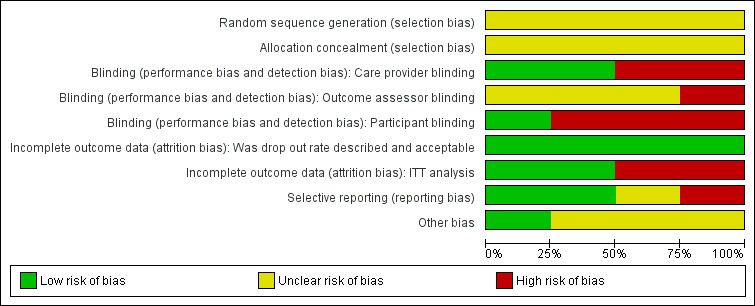
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
In all four studies the authors stated that patients were allocated randomly to treatment. In all studies individual patients (i.e. not wounds) were allocated to treatment groups. None of the studies reported how their allocation sequence was generated, nor did they describe whether the allocation was concealed, which does not exclude the possibility of selection bias.
Blinding
Perrins 1967 blinded the surgeon to treatment allocation. Bouachour 1996 blinded surgeons and patients to treatment allocation. In the trial of Vishwanath 2011 the treatment (HBOT) and control groups (no HBOT) were distinguishable for patients and caregivers. Blinding of the outcome assessor for each outcome was unclear in all studies and it was unclear who actually conducted the outcome assessment. Xie 2007 reported that the study was not blinded. Inadequate blinding may affect our first primary outcome 'complete wound healing' more than our second primary outcome 'adverse events'.
Incomplete outcome data
Participants in all studies completed the follow‐up period and none reported missing outcome data. Bouachour 1996 and Xie 2007 analysed all randomised participants in the groups to which they had been allocated. However Perrins 1967 had a complete follow‐up data set, but excluded two patients from the analysis. For this study we were able to perform an intention‐to‐treat analysis post hoc by calculating the number of patients from the percentages given. Perrins reported percentages for the intervention group (n = 22; two patients excluded) and the control group (n = 24). The grafts of the two patients who were excluded failed completely and we have added these two results to the intervention group as a less than 60% healthy graft area. Thus, all patients were analysed in the groups to which they were assigned originally. Furthermore, Vishwanath 2011 also excluded two patients from the analysis since flap loss had occurred even before HBOT was exhibited to the case group. It was not possible to perform an ITT analysis post hoc. This last study may introduce bias due to excluding patients from analysis.
Selective reporting
No study protocols were available for any of the included trials.
Perrins 1967 measured percentage wound healing at day seven with multiple sub endpoints. This increases the risk of a type 1 error. Bouachour 1996 and Vishwanath 2011 described all outcome measurements in the methods section and these were subsequently reported in the results section. However, Bouachour 1996 presented subgroup analyses, which were not mentioned a priori in the methods section, but performed in the results section. Xie 2007 reported complete survival of the graft at day seven after the operation, but no other outcome measures.
Other potential sources of bias
Perrins 1967 and Xie 2007 did not report the characteristics of the participants at baseline, therefore we were unable to judge whether there was baseline comparability between groups in these two studies. Bouachour 1996 was supported by a research grant received from their hospital; there was no financial conflict of interest with respect to the trial outcome. In the trial of Vishwanath 2011 the co‐interventions were similar. Baseline comparability remained unclear. Many important baseline prognostic variables were not described.
Effects of interventions
Comparison 1: hyperbaric oxygen therapy (HBOT) compared with usual care (two RCTs, totaling 58 people)
Two studies (Perrins 1967; Vishwanath 2011) compared HBOT treatment with usual care for different indications. Perrins 1967 randomised 48 patients with split‐skin grafting (SSG) (HBOT n = 24; usual care n = 24). Vishwanath 2011 randomised 10 patients who required free flap grafting (HBOT n = 5; usual care n = 5).
Primary outcomes
Wound healing was not reported for this comparison in any study.
(1) Adverse effects
Flap oedema was measured as the duration to resolution of oedema (in days) in one study (Vishwanath 2011). Only average scores were given, which was two days in the HBO group compared to 2.5 days in the usual care group. Furthermore, they measured venous congestion, but found that venous congestion had settled before the case group was given HBOT (average HBOT 0.25 days; average usual care 0.5 days). For both outcomes, no significant differences were found. In each group one patient was excluded from the analysis since flap loss had occurred before HBOT was administered to the intervention group.
Secondary outcomes
Two different measures of graft survival were used:
(1) Complete survival (defined as at least 95% take)
Perrins 1967 assessed the healthy graft area after seven days. The area grafted was traced on sterile cellophane and similar tracings were made of the areas that were covered by permanently healthy graft area.The cellophane represented a successful 'take'. Fourteen of the 24 participants (58%) treated with HBOT compared with four of 24 control participants (17%) had a >95% healthy graft area (RR 3.50, 95% CI 1.35 to 9.11, NNT 2) which is statistically significant in favour of HBOT (Analysis 1.1).
1.1. Analysis.
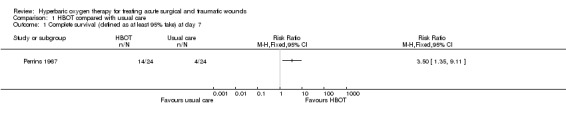
Comparison 1 HBOT compared with usual care, Outcome 1 Complete survival (defined as at least 95% take) at day 7.
(2) Graft loss
Mortality, pain scores, quality of life, patient satisfaction, activities of daily living, TcpO2 increase, amputation, length of hospital stay and costs were not reported for this comparison. Vishwanath 2011 defined graft loss as an unviable segment of tissue of any size in the flap. One flap in each group failed to survive beyond 24 hours of surgery. The authors of the trial excluded these two patients from the analysis because HBOT was not yet administered to the intervention group. No other data were available. However, the authors stated that there was no clinically relevant difference between the two treatment arms.
Vishwanath 2011 also reported on the period of post‐operative recovery, which was defined as the period of complete epithelialization and cessation of all discharge. However, this was not one of our predefined outcome parameters.
Comparison 2: HBOT compared with sham HBOT (one RCT, 36 people)
One study (Bouachour 1996) compared HBOT treatment (n = 18) with sham HBOT treatment (n = 18) in patients with crush injuries.
Primary outcomes
(1) Complete healing
Complete healing was described as the number of wounds healed without tissue necrosis requiring surgical excision (exact time point unclear). Complete healing was achieved in 17 patients (94%) treated with HBOT compared with 10 patients (56%) in the sham HBOT group, showing a statistically significant difference in favour of HBOT (RR 1.70, 95% CI 1.11 to 2.61, NNT 3) (Analysis 2.1).
2.1. Analysis.
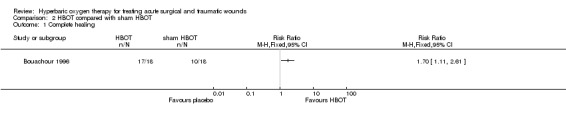
Comparison 2 HBOT compared with sham HBOT, Outcome 1 Complete healing.
(2) Time to healing
Time to complete healing was measured in days and presented as means with standard deviations. Whilst this approach is not ideal as it does not account for censoring, all participants were followed and included in the final analysis (Deeks 2011). There was no difference between the groups in mean time to healing: 50.2 (SD 21.1) days with HBOT versus 55.8 (SD 19.9) days with sham HBOT (MD ‐5.60, 95% CI ‐19.00 to 7.80) (Analysis 2.2).
2.2. Analysis.
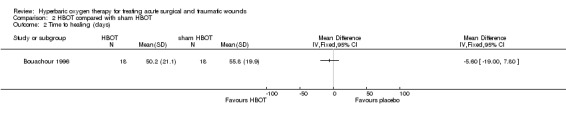
Comparison 2 HBOT compared with sham HBOT, Outcome 2 Time to healing (days).
(3) Adverse effects
Two additional surgical procedures (in one patient) were needed in the HBOT group compared with eight additional surgical procedures (in six patients) in the sham HBOT group, as the first operation did not have the desired effect (RR 0.17, 95% CI 0.02 to 1.25) (Analysis 2.3). One patient in the HBOT group and eight patients in the sham HBOT group developed necrotic tissue showing a statistically significant difference in favour of HBOT (tissue necrosis: RR 0.13, 95% CI 0.02 to 0.90, NNH 3) (Analysis 2.4).
2.3. Analysis.
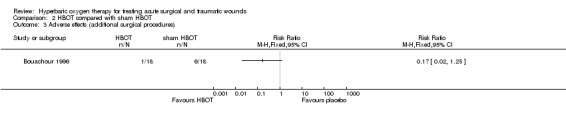
Comparison 2 HBOT compared with sham HBOT, Outcome 3 Adverse effects (additional surgical procedures).
2.4. Analysis.
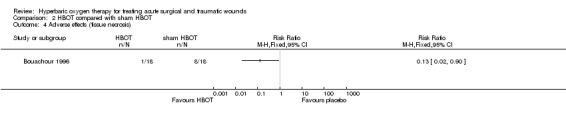
Comparison 2 HBOT compared with sham HBOT, Outcome 4 Adverse effects (tissue necrosis).
Secondary outcomes
(1) Amputation
There were no amputations in the HBOT group compared with two amputations in the sham HBOT group. No significant difference was found (RR 0.20, 95% CI 0.01 to 3.89) (Analysis 2.5).
2.5. Analysis.
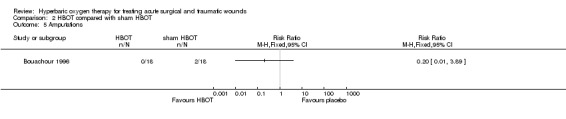
Comparison 2 HBOT compared with sham HBOT, Outcome 5 Amputations.
(2) Length of hospital stay
Length of hospital stay was measured in days and presented as means with standard deviations. Length of hospital stay was 22.4 (±12.4) days in the HBOT group compared with 22.9 (±16.3) days in the sham HBOT group and was not significantly different (MD ‐0.50, 95% CI ‐9.96 to 8.96) (Analysis 2.6).
2.6. Analysis.

Comparison 2 HBOT compared with sham HBOT, Outcome 6 Length of hospital stay.
Mortality, pain scores, quality of life, patient satisfaction, activities of daily living and costs were not reported. The study compared TcpO2 measurements between healing and non‐healing wounds, but they did not make a comparison between the HBOT and the sham HBOT group. The authors mentioned the Bilateral Perfusion Index (BPI = PtCO2 of the injured limb/PtCO2 of the uninjured limb), but this was not one of our predefined outcome parameters.
Comparison 3: HBOT compared with dexamethasone or heparin (one RCT, 135 people)
One study (Xie 2007) compared HBOT (n = 45) with dexamethasone (n = 45) and local heparin (n = 45) in patients with skin defects in the limbs and who underwent flap grafting.
Primary outcomes
Objective measurements of wound healing (such as time to complete healing and number of wounds completely healed) and adverse effects were not reported for this comparison.
Secondary outcomes
Two different comparisons of flap survival were reported.
(1) Complete survival of the flap, HBOT versus dexamethasone
Complete survival was measured at day seven after the operation. Forty out of 45 patients (89%) treated with HBOT compared with 35 out of 45 patients (78%) treated with dexamethasone had a complete survival of the graft (RR 1.14, 95% CI 0.95 to 1.38) (Analysis 3.1). No significant difference was found.
3.1. Analysis.
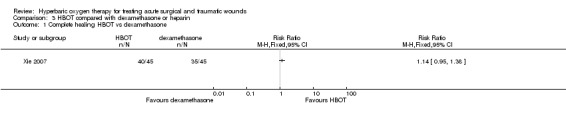
Comparison 3 HBOT compared with dexamethasone or heparin, Outcome 1 Complete healing HBOT vs dexamethasone.
(2) Complete survival of the flap, HBOT versus heparin
Forty out of 45 patients (89%) treated with HBOT compared with 33 out of 45 patients (73%) treated with local heparin (NSD) had a complete survival of the graft (Analysis 3.2 RR 1.21; 95% CI 0.99 to 1.49). Again, no significant difference was found.
3.2. Analysis.
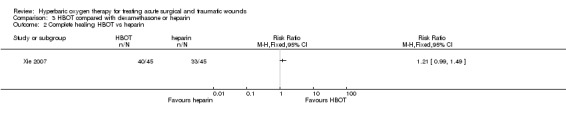
Comparison 3 HBOT compared with dexamethasone or heparin, Outcome 2 Complete healing HBOT vs heparin.
Mortality, pain scores, quality of life, patient satisfaction, activities daily living, TcpO2 increase, amputation, length of hospital stay and costs are not reported for this comparison.
Discussion
Summary of main results
This systematic review has identified insufficient evidence to support or refute the effectiveness of HBOT for the management of acute surgical or traumatic wounds. Only four RCTs were found, which could not be pooled due to clinical heterogeneity (difference comparisons and diverse outcome measures). Whilst two trials were suggestive of a benefit of HBOT for the healing of crush injuries (Bouachour 1996) and split skin graft survival (Perrins 1967), these trials were at unclear or high risk of bias. Therefore, we cannot be confident that these results reflect the true effect of HBOT. Furthermore the outcome of percentage graft survival is not meaningful for the patient who is interested in complete wound healing. Another small trial Vishwanath 2011 did not found differences in graft loss. The potentially beneficial effects of HBOT could not be weighed against its cost and possible logistical difficulties due to lack of data.
Overall completeness and applicability of evidence
There are some limitations to this review. Firstly the studies were small and had methodological limitations, making it difficult to draw conclusions about the effectiveness of HBOT. Second, the studies were not comparable; they evaluated different wound types and had different comparator treatments and reported outcomes. Due to of this lack of clinical homogeneity a meta‐analysis was inappropriate. Third, the studies did not all describe the primary outcomes of our review, as well as many predefined secondary outcomes, such as mortality, pain scores, quality of life, patient satisfaction, activities of daily living, TcpO2 increase, amputation, length of hospital stay and costs.
In addition to these methodological limitations there are practical issues: facilities capable of administering HBOT are relatively scare and conducting a large randomised controlled trial could therefore be logistically difficult in an acute setting. The applicability of the evidence may be limited by the fact that the four included studies were published over a 40‐year period up to 2011. Although the HBO technique is well‐established and has not changed substantially over time the effectiveness of the comparators may well have changed substantially over time, making the relative benefits of HBOT change.
Quality of the evidence
The included studies were generally small with a short follow‐up period (six to seven days). Several reporting and methodological problems were observed in the assessment of the studies, particularly in the study by Xie 2007). The first problem is that none of the included studies described the method of allocation concealment, which is considered to be one of the most important causes of bias in a randomised controlled trial (Altman 2001). Without allocation concealment a properly developed random allocation sequence can be subverted. Proper allocation concealment secures strict implementation of a random allocation sequence without foreknowledge of treatment assignments (Schulz 2002a). The second problem is that in some of the trials the outcome assessor was not blinded to the intervention and this can result in measurement bias (Schulz 2002b). Another methodological problem was the lack of an ITT analysis in one study (Perrins 1967; Vishwanath 2011), which may have resulted in an overestimation of the treatment effect (Ruiz‐Canela 2000).
Potential biases in the review process
Although we have made efforts to locate unpublished studies, it remains possible that this review is subject to positive publication bias, with generally favourable studies more likely to be published. Many of the predefined (primary and secondary) outcomes were not reported in the included studies. Therefore we cannot report data relating to risk, cost or patient satisfaction to weigh against the benefit estimated.
Agreements and disagreements with other studies or reviews
We have excluded burns and fractures from the acute wounds we aimed for. Both have already been the subject of separate Cochrane Reviews. A systematic review by Villanueva focused on the treatment of HBOT for burns. Based on data from two trials, the review found little evidence that burn patients benefit from HBOT (Villanueva 2004). The Bennett review focused on HBOT for the management of delayed union or established non‐union of bony fractures, but found no relevant clinical evidence (Bennett 2005b). In our study we also found a small number of studies, but we did find some evidence that patients with crush injuries appear to benefit from HBOT. We also found a significant beneficial result for the percentage survival of split skin grafts. Previously published reviews support our results (Goldman 2009; Wang 2003). They concluded that HBOT may be beneficial as an adjunctive therapy for compromised skin grafts. They found a low to moderate level of evidence that HBOT promotes successful 'take' in such wounds. Both reviews included studies with different designs and did not focus on RCTs only. Stronger evidence is available for the effectiveness of HBOT in chronic wounds, in particular diabetic foot ulcers (Cochrane Review: Kranke 2012).
Authors' conclusions
Implications for practice.
This review offers insufficient evidence to support the routine use of HBOT for patients with acute surgical or traumatic wounds. Based on a single trial there is some evidence that HBOT may improve wound healing and reduce adverse effects in the treatment of crush injuries, a traumatic wound. However, this study had several methodological flaws which makes it hard to generalise these results to clinical practice. HBOT also tends to improve the percentage survival of split skin grafts. However, to draw proper conclusions new well‐designed larger RCTs are needed.
Implications for research.
Further evaluation through high quality randomised controlled trials is needed to determine whether there are clinically significant effects from the use of HBOT for acute surgical and traumatic wounds (Eskes 2012). Studies should report according to the CONSORT statement.
Future studies should be adequately powered for the primary endpoints as proposed by the FDA, like complete wound closure and accelerated wound closure (FDA 2006). The primary and secondary outcomes used in this review should be among the endpoints. New studies need to use an appropriate comparator therapy or an effective sham therapy to assess the true additional effect of HBOT over standard treatment options.
What's new
| Date | Event | Description |
|---|---|---|
| 21 August 2013 | New search has been performed | For this first update one additional study included (Vishwanath 2011). Dr Lubbers is no longer involved as an author. Dirk Ubbink assumes the role of contact person for this review. |
| 21 August 2013 | New citation required but conclusions have not changed | New search. No change to conclusions of the review. |
Acknowledgements
We thank all the peer reviewers for reviewing the protocol and review for relevance, rigour and readability (Mike Bennett, Andrew Jull, Caroline Main, Frank Peinemann, Joan Webster, Durhane Wong‐Rieger and Gill Worthy). We thank Faridi van Etten for help with developing our search strategy and Jenny Bellorini who copy edited the text of our review. We also thank Maarten Lubbers for his contribution to the original review.
Appendices
Appendix 1. Search methods section from the original review
For this original review we searched the following databases for reports of eligible trials over all years:
Cochrane Wounds Group Specialised Register (searched 25 August 2010)
The Cochrane Central Register of Controlled Trials (CENTRAL) ‐ The Cochrane Library 2010 Issue 3
Ovid MEDLINE ‐ 1950 to August Week 2 2010
Ovid MEDLINE ‐ In‐Process & Other Non‐Indexed Citations August 24, 2010
Ovid EMBASE ‐ 1980 to 2010 Week 33
EBSCO CINAHL ‐ 1982 to 20 August 2010
The following search strategy was used in the Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor Acute Disease explode all trees #2 MeSH descriptor Wounds and Injuries explode all trees #3 (#1 AND #2) #4 MeSH descriptor Surgical Wound Infection explode all trees #5 MeSH descriptor Surgical Wound Dehiscence explode all trees #6 MeSH descriptor Wounds, Penetrating explode all trees #7 MeSH descriptor Lacerations explode all trees #8 MeSH descriptor Burns explode all trees #9 MeSH descriptor Bites and Stings explode all trees #10 MeSH descriptor Skin Transplantation explode all trees #11 MeSH descriptor Fractures, Open explode all trees #12 MeSH descriptor Gas Gangrene explode all trees #13 ((surgical NEXT wound*) or (incised NEXT wound*)):ti,ab,kw #14 (laceration* or gunshot or (gun NEXT shot) or stab or stabbing or stabbed or bite* or bitten):ti,ab,kw #15 ((traumatic NEXT wound*) or (acute NEXT wound*)):ti,ab,kw #16 ((mechanical NEXT trauma) or polytrauma):ti,ab,kw #17 ((thermal or blast or crush or avulsion) NEXT injur*):ti,ab,kw #18 ("burn" or "burns" or burned or scald*):ti,ab,kw #19 acute NEXT wound*:ti,ab,kw #20 acute NEXT ulcer*:ti,ab,kw #21 ((donor NEXT site*) or (skin NEXT graft*)):ti,ab,kw #22 ((open NEXT fracture*) or (compound NEXT fracture*)):ti,ab,kw #23 "gas gangrene":ti,ab,kw #24 experimental NEXT wound*:ti,ab,kw #25 "skin infarction":ti,ab,kw #26 skin NEXT flap*:ti,ab,kw #27 (#3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26) #28 MeSH descriptor Hyperbaric Oxygenation explode all trees #29 (hyperbaric* NEXT oxygen*):ti,ab,kw #30 (HBO or HBOT):ti,ab,kw #31 (high NEXT pressure NEXT oxygen*):ti,ab,kw #32 (#28 OR #29 OR #30 OR #31) #33 (#27 AND #32)
This strategy was adapted where necessary to search Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL. These search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL are shown in Appendix 2; Appendix 3; Appendix 4 respectively. The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision). The Ovid EMBASE and EBSCO CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network. There were no restrictions on the basis of date or language of publication. We contacted the Trials Search Co‐ordinator of the Cochrane Wounds Group to assist with the development of the various search strategies.
Appendix 2. Ovid MEDLINE search strategy
1 exp Acute Disease/ 2 exp "Wounds and Injuries"/ 3 1 and 2 4 exp Surgical Wound Infection/ 5 exp Surgical Wound Dehiscence/ 6 exp Wounds, Penetrating/ 7 exp Lacerations/ 8 exp Burns/ 9 exp "Bites and Stings"/ 10 exp Skin Transplantation/ 11 exp Fractures, Open/ 12 exp Gas Gangrene/ 13 (surgical wound$ or incised wound$).ti,ab. 14 (laceration$ or gunshot or gun shot or stab or stabbing or stabbed or bite$1 or bitten).ti,ab. 15 (traumatic wound$ or acute wound$).ti,ab. 16 (mechanical trauma or polytrauma).ti,ab. 17 ((thermal or blast or crush or avulsion) adj injur$).ti,ab. 18 (burn or burns or burned or scald$).ti,ab. 19 acute wound$.ti,ab. 20 acute ulcer$.ti,ab. 21 (donor site$ or skin graft$).ti,ab. 22 (open fracture$ or compound fracture$).ti,ab. 23 gas gangrene.ti,ab. 24 experimental wound$.ti,ab. 25 skin infarction.ti,ab. 26 skin flap$.ti,ab. 27 or/3‐26 28 exp Hyperbaric Oxygenation/ 29 (hyperbaric$ adj oxygen$).ti,ab. 30 (HBO or HBOT).ti,ab. 31 high pressure oxygen$.ti,ab. 32 or/28‐31 33 27 and 32
Appendix 3. Ovid EMBASE search strategy
1 exp Acute Disease/ 2 exp Wound/ 3 1 and 2 4 exp Surgical Wound/ 5 exp Wound Dehiscence/ 6 exp Penetrating Trauma/ 7 exp Laceration/ 8 exp Burn/ 9 exp Bite/ 10 exp Bite Wound/ 11 exp Dog Bite/ 12 exp Skin Transplantation/ 13 exp Open Fracture/ 14 exp Gas Gangrene/ 15 (surgical wound$ or incised wound$).ti,ab. 16 (laceration$ or gunshot or gun shot or stab or stabbing or stabbed or bite$1 or bitten).ti,ab. 17 (traumatic wound$ or acute wound$).ti,ab. 18 (mechanical trauma or polytrauma).ti,ab. 19 ((thermal or blast or crush or avulsion) adj injur$).ti,ab. 20 (burn or burns or burned or scald$).ti,ab. 21 acute wound$.ti,ab. 22 acute ulcer$.ti,ab. 23 (donor site$ or skin graft$).ti,ab. 24 (open fracture$ or compound fracture$).ti,ab. 25 gas gangrene.ti,ab. 26 experimental wound$.ti,ab. 27 skin infarction.ti,ab. 28 skin flap$.ti,ab. 29 or/3‐28 30 exp Hyperbaric Oxygen/ 31 (hyperbaric$ adj oxygen$).ti,ab. 32 (HBO or HBOT).ti,ab. 33 high pressure oxygen$.ti,ab. 34 or/30‐33
Appendix 4. EBSCO CINAHL search strategy
S32 S26 and S31 S31 S27 or S28 or S29 or S30 S30 TI high pressure oxygen* or AB high pressure oxygen* S29 TI ( HBO or HBOT ) or AB ( HBO or HBOT ) S28 TI hyperbaric oxygen* or AB hyperbaric oxygen* S27 (MH "Hyperbaric Oxygenation") S26 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 S25 TI skin flap* or AB skin flap* S24 TI skin infarction or AB skin infarction S23 TI experimental wound* or AB experimental wound* S22 TI gas gangrene or AB gas gangrene S21 TI ( open fracture* or compound fracture* ) or AB ( open fracture* or compound fracture* ) S20 TI ( donor site* or skin graft* ) or AB ( donor site* or skin graft* ) S19 TI ( acute wound* or acute ulcer* ) or AB ( acute wound* or acute ulcer* ) S18 TI ( burn or burns or burned or scald* ) or AB ( burn or burns or burned or scald* ) S17 TI ( thermal injur* or blast injur* or crush injur* or avulsion ) or AB ( thermal injur* or blast injur* or crush injur* or avulsion ) S16 TI ( mechanical trauma or polytrauma ) or AB ( mechanical trauma or polytrauma ) S15 TI ( traumatic wound* or acute wound* ) or AB ( traumatic wound* or acute wound* ) S14 TI ( laceration* or gunshot or gun shot or stab or stabbing or stabbed or bite* or bitten ) or AB ( laceration* or gunshot or gun shot or stab or stabbing or stabbed or bite* or bitten ) S13 TI ( surgical wound* or incised wound* ) or AB ( surgical wound* or incised wound* ) S12 (MH "Gas Gangrene") S11 (MH "Fractures, Open") S10 (MH "Skin Transplantation") S9 (MH "Burns+") S8 (MH "Bites and Stings+") S7 (MH "Tears and Lacerations") S6 (MH "Wounds, Penetrating+") S5 (MH "Surgical Wound Dehiscence") S4 (MH "Surgical Wound Infection") S3 S1 and S2 S2 (MH "Wounds and Injuries+") S1 MH "Acute Disease")
Appendix 5. Risk of bias definitions
Criteria for judgements about the sources of bias
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process, such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of ‘Yes’ or ‘No’.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes used without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of ‘Yes’ or ‘No’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following:
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Any one of the following:
Insufficient information to permit judgement of ‘Yes’ or ‘No’.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation;
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following:
Insufficient reporting of attrition/exclusions to permit judgement of ‘Yes’ or ‘No’ (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Any of the following:
The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following:
Not all of the study’s pre‐specified primary outcomes have been reported.
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered into a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of ‘Yes’ or ‘No’. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias, for example the study:
had a potential source of bias related to the specific study design used; or
stopped early due to some data‐dependent process (including a formal‐stopping rule); or
had extreme baseline imbalance; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias
Data and analyses
Comparison 1. HBOT compared with usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete survival (defined as at least 95% take) at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. HBOT compared with sham HBOT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Time to healing (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse effects (additional surgical procedures) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse effects (tissue necrosis) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Amputations | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. HBOT compared with dexamethasone or heparin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete healing HBOT vs dexamethasone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Complete healing HBOT vs heparin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bouachour 1996.
| Methods | Randomised clinical trial Study period: not stated |
|
| Participants | Inclusion criteria: acute crush injury of the limb classified as type 2 or 3 according to Gustillo; surgical management within 6 hours after injury; no history of peripheral arterial occlusive disease Exclusion criteria: enrolment in any other trial; (suspected) pregnancy; neurologic, pulmonary or otorhinolaryngologic diseases contraindicating HBOT Baseline wound size: not stated Mean age (SD): HBOT 45.8 (16.1); Control 51.5 (20.9) HBOT n = 18 Control n =18 Total number of patients: 36 Duration of follow‐up: to complete wound healing |
|
| Interventions | Intervention Group 1: HBOT treatment in a multiplace chamber, sessions of 100% O2 at 2.5 atmosphere absolute (ATA) for 90 minutes, twice daily over 6 days Comparison Group 2: sham HBOT, sessions of 21% O2 at 1.1 ATA for 90 minutes, twice daily over 6 days | |
| Outcomes | Primary outcomes: Complete wound healing without tissue necrosis requiring surgical excision Time of healing (days) Other outcomes: Adverse effects; amputation; length of hospital stay | |
| Notes | Location: Centre Hospitalier Universitaire, Angers, France France Setting: university hospital Financial support: research grants from the Centre of Hospitalier Universitaire of Angers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned to receive HBO therapy or placebo". Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: the method of concealment is not described |
| Blinding (performance bias and detection bias) Care provider blinding | Low risk | Quote: "the surgeons and the patients were not informed of the treatment protocol performed during the study" Comment: probably done; surgeons are blinded |
| Blinding (performance bias and detection bias) Outcome assessor blinding | Unclear risk | Comment: it is not certain that the outcome assessor was unaware of the treatment. They did not describe clearly who did the outcome assessments (surgeon or someone else). |
| Blinding (performance bias and detection bias) Participant blinding | Low risk | Quote: "the surgeons and the patients were not informed of the treatment protocol performed during the study" Comment: probably done; patients are blinded |
| Incomplete outcome data (attrition bias) Was drop out rate described and acceptable | Low risk | Comment: there were no drop‐outs and all patients were assessed at the 6‐days follow‐up time point |
| Incomplete outcome data (attrition bias) ITT analysis | Low risk | Quote: "A total of 36 patients were enrolled in the trial: 18 in the HBO group and 18 in the placebo group" Comment: all patients were analysed in the groups to which they were originally randomly assigned. There were no patients excluded after randomisation. |
| Selective reporting (reporting bias) | Low risk | Comment: no protocol available, but the trial report lists the outcomes of interest in both the methods and the results section |
| Other bias | Low risk | Comment: co‐interventions similar, groups comparable at baseline |
Perrins 1967.
| Methods | Randomised clinical trial Study period: not stated |
|
| Participants | Inclusion criteria: every patient with split skin grafting Exclusion criteria: infants Baseline wound size: not stated Mean age (SD): not stated HBOT n = 24 Control n =24 Total number of patients = 48 Duration of follow‐up: 7 days Setting: Burns Unit, Queen Mary's Hospital, Roehampton, London, United Kingdom |
|
| Interventions | Intervention: HBOT treatment in a Vicker's clinical transparent pressure chamber, sessions of 100% O2 at 2 ATA for 2 hours, 1 day twice and the next 3 days once Comparison: usual care, clarification of usual care was not stated All cases had closed dressings of paraffin gauze, cotton wool and bandages applied to the operation and donor sites | |
| Outcomes | Graft survival (defined as at least 95% take) at day 7 | |
| Notes | Location: Queen Mary's Hospital, Roehampton, London, United Kingdom Setting: Burns Unit Financial support: not stated Estimating patches is not a reliable and validated outcome measurement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were allotted randomly to treatment or control groups" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: the method of concealment is not described |
| Blinding (performance bias and detection bias) Care provider blinding | Low risk | Quote: "routine surgery was performed by the surgeon, who did not know if the case was subsequently to be treated". Comment: probably done; surgeon is blinded |
| Blinding (performance bias and detection bias) Outcome assessor blinding | Unclear risk | Comment: the surgeon who performed the operation was blinded, but it is not clear whether he was also the outcome assessor |
| Blinding (performance bias and detection bias) Participant blinding | High risk | Comment: only the surgeon who performed the operation was blinded |
| Incomplete outcome data (attrition bias) Was drop out rate described and acceptable | Low risk | Comment: there were no drop‐outs and all patients were assessed at the 6‐day follow‐up time point. |
| Incomplete outcome data (attrition bias) ITT analysis | High risk | Comment: 2 grafts failed completely in the treated group. These 2 cases were excluded from analysis, on the grounds that a successful 'take' could not be expected. |
| Selective reporting (reporting bias) | High risk | Comment: no protocol available, but percentage wound healing was measured at day seven with multiple sub‐endpoints. This increases the risk of a type 1 error. |
| Other bias | Unclear risk | Comment: baseline comparability not stated; same treatment apart from intervention |
Vishwanath 2011.
| Methods | Randomised clinical trial Study period: not stated |
|
| Participants | Inclusion criteria: (1) patients with a major defect requiring a free flap in which conventional techniques were considered as inapplicable or sub optimal and (2) fitness to undergo up to 12 hours of anaesthesia and surgery. Exclusion criteria: none reported Baseline wound size: not stated Mean age (SD): not stated HBOT n = 5 Control n = 5 Total number of participants = 10 Duration of follow‐up: 14 days |
|
| Interventions | Intervention: HBOT treatment, sessions once a day for seven days (starting from the first postoperative day) at 2.5 ATA (type of chamber and duration not reported), Comparison: "usual care", clarification of usual care was not stated. | |
| Outcomes | Flap loss (i.e. unviable segment of tissue of any size in the flap) (number); Adverse effects; flap oedema and venous congestion. |
|
| Notes | Location: INHS Asvini, Mumbai, India Setting: Department of surgery and reconstructive surgery, hospital Financial support: Research grants from the office of DGAFMS |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised into two groups by random chit method" Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: The method of concealment is not described |
| Blinding (performance bias and detection bias) Care provider blinding | High risk | Comment: The treatment and control groups are distinguishable for the patients |
| Blinding (performance bias and detection bias) Outcome assessor blinding | Unclear risk | Comment: Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) Participant blinding | High risk | Comment: The treatment and control groups are distinguishable for the patients |
| Incomplete outcome data (attrition bias) Was drop out rate described and acceptable | Low risk | Comment: there were no drop‐outs and all patients were assessed up to 14 days postoperatively |
| Incomplete outcome data (attrition bias) ITT analysis | High risk | Quote: "One flap each in groups 1 and 2 failed to survive beyond 24 hours of surgery. These patients were excluded from the analysis since flap loss had occurred even before HBO was exhibited to the case group" Comment: No ITT‐analysis was performed |
| Selective reporting (reporting bias) | Low risk | Comment: no protocol available; but the trial report list the outcomes of interest in both the methods and the results section |
| Other bias | Unclear risk | Comment: baseline comparable unclear; same treatment apart from intervention |
Xie 2007.
| Methods | Randomised clinical trial Study period: August 2002 to August 2006 |
|
| Participants | Inclusion criteria: patients with skin defects in the limbs who underwent flap grafting Exclusion criteria: none stated Baseline wound size: not stated, range 8 cm² to 40 cm² Mean age (SD): not stated HBOT = 45 Dexamethasone = 45 Local heparin = 45 Total number of patients = 135 Duration of follow‐up: 7 days |
|
| Interventions | Intervention: HBOT treatment in a hyperbaric oxygen chamber made in China, sessions 2 times a day for 3 days and thereafter once a day resulting in a total of 6 to 12 treatments Comparison 1: dexamethasone treatment started at the day of operation with a dose of 0.4 mg/kg per intravenous infusion; continued on day 1 with a single dose of 0.2 mg/kg; day 2 and 3 0.1 mg/kg; day 4 and 5 0.05 mg/kg; treatment stopped at day 6 Comparison 2: subcutaneous local heparin treatment (200 U/ml NS) was given for 6 days |
|
| Outcomes | Flap survival at day 7, clinical assessment of skin colour, temperature of the skin, capillary refill test, arterial pulsations and bleeding | |
| Notes | Location: Baoon People’s Hospital of Southern Medical University, Guangdong, China Setting: Department of Orthopedics, hospital Financial support: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "all patients were randomly divided" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding (performance bias and detection bias) Care provider blinding | High risk | Quote: "blind evaluation methods has not been applied" Comment: not done |
| Blinding (performance bias and detection bias) Outcome assessor blinding | High risk | Quote: "blind evaluation methods has not been applied" Comment: not done |
| Blinding (performance bias and detection bias) Participant blinding | High risk | Quote: 'blind evaluation methods has not been applied' Comment: not done |
| Incomplete outcome data (attrition bias) Was drop out rate described and acceptable | Low risk | Comment: there were no drop‐outs and all patients were assessed at the 7‐day follow‐up time point |
| Incomplete outcome data (attrition bias) ITT analysis | Low risk | Comment: all patients were analysed in the groups to which they were originally randomly assigned. There were no patients excluded after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available; they merely reported complete survival of the graft at day seven after the operation, but no other outcome measures. |
| Other bias | Unclear risk | Comment: baseline comparability not stated |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brannen 1997 | Included in Cochrane Review of thermal burns (Villanueva 2004) |
| Hart 1974 | Included in Cochrane Review of thermal burns (Villanueva 2004) |
| Jian 2011 | Not RCT |
| Niezgoda 1997 | Included in Cochrane Review of thermal burns (Villanueva 2004) |
| Xu N 1999 | Included in Cochrane Review of thermal burns (Villanueva 2004) |
Characteristics of ongoing studies [ordered by study ID]
Brismar.
| Trial name or title |
NCT01002209 Postoperative Hyperbaric Oxygen Treatments to Reduce Complications in Diabetic Patients Undergoing Vascular Surgery (HODiVA) |
| Methods | Randomized controlled trial |
| Participants | Inclusion Criteria: Patients are eligible for inclusion if the following criteria are fulfilled ‐ informed consent obtained; scheduled for lower extremity open vascular surgery; diabetes treated with insulin or oral antidiabetic medicine; age ≥ 18 years Exclusion Criteria: Contraindications to HBO therapy; pregnancy (women of childbearing potential will undergo pregnancy test before inclusion); patients already in HBO treatment; vascular reoperation; creatinine > 250 mmol/L; NYHA class IV heart failure or severe cardiopulmonary disease with desaturation judged to be incompatible with safe HBO/ placebo therapy in a monoplace chamber; clinically significant chronic obstructive pulmonary disease. Acute sepsis; malignancy or other serious medical condition where it is likely that the patient will significantly deteriorate or not survive within the two years of follow up. Simultaneous or previous (within 30 days prior to study entry participation in a clinical study using experimental drugs or devices. Mental condition making the subject unable to understand the concepts and risk of the study |
| Interventions | Intervention: HBO treatment will be given in a monoplace chamber and will start on first postoperative day (study day 1). The HBO group will be treated with 100% oxygen at 2.5 bar for 100 min with two 10 min air brakes (without mask). HBO treatment will be given twice daily first three days (study day 1‐3) and then once daily for up to three days (study day 4‐6). The total number of treatments will be at least 6 and at most 9 treatments. Patients who have received at least three days HBO/placebo treatment and who have a clearly uncomplicated postoperative course will terminate HBO/placebo treatment on the day of discharge from hospital. Comparison: HBO sham treatment will start on first postoperative day (study day 1). HBO sham treatment will be given twice daily first three days (study day 1‐3) and then once daily for up to three days (study day 4‐6). The total number of treatments will be at least 6 and at most 9 treatments. Patients who have received at least three days HBO sham treatments and who have a clearly uncomplicated postoperative course will terminate HBO sham treatment on the day of discharge from hospital. For patient blinding purposes, the sham group will breathe air and will be given a brief compression to 1.5 bar at the beginning of each treatment after which the chamber is slowly decompressed to 1.1, 1.2, 1.3, or 1.4 bar corresponding to 0.23 ,0.25, 0.27, and 0.29 bar inspired oxygen. Two 10 min "airbrakes" will also be included. |
| Outcomes | Time to complete healing of operative wounds (Time Frame: 7‐365 days), HBO complications (confinement anxiety, barotrauma, oxygen convulsions) (Time Frame: During HBO treatment up to day 6) infections (numbers at 7 and 30 days assessed by ASEPSIS score), severity of infections (ASEPSIS score), a combination of any wound infection and/or unhealed wounds at 30 days (combined endpoint) (Time Frame: 30 days (plus minus 3 days), SF‐36 score (Time Frame: 7, 14, 28, 365 days (plus minus 3 days), morbidity, major amputation (Time frame: 0‐365 days), tissue perfusion and oxygenation on dorsum of foot on operated extremity as assessed by Transcutaneous oximetry during normobaric air breathing and after 6 min normobaric 100% oxygen challenge (Time Frame: day 3‐5, 7 and 14, 28, 365 (plus minus 3 days) |
| Starting date | October 2009 ‐ Anticipated end date: October 2014 |
| Contact information | Contact: Kerstin Brismar, MD, Prof (kerstin.brismar@ki.se) Contact: Jonas Malmstedt, MD (jonas.malmstedt@karolinska.se), Karolinska University Hospital, Stockholm, Sweden, 17176 |
| Notes | Location: Sweden Source of funding: self‐funded |
Millar.
| Trial name or title |
NCT00264511 Hyperbaric Oxygen in Lower Leg Trauma |
| Methods | Randomized controlled trial |
| Participants | Inclusion Criteria: Acute fracture of the tibia with significant soft tissue injury of Gustilo Grade 3. Enrolment within 48 hours of injury with expectation of commencement of HBO therapy within 48 hours of injury. Valid consent Exclusion Criteria: significant head injury, injuries incompatible with HBO, resuscitation requirements incompatible with HBO, follow up not possible, hyperbaric contra indications |
| Interventions | Intervention: Hyperbaric oxygenation (not further specified) Comparison: No hyperbaric oxygenation. Patients randomised to this group will receive standard trauma care. |
| Outcomes | Acute phase complication rate, soft tissue necrosis, infection (number), compartment syndrome, amputations, radiological union, quality of life score (method unclear), functional outcome scores. |
| Starting date | February 2006 ‐ Anticipated end date: December 2013 |
| Contact information | Contact: Ian L Millar, MBBS (I.millar@alfred.org.au), Bayside Health |
| Notes | Location: Worldwide Source of funding: Bayside Health |
Sires.
| Trial name or title | NCT01605110 Effects of Hyperbaric Oxygen Therapy on Surgical Wound Healing (BLEPH) |
| Methods | Randomized controlled trial |
| Participants | Inclusion Criteria: Patients that are able to undergo surgery at the Allure Clinic are capable of undergoing exposure to HBOT, as the contraindications of HBOT are similar to eyelid surgery, with few exceptions. Exclusion Criteria: Active smokers and those who have quit smoking in the last 12 months, those with known lung disease, seizure disorder, congestive heart failure, known active cancer, previous treatment with specific chemotherapy agents (Doxorubicin, Bleomycin, Disulfiram, Cis‐platinum, Mafenide acetate), those who cannot undergo pressurization/ depressurization because of eustachian‐tube dysfunction and confinement anxiety. |
| Interventions | Intervention: Hyperbaric oxygen therapy: two HBOT treatments (one before and one after surgery) with patients who have undergone upper eyelid surgery. Patients that volunteer to participate in this study will be exposed to two treatments of 100% oxygen at 2.0 atmospheres absolute (ATA) Comparison: air (sham) 1.2 ATA inside mono‐place chambers for 90 minutes. |
| Outcomes | Reduction of ecchymosis grade (Time Frame: 21 days) clinical signs, reduction of edema (Time Frame: 21 days) |
| Starting date | August 2011 ‐ Anticipated end date: November 2013 |
| Contact information | Bryan Sires, MD, FACS (no email address given), Allure Laser Center & Medispa |
| Notes | Location: Washington, USA Source of funding: Restorix Research Institute, LLLP |
Contributions of authors
Anne Eskes: conceived the review question and developed the protocol; completed the first draft and edited the protocol; made an intellectual contribution to the protocol and approved the final version prior to submission; updated the original review. Dirk T Ubbink: conceived the review question, secured funding and co‐ordinated the protocol development; edited and advised on the protocol; made an intellectual contribution and approved the final version prior to submission; updated the original review; and is guarantor of the work. Cees Lucas: completed the first draft of the revision and advised on part of the protocol; made an intellectual contribution to the protocol, the original review and updated version, approved the final version prior to submission. Hester Vermeulen: conceived the review question, secured funding and co‐ordinated the protocol development; edited and advised on the protocol; made an intellectual contribution and approved the final version prior to submission; updated the original review; and is guarantor of the work.
Contributions of editorial base
Nicky Cullum: edited the protocol and review; advised on methodology, interpretation and content; and approved the final review prior to submission. Sally Bell‐Syer: co‐ordinated the editorial process; advised on methodology, interpretation and content; edited the protocol, review and review update. Ruth Foxlee: designed the search strategy, edited the search methods section and ran the searches. Rachel Richardson: edited and checked the review update.
Sources of support
Internal sources
No sources of support supplied
External sources
Infrastructure supplied by the hospital, Academic Medical Centre at the University of Amsterdam, Netherlands.
NIHR/Department of Health (England), (Cochrane Wounds Group), UK.
Declarations of interest
None of the authors have any conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bouachour 1996 {published data only}
- Bouachour G, Cronier P, Gouello JP, Toulemonde JL, Talha A, Alquier P. Hyperbaric oxygen therapy in the management of crush injuries: a randomized double‐blind placebo‐controlled clinical trial. Journal of Trauma 1996;41(2):333‐9. [DOI] [PubMed] [Google Scholar]
Perrins 1967 {published data only}
- Perrins DJ. Influence of hyperbaric oxygen on the survival of split skin grafts. Lancet 1967;289(7495):868‐71. [DOI] [PubMed] [Google Scholar]
Vishwanath 2011 {published data only}
- Vishwanath G. Hyperbaric oxygen therapy in free flap surgery: Is it meaningful?. Medical Journal Armed Forces India 2011;67(3):253‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xie 2007 {published data only}
- Xie Z‐X, Li C‐Y. Changes in arterial flow after flap grafting under various tensions. Journal of Clinical Rehabilitative Tissue Engineering Research 2007;11(25):5004‐5. [Google Scholar]
References to studies excluded from this review
Brannen 1997 {published data only}
- Brannen AL, Still J, Haynes M, Orlet H, Rosenblum F, Law E, et al. A randomized prospective trial of hyperbaric oxygen in a referral burn center population. The American Surgeon 1997;63(3):205‐8. [PubMed] [Google Scholar]
Hart 1974 {published data only}
- Hart GB, O'Reilly RR, Broussard ND, Cave RH, Goodman DB, Yanda RL. Treatment of burns with hyperbaric oxygen. Surgery, Gynecology and Obstetrics 1974;139(5):693‐6. [PubMed] [Google Scholar]
Jian 2011 {published data only}
- Jian W, Liang D. Zhang H. Shang W, Wang P, Li W. The effect of hyperbaric oxygen therapy on treatment of late healed wounds after pharyngeal and laryngeal surgery. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2011;25(1):14‐15. [PubMed] [Google Scholar]
Niezgoda 1997 {published data only}
- Niezgoda JA, Cianci P, Folden BW, Ortega RL, Slade JB, Storrow AB. The effect of hyperbaric oxygen therapy on a burn wound model in human volunteers. Plastic and Reconstructive Surgery 1997;99(6):1620‐5. [PubMed] [Google Scholar]
Xu N 1999 {published data only}
- Xu N, Li Z, Luo X. Effects of hyperbaric oxygen therapy on the changes in serum sIL‐2R and Fn in severe burn patients. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi 1999;15(3):220‐3. [PubMed] [Google Scholar]
References to ongoing studies
Brismar {published data only}
- NCT01002209Postoperative Hyperbaric Oxygen Treatments to Reduce Complications in Diabetic Patients Undergoing Vascular Surgery (HODiVA). Ongoing study October 2009 ‐ Anticipated end date: October 2014.
Millar {published data only}
- NCT00264511Hyperbaric Oxygen in Lower Leg Trauma. Ongoing study February 2006 ‐ Anticipated end date: December 2013.
Sires {published data only}
- NCT01605110 Effects of Hyperbaric Oxygen Therapy on Surgical Wound Healing (BLEPH). Ongoing study August 2011 ‐ Anticipated end date: November 2013.
Additional references
Altman 2001
- Altman DG, Schulz KF. Statistics notes: concealing treatment allocation in randomised trials. BMJ 2001;323(7310):446‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 2005a
- Bennett MH, Feldmeier J, Hampson N, Smee R, Milross C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005005.pub2] [DOI] [PubMed] [Google Scholar]
Bennett 2005b
- Bennett MH, Stanford R, Turner R. Hyperbaric oxygen therapy for promoting fracture healing and treating fracture non‐union. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004712.pub2] [DOI] [PubMed] [Google Scholar]
Bennett 2008
- Bennett MH, French C, Schnabel A, Wasiak J, Kranke P. Normobaric and hyperbaric oxygen therapy for migraine and cluster headache. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD005219.pub2] [DOI] [PubMed] [Google Scholar]
Boerema 1964
- Boerema I. High tension oxygen therapy. Hyperbaric oxygen. Proceedings of the Royal Society of Medicine 1964;57:817‐8. [PMC free article] [PubMed] [Google Scholar]
Boerema 1965
- Boerema I. The use of hyperbaric oxygen. American Heart Journal 1965;69:289‐92. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT and Altman DG on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (Editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Eskes 2010
- Eskes A, Ubbink DT, Lubbers M, Lucas C, Vermeulen H. Hyperbaric oxygen therapy for treating acute surgical and traumatic wounds. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD008059.pub2] [DOI] [PubMed] [Google Scholar]
Eskes 2012
- Eskes AM, Brölmann FE, Sumpio BE, Mayer D, Moore Z, Agren MS, et al. Fundamentals of randomized clinical trials in wound care: design and conduct. Wound Repair and Regeneration 2012;20(4):449‐55. [DOI] [PubMed] [Google Scholar]
FDA 2006
Franz 2007
- Franz MG, Steed DL, Robson MC. Optimizing healing of the acute wound by minimizing complications. Current Problems in Surgery 2007;44(11):691‐763. [DOI] [PubMed] [Google Scholar]
Goldman 2009
- Goldman RJ. Hyperbaric oxygen therapy for wound healing and limb salvage: a systematic review. PM&R: The Journal of Injury, Function, and Rehabilitation 2009;1(5):471‐89. [DOI] [PubMed] [Google Scholar]
Hess 2003
- Hess CL, Howard MA, Attinger CE. A review of mechanical adjuncts in wound healing: hydrotherapy, ultrasound, negative pressure therapy, hyperbaric oxygen, and electrostimulation. Annals of Plastic Surgery 2003;51(2):210‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (Editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ioannidis 2008
- Ioannidis JP, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta‐analysis in forest plots. BMJ 2008;336(7658):1413‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jull 2008
- Jull AB, Rodgers A, Walker N. Honey as a topical treatment for wounds. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD005083.pub2] [DOI] [PubMed] [Google Scholar]
Kindwall 2002
- Kindwall EP. Hyperbaric Medicine Practice. 2nd Edition. Best Publishing Company, 2002:4‐8. [Google Scholar]
Kranke 2012
- Kranke P, Bennett MH, Martyn‐St James M, Schnabel A, Debus SE. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD004123.pub3] [DOI] [PubMed] [Google Scholar]
Lazarus 1994
- Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Pecoraro RE, Rodeheaver G, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Archives of Dermatology 1994;130(4):489‐93. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
MacFarlane 2001
- MacFarlane C, Cronjé FJ. Hyperbaric oxygen and surgery. South African Journal of Surgery 2001;39(4):117‐21. [PubMed] [Google Scholar]
Niinikoski 2003
- Niinikoski J. Hyperbaric oxygen therapy of diabetic foot ulcers, transcutaneous oxymetry in clinical decision making. Wound Repair and Regeneration 2003;11(6):458‐61. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1.7. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Roeckl‐Wiedmann 2005
- Roeckl‐Wiedmann I, Bennett M, Kranke P. Systematic review of hyperbaric oxygen in the management of chronic wounds. British Journal of Surgery 2005;92(1):24‐32. [DOI] [PubMed] [Google Scholar]
Ruiz‐Canela 2000
- Ruiz‐Canela M, Martínez‐González MA, Irala‐Estévez J. Intention to treat analysis is related to methodological quality. BMJ 2000;320(7240):1007‐8. [PMC free article] [PubMed] [Google Scholar]
Schulz 2002a
- Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet 2002;359(9306):614‐8. [DOI] [PubMed] [Google Scholar]
Schulz 2002b
- Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet 2002;359(9307):696‐700. [DOI] [PubMed] [Google Scholar]
Sign 2012
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed 6 November 2012).
Ubbink 1997
- Ubbink DT, Tulevski II, Hartog D, Koelemay MJ, Legemate DA, Jacobs MJ. The value of non‐invasive techniques for the assessment of critical limb ischaemia. European Journal of Vascular and Endovascular Surgery 1997;13(3):296‐300. [DOI] [PubMed] [Google Scholar]
Vermeulen 2004
- Vermeulen H, Ubbink D, Goossens A, Vos R, Legemate D. Dressings and topical agents for surgical wounds healing by secondary intention. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD003554.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Villanueva 2004
- Villanueva E, Bennett MH, Wasiak J, Lehm JP. Hyperbaric oxygen therapy for thermal burns. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004727.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2003
- Wang C, Schwaitzberg S, Berliner E, Zarin DA, Lau J. Hyperbaric oxygen for treating wounds: a systematic review of the literature. Archives of Surgery 2003;138(3):272‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Eskes 2011
- Eskes AM, Ubbink DT, Lubbers MJ, Lucas C, Vermeulen H. Hyperbaric oxygen therapy: solution for difficult to heal acute wounds? Systematic review. World Journal of Surgery 2011;35(3):535‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]


