Abstract
ABSTRACT
Introduction
Sedation in mechanically ventilated adults in the intensive care unit (ICU) is commonly achieved with intravenous infusions of propofol, dexmedetomidine or benzodiazepines. Significant limitations associated with each can impact their usage. Inhaled isoflurane has potential benefit for ICU sedation due to its safety record, sedation profile, lack of metabolism and accumulation, and fast wake-up time. Administration in the ICU has historically been restricted by the lack of a safe and effective delivery system for the ICU. The Sedaconda Anaesthetic Conserving Device-S (Sedaconda ACD-S) has enabled the delivery of inhaled volatile anaesthetics for sedation with standard ICU ventilators, but it has not yet been rigorously evaluated in the USA. We aim to evaluate the efficacy and safety of inhaled isoflurane delivered via the Sedaconda ACD-S compared with intravenous propofol for sedation of mechanically ventilated ICU adults in USA hospitals.
Methods and analysis
INhaled Sedation versus Propofol in REspiratory failure in the ICU (INSPiRE-ICU1) is a phase 3, multicentre, randomised, controlled, open-label, assessor-blinded trial that aims to enrol 235 critically ill adults in 14 hospitals across the USA. Eligible patients are randomised in a 1.5:1 ratio for a treatment duration of up to 48 (±6) hours or extubation, whichever occurs first, with primary follow-up period of 30 days and additional follow-up to 6 months. Primary outcome is percentage of time at target sedation range. Key secondary outcomes include use of opioids during treatment, spontaneous breathing efforts during treatment, wake-up time at end of treatment and cognitive recovery after treatment.
Ethics and dissemination
Trial protocol has been approved by US Food and Drug Administration (FDA) and central (Advarra SSU00208265) or local institutional review boards ((IRB), Cleveland Clinic IRB FWA 00005367, Tufts HS IRB 20221969, Houston Methodist IRB PRO00035247, Mayo Clinic IRB Mod22-001084-08, University of Chicago IRB21-1917-AM011 and Intermountain IRB 033175). Results will be presented at scientific conferences, submitted for publication, and provided to the FDA.
Trial registration number
Keywords: Randomized Controlled Trial, INTENSIVE & CRITICAL CARE, Clinical Trial, Propofol
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study is a multicentre, randomised, controlled, open-label, assessor-blinded trial to evaluate the efficacy and safety of inhaled isoflurane delivered via the Sedaconda Anaesthetic Conserving Device-S compared with intravenous propofol for sedation of mechanically ventilated adults intensive care unit (ICU) patients.
The inclusion of a diverse group of medical and surgical mechanically ventilated adult ICU patients requiring continuous sedation across a large number of geographically diverse US ICUs enhances generalisability of the trial results.
The use of guideline-driven best sedation practices, including the ABCDEF bundle, in all participating ICUs ensures the trial results are applicable to modern, evidence-based care environments.
Although outcome assessors are blinded to treatment group assignment, patients, families and clinicians are not; clinician knowledge of sedation strategy assignment may potentially influence clinical decision-making and reporting.
Introduction
Pain, agitation and delirium are commonly experienced by critically ill adults, especially those requiring invasive mechanical ventilation.1 A significant proportion of mechanically ventilated adults admitted to the intensive care unit (ICU) require sedation, and often analgesia, to optimise their comfort, safety and clinical management. This therapy is most commonly achieved with intravenous infusions of propofol, dexmedetomidine or benzodiazepines (eg, midazolam). These sedatives, even when titrated to an established sedation goal, have significant limitations and potential harmful effects in the critically ill—a population with frequent underlying comorbidity and end-organ dysfunction that impact drug response and clearance. Propofol can cause hypotension, hyperlipidaemia, respiratory depression, is immunosuppressive, and carries the risk of propofol-related infusion syndrome, a potentially lethal side effect.2,4 Dexmedetomidine has been associated with bradycardia and hypotension, potentially increased mortality in younger adults, and may be insufficient when deeper levels of sedation are required.5,8 Midazolam infusions are associated with drug accumulation leading to coma, prolonged wake-up times, increased length of mechanical ventilation and hospital stay, tolerance necessitating dose-escalation, pharmacogenomic variability, dose-related incident delirium, withdrawal symptoms after discontinuation, and increased mortality.9,15
Current guidelines1 recommend the use of non-benzodiazepine sedatives, such as propofol or dexmedetomidine, when continuous sedation is required based on several studies demonstrating more favourable short- and long-term outcomes with their use.12,1416 Recent trials evaluating propofol and dexmedetomidine, however, have found patients frequently require the administration of additional sedatives (each with their own safety concerns) and spend significant time outside of the desired sedation target despite the protocolised use of daily spontaneous awakening studies and the prioritisation of light sedation goals.17,19 While multidomain approaches like the ABCDEF bundle20 have been shown to reduce delirium and coma, facilitate mechanical ventilation and ICU liberation, and reduce mortality, they can be challenging to adopt on a routine basis, particularly during surges of ICU care as was recently experienced during the COVID-19 pandemic.21,23 None of the currently available intravenous sedatives in the USA meet all the criteria for the ideal sedative in mechanically ventilated ICU adults, and, thus, there remains an unmet medical need for additional safe and highly efficacious sedative agents for this population.
Inhaled volatile anaesthetics, such as isoflurane, have long been used for general anaesthesia in operating rooms and possess many advantageous properties for ICU sedation in mechanically ventilated adults including the ability to be titrated to a full range of sedation depths, perform rapid wake-ups, avoid drug tolerance and withdrawal, provide analgesia, reduce respiratory depression and avoid drug accumulation, even in the setting of end-organ dysfunction given their minimal metabolism and lack of renal clearance.24,27 Use of inhaled volatile agents in the ICU has traditionally been limited to rescue scenarios (eg, refractory bronchospasm, status asthmaticus and status epilepticus) due to the requirement of anaesthesia personnel and machines for administration.
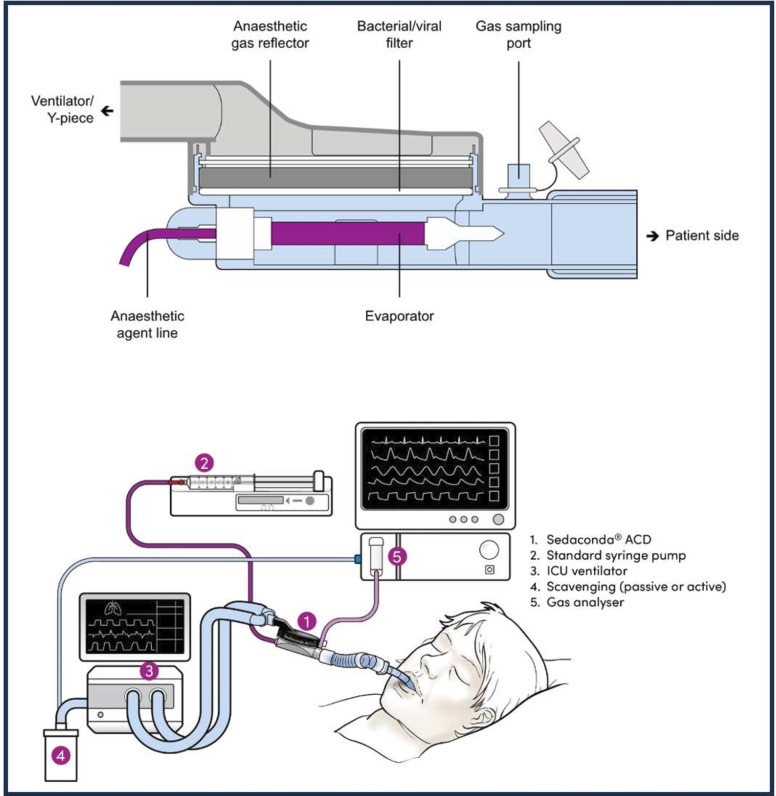
Recent technological advances, however, have greatly simplified the administration of inhaled volatile anaesthetics in the ICU through the introduction of volatile anaesthetic reflection filters to minimise anaesthetic vapour loss and enable inhaled sedation to be performed with standard ICU ventilators. The Sedaconda Anaesthetic Conserving Device-S (Sedaconda ACD-S, Sedana Medical AB, Danderyd, Sweden) is a small, disposable, volatile anaesthetic agent delivery and reflection system, developed for the administration of isoflurane (or sevoflurane) primarily for ICU sedation by non-anaesthesia personnel (figure 1). The anaesthetic is continuously infused via a syringe pump by the bedside nurse into the ACD-S device, and titration to the targeted sedation goal is achieved by changing the syringe pump infusion rate. The ACD-S device is inserted into the ventilator circuit between the patient’s endotracheal tube or tracheostomy and the Y-piece in place of a passive heat and moisture exchanger. The small amount of isoflurane that is not reflected by the ACD-S device is captured by gas scavenging on the ventilator exhaust port, resulting in a closed administration circuit shown to result in minimal environmental release of isoflurane and at an amount that is far below current US Occupational Safety and Health Administration exposure limits.28
Figure 1. The Sedaconda Anaesthetic Conserving Device-S (Sedaconda ACD-S) is a small, disposable volatile anaesthetic agent delivery and reflection system capable of administering isoflurane to mechanically ventilated patients. Standard placement is integrated into the ventilator circuit in place of the commonly used passive heat and moisture exchanger, between the patient’s endotracheal tube or tracheostomy and the Y-piece of the ventilator tubing. The evaporator is a porous plastic rod with a large surface area that vaporises isoflurane with the airflow. Isoflurane exhaled by the patient enters the reflection medium, is absorbed to the active carbon filter, and is desorbed and returned to the patient in the next breath with approximately 90% reflection. The syringe is a standard 50–60 mL syringe with a unique connector system to prevent unintentional intravenous administration. Sedation depth is adjusted by increasing or decreasing the syringe pump rate. A FlurAbsorb active carbon filter is used to scavenge the small amounts of exhaled isoflurane not reflected by the ACD-S device to create a closed administration circuit. End-tidal carbon dioxide (CO2) concentration is measured with standard gas analyser and presented with capnography. ICU, intensive care unit.
The Sedaconda ACD-S has been approved and used for inhaled sedation in the ICU in over 40 countries in Europe, Asia and South America for several years. Isoflurane via the Sedaconda ACD-S was recently approved for sedation of mechanically ventilated adults in 17 European countries. Growing evidence and clinical experience from this increased use within the ICU indicate sedation efficacy reduced opioid requirements, ability to maintain spontaneous breathing, fast predictable wake-up regardless of sedation depth, limited side effects and less need for additional sedatives.29,33 Clinically insignificant drug accumulation and rapid wake-up suggest isoflurane may potentially improve delirium in the ICU and long-term cognition afterwards,34 and inhaled anaesthetics in critically ill patients have been associated with fewer hallucinations and faster psychomotor recovery.35 However, most studies to date are small, and the impact of inhaled anaesthetics on delirium and cognitive outcomes in ICU populations remain unclear. Isoflurane administered via the Sedaconda ACD-S for inhaled sedation in the ICU is currently not approved by the US Food and Drug Administration (FDA) and has not been evaluated within the US healthcare system where ICU personnel and management practices are different from Europe.36 37 Therefore, the INhaled Sedation versus Propofol in REspiratory failure in the ICU 1 trial (INSPiRE-ICU1, NCT05312385) was designed to evaluate the efficacy and safety of inhaled isoflurane delivered via the Sedaconda ACD-S compared with intravenous propofol for sedation of mechanically ventilated ICU adult patients in the USA.
Methods and analysis
Trial design, setting and registration
Trial design
INSPiRE-ICU1 is a phase 3, multicentre, randomised, controlled, open-label, assessor-blinded trial evaluating the efficacy and safety of inhaled isoflurane delivered via the Sedaconda ACD-S compared with intravenous propofol for the sedation of mechanically ventilated adults ICU patients. INSPiRE-ICU1 is the first of two, methodologically identical, parallel phase 3 trials (INSPiRE-ICU1 and INSPiRE-ICU2). Reporting of the INSPiRE-ICU1 protocol herein adheres to the Standard Protocol Items for Randomised Trials (SPIRIT)38 statement as delineated in online supplemental materials, SPIRIT checklist.
Patient and public involvement
Patients or the public were not involved in trial design, conduct or dissemination plans of our research.
Setting
INSPiRE-ICU1 will include 235 adult patients (≥18 years) from 14 academic hospital systems in the USA.
Trial registration
The trial is registered on ClinicalTrials.gov and under FDA Investigational New Drug Application Number 141407.
Population, eligibility, screening and consent
Study population and eligibility
The target population includes adults in the ICU anticipated to require invasive mechanical ventilation and continuous sedation to achieve a clinically indicated Richmond Agitation Sedation Scale score (RASS)39 range of −1 to −4 for >12 hours without concomitant conditions or considerations that confound assessment of sedation depth. Eligible patients include those admitted to an ICU with an anticipated need for mechanical ventilation and continuous sedation or whom have upcoming planned surgery and a similar anticipated need for postoperative continuous sedation and mechanical ventilation. Exclusion criteria included severe neurological condition causing inability to participate in the trial or contraindication to propofol or isoflurane including severe haemodynamic compromise defined by norepinephrine >0.3 µg/kg/min or equivalent as defined in online supplemental appendix A. Full inclusion and exclusion criteria are delineated in box 1.
Box 1. Inclusion and exclusion criteria.
Inclusion criteria
Adults ≥18 years of age.
Anticipated to require >12 hours of invasive mechanical ventilation and continuous sedation in the ICU.
Receipt of continuous sedation due to clinical need for sedation to RASS<0.
Exclusion criteria
Need for RASS −5.
Sedation for invasive mechanical ventilation for >72 hours.1
Severe neurological condition causing inability to participate in the trial, namely inability to assess RASS and CPOT.2
Ventilator tidal volume <200 or >1000 mL.
Need for ECMO, ECCO2R, HFOV or HFPV.
Comfort care only (ie, end of life care).
-
Contraindication to propofol or isoflurane, including:
Severe haemodynamic compromise, defined as the need for norepinephrine ≥0.3 µg/kg/min (or equivalents) to maintain blood pressure within a clinically acceptable range (eg, ≥65 mm Hg).3
Known or suspected personal/family MH history, high MH risk or acute drug-induced muscle injury.
Allergy to isoflurane or propofol, or propofol infusion syndrome.
History of ventricular tachycardia and/or long QT syndrome.
Intravenous benzodiazepine or barbiturate requirements for seizures or dependencies, including alcohol withdrawal.
Neuromuscular disease that impairs spontaneous ventilation.4
Concurrent enrolment in another study that, in the investigator’s opinion, would impact the patient’s safety or study assessments.
Participation in another study involving investigational drug(s) or devices(s) within 30 days.
Previous randomisation or receipt of treatment in INSPiRE-ICU1 or 2.
Anticipated requirement of treatment with continuous infusion of an NMBA for >4 hours.
Female patients who are pregnant or breastfeeding.
Imperative need for continuous active humidification through mechanical ventilation circuit.
Attending physician’s refusal to include the patient.
Inability to obtain informed consent.
CPOT, Critical Care Pain Observation Tool score; ECCO2R, extracorporeal CO2 removal; ECMO, extracorporeal membrane oxygenation; HFOV, high -frequency oscillation ventilation; HFPV, high -frequency percussive ventilation; ICU, intensive care unit; IV, intravenous; MH, malignant hyperthermia; NMBA, neuromuscular blocking agent; RASS, Richmond Agitation Sedation Scale score.
(1) 1For patients extubated ≥24 hours and subsequently re-intubated, the start time of sedation for invasive mechanical ventilation is considered the time of re-intubation. For patients extubated and re-intubated within 24 hours, the start time of sedation for invasive mechanical ventilation is considered to be the time of the original intubation. (2) 2Examples include including acute stroke, severe head trauma, meningitis, suspected or known intracranial pressure elevation, or the need for intracranial pressure monitoring. (3) 3Vasopressor doses are summed into norepinephrine equivalents according to the approach delineated in the Supplemental Materials, Appendix Aonline supplemental appendix A. (4) 4Examples include C5 or higher spinal cord injury, amyotrophic lateral sclerosis, etc.
Screening and consent
Patients in the ICU fulfilling all inclusion criteria and no exclusion criteria are considered for enrolment. Initial informed consent is most commonly obtained from a legally authorised representative and then re-confirmed with the patient when their clinical condition permits. Initial screening is permitted up to 30 days prior to any study treatments to allow for the identification and potential enrolment of patients with planned postoperative mechanical ventilation. In such instances, informed consent is obtained initially from the patient.
Timeline, sample size and recruitment
Study timeline and flow
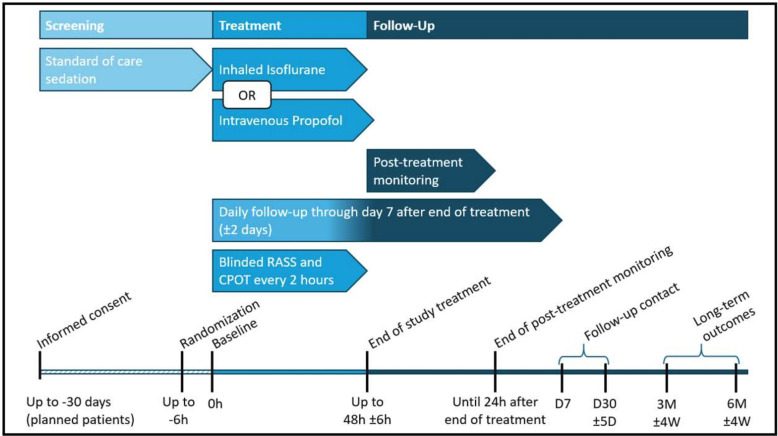
The study timeline and participant flow are summarised in figure 2.
Figure 2. Trial scheme for patient progression. Patients are screened between days −30 and 0 hours prior to initiation of study drug administration to determine trial eligibility. Baseline values are obtained during the baseline phase. At randomisation, ongoing sedation and opioid infusions are reduced to half. Initiating study drug treatment is performed as close to randomisation as possible, no later than 6 hours after randomisation. Study drug titration is performed by the clinical team to reach targeted sedation depth. Blinded RASS assessments occur every 2 hours with pain levels assessed in parallel with CPOT assessments. Study drug treatment is stopped when patient is planned for extubation or reaches maximum treatment duration of 48 (±6) hours, whichever occurs first. Patients are monitored until 24 hours after end of treatment, followed-up until day 7 and day 30, and receive long-term assessments at 3 and 6 months. COPT, Critical Care Pain Observation Tool; RASS, Richmond Agitation Sedation Scale.
Sample size
The trial is powered to evaluate the non-inferiority of inhaled isoflurane via the Sedaconda ACD-S compared with intravenous propofol in maintaining the depth of sedation within the target RASS range of −1 to −4. Based on previous studies and accounting for lack of familiarity in the USA with inhaled sedation in the ICU, anticipated time spent in the target RASS range is on average 70% with isoflurane and 75% with propofol with an SD of approximately 20%.32 40 Assuming an attrition rate of 5%, a total of 235 randomised patients will provide 95% power for a non-inferiority test with a one-sided alpha of 0.025.
Recruitment
Recruitment started 28 April 2022 with completion of recruitment expected in 2024. Queries to investigators, data cleaning and closure of the database will follow. Data analysis, manuscript preparation and submission for publication are anticipated to occur in 2025.
Assignment of interventions, blinding and masking
Randomisation
Owing to the novelty of inhaled sedation in the US ICUs, sites are allowed approximately 3–5 run-in training patients per the full inclusion and exclusion criteria who receive isoflurane in a manner consistent with the remainder of the trial protocol, with the exception that all assessments are non-blinded. These patients are not included in the power calculation of sample size, and data from the run-in patients will not contribute to efficacy analyses. Patients are otherwise randomised in a 1.5:1 ratio to receive isoflurane or propofol as determined by a computer-generated sequence using a central online management system. The approach to randomisation is stratified according to the Simplified Acute Physiology Score (SAPS) III score41 and patient type. SAPS is categorised as 0 to <40, 40 to <60 or ≥60 and patient type is categorised as medical or surgical, as assessed at the time of screening. Surgical patients are considered those falling into at least one of the following categories: trauma; surgery within the two prior weeks and for whom respiratory failure is related to that surgery, surgical disease process or a complication thereof; and patients anticipated to undergo surgery within the next 2 days for a condition that is related to the aetiology of respiratory failure. Study drug is then provided according to randomisation by the local investigational pharmacy.
Blinding and masking
Owing to the complexity of clinical care in the ICU, differing routes and mechanisms of study drug administration (ie, inhaled vs intravenous), and differing pharmacological effects of the study drugs, including their onset and offset, bedside staff are not blinded to the treatment assignment. Treatment assignments are not disclosed to members of the care team not involved in the direct care of the patient. Key study assessments are conducted by blinded assessors trained in the standardised assessment of RASS and Critical Care Pain Observation Tool (CPOT)42 to mitigate the risk of bias. As such, the trial is open-label with blinded assessments. Masking is accomplished by establishing both an inhaled and intravenous drug delivery setup with one being active and the other being non-functional according to treatment assignment. Recognisable components of both setups are physically concealed to mitigate the risk of assessors becoming aware of the treatment assessment (ie, unblinded). Additionally, the standalone gas monitor (see next section for details) is configured to display only capnography, with end-tidal agent concentrations obscured by default but available to the treating clinical team on-demand. If a potential assessor becomes unblinded, this assessor will not participate in further assessments.
Treatment approach, intervention, control and concomitant care
Treatment approach
After randomisation but before study drug initiation, standard of care (SOC) sedative and opioid analgesics are reduced by half unless contraindicated by agitation or pain. Sedative agents are then stopped on initiation of the study drug (isoflurane or propofol) to further minimise confounding of sedation depth assessments and other measures by residual SOC sedative agent use. Opioid medications for analgesia are allowed throughout the study drug treatment period per unit standard pain assessment protocols. All patients receive the allocated study drug for up to 48±6 hours as the primary sedative agent, titrated to achieve the target RASS range of −1 to −4 based on clinical assessments. A standalone end-tidal carbon dioxide and anaesthetic agent monitor (BeneVision N12, Mindray, China) is used for monitoring of exhaled gasses in both arms of the trial alongside SOC physiologic and clinical monitoring.
Intervention: inhaled isoflurane
Inhaled isoflurane is administered via the Sedaconda ACD-S, previously known as the AnaConDa-S. The Sedaconda ACD-S contains a porous plastic evaporator rod, facilitating isoflurane vaporisation and an interwoven lipophilic active carbon filter, facilitating agent conservation as well as heat and moisture exchange. The Sedaconda ACD-S is placed in the ventilator circuit between the tracheal tube and the Y-piece, and isoflurane is delivered to the Sedaconda ACD-S continuously via a syringe pump (figure 1). Unique connectors and fittings on the isoflurane syringe and delivery tubing are designed to prevent unintentional intravenous drug delivery, and the low priming volume (1.2 mL) delivery tubing is permanently fused to the Sedaconda ACD-S. Scavenging of exhaled gasses is accomplished by diverting exhaled gasses from the ventilator exhaust port through an active carbon filter (FlurAbsorb, Sedana Medical AB, Sweden) before exhausting to the ambient atmosphere. The Sedaconda ACD-S introduces approximately 50 mL of dead space into the ventilator circuit; therefore, patients with tidal volumes <200 mL are excluded from the trial given their risk for clinically significant rebreathing. The Sedaconda ACD-S is changed every 24 hours or more often when clinically indicated (eg, secretion burden).
After priming the Sedaconda ACD-S, isoflurane is administered at a starting dose up to 3 mL/hour, and a lower starting dose may be used when clinically indicated (eg, deep sedation or hypotension at baseline). Isoflurane is then titrated according to clinical assessment of sedation depth based on RASS score assessment in recommended increments of 0.5–1 mL/hour to reach the RASS score target. Peak clinical effects are typically noticeable within 10–15 min after an isoflurane dose change. Isoflurane is administered up to a maximum dose of 15 mL/hour, and bolus doses are administered at 0.3–0.5 mL to rapidly deepen sedation when clinically indicated.
Control: intravenous propofol
Propofol is administered intravenously via a conventional infusion pump channel and intravenous tubing at a starting dose of 10–25 µg/kg/min or the pre-randomised dose in patients already receiving propofol for SOC sedation. Propofol is then titrated according to clinical assessment of sedation depth based on RASS score assessment in recommended increments of 5 –10 µg/kg/min every 5–10 min up to a maximum dose of 66 µg/kg/min to reach the RASS score target. Boluses of propofol can be administered in doses of 0.3–0.5 mg/kg to rapidly deepen sedation when clinically indicated.
Spontaneous awakening trials and the wake-up test
Sedation may be interrupted for clinical purposes, such as neurological examinations and/or daily spontaneous awakening trials (SATs), in accordance with SOC. Prior to the end of treatment (EOT), the time to wake-up is measured for all patients guided by a safety screen as delineated in online supplemental appendix B unless prohibited by patient safety considerations in the judgement of the clinical team or investigator. The wake-up test starts when the study drug is stopped and continues until one of four scenarios occurs: RASS≥0 is confirmed by blinded assessment, 4 hours pass after EOT, re-sedation is clinically indicated (eg, due to cardiorespiratory distress), or occurrence of a new-onset neurological deficit or detection of an intracranial event.
During SAT or the wake-up test, medications intended to reduce autonomic stress (eg, agitation), such as antipsychotics, analgesics or α2-adrenergic agonists (eg, dexmedetomidine), are permitted in patients who do not otherwise tolerate awakening. If the patient is deemed to require re-sedation after awakening, the study drug is restarted, and bolus doses of the assigned study drug may be given.
Spontaneous breathing tests and extubation
Daily paired SAT and spontaneous breathing trial (SBT) are considered elements of SOC as described, and extubation is to be performed per the SOC.
End of treatment
Cessation of the study drug is considered EOT when one of three scenarios are met:
Study drug is stopped for extubation,
48±6 hours of study drug treatment, or
Study drug discontinuation based on investigator judgement (ie, when continued treatment is not in the patient’s best interest).
Concomitant care
ABCDEF bundle
Care of patients in the ICU is managed as per the SOC guided by the widely adopted ABCDEF bundle, which has been shown to improve patient outcomes proportional to the compliance of bundle element delivery.20 43 The assessment and monitoring of pain is accomplished via the validated CPOT scale.42 Assessment of both awakening and breathing with paired SAT and SBT is conducted daily when clinically appropriate, guided by a safety screen delineated in online supplemental appendix B. The choice of analgesic and sedative approaches is informed by continual clinical assessment and efforts to decrease unnecessary exposure to relevant agents. Delirium monitoring and management is aimed at maximising non-pharmacological strategies for its prevention and treatment. Strategies that promote early mobility and exercise are associated with reduced delirium and other favourable clinical outcomes. Family engagement is the final component of the ABCDEF bundle, in recognition of the key role that family members and/or surrogate decision makers play in decision-making, treatment planning and support for the patient.
Treatment of pain
Treatment of pain throughout the trial follows the SOC and may include opioids, including infusions, or other non-opioid analgesic medications (eg, acetaminophen, non-steroidal anti-inflammatories). Decisions about analgesic approaches are at the discretion of the clinical team and investigator but should be guided by CPOT assessments. To minimise the potential adverse effects of opioids, it is recommended to use the lowest possible doses to achieve adequate analgesic and comfort.
Rescue sedation and treatment failure
Sedative requirements, whenever possible, are met using the assigned study drug, including active titration and/or bolus dosing up to two times per hour. Rescue sedation, in the context of the trial, is defined as the need for sedative agents other than the assigned study drug to address acute agitation despite adequate analgesia. If the assigned study drug is insufficient to alleviate inadequate sedation despite administration at the maximum dose, adequate analgesia and optimised clinical care (eg, ventilator settings that promote synchrony, positioning to promote comfort), permitted second line rescue sedative approaches include a dexmedetomidine infusion (for no more than 3 hours in a 24-hour period) and/or midazolam bolus doses (no more than three doses in 24 hours). When the assigned study drug and second line rescue sedative maximum doses are exceeded, the patient is considered to have failed treatment, the assigned study drug is stopped and sedation reverts back to the SOC. Full details about rescue sedation and treatment failure are delineated in online supplemental appendix C.
Prohibited and restricted medications
Medications used for the purpose of sedation or paralysis other than the assigned study and rescue drugs are prohibited during the treatment period. Examples of such medications include: barbiturates, chloral hydrate, chlorpromazine, clonidine, gamma-hydroxybutyrate and ketamine. Additionally, paralytics or neuromuscular blocking medications for >4 hours during the treatment period are prohibited as these preclude the ability to maintain the target RASS sedation range of −1 to −4. If prohibited medications are required for patient safety during the treatment period, the patient meets criteria for early study drug discontinuation and transition to SOC. Other sedative and paralytic medications are restricted except in specific circumstances including but not limited to propofol outside of its assignment as a study drug and specific types of procedural sedation in the ICU, benzodiazepines outside of midazolam as a rescue sedative per the protocol, α2-adrenergic agonists outside of dexmedetomidine as a rescue sedative or as part of SAT and wake-up testing, antipsychotics apart from those prescribed prior to ICU admission or as part of SAT and wake-up testing, and neuromuscular blocking drugs outside of application for ≤4 hours for procedures. Full details about prohibited and restricted medications are delineated in online supplemental appendix D.
Sedation for diagnostic or therapeutic procedures in the ICU
The assigned study drug is the primary modality by which to accomplish adequate procedural sedation in the ICU in addition to the treatment of pain, as applicable, per the SOC. Patients assigned to isoflurane requiring airway procedures (eg, bronchoscopy and endotracheal tube suctioning) may receive propofol in either bolus doses of 1–2 mg/kg or as an infusion up to 66 µg/kg/min.
Sedation for diagnostic or therapeutic procedures outside of the ICU
For purposes of this trial, administration of the assigned study drug is confined to the ICU during the treatment period. When sedation outside of the ICU is required (eg, for transfers to operating room or imaging) SOC sedation at the discretion of the clinical team will be administered.
Primary, secondary and exploratory outcomes
Primary outcome
The primary outcome is the percentage of time sedation depth is maintained within the prescribed RASS target range of −1 to −4, in the absence of rescue sedation, through the end of study drug treatment. This parameter will be derived for each patient, as follows:
% adequate sedation=(success time)/(success time+failure time)
‘Success time’ is the time during study drug treatment when blinded RASS falls within −1 to −4.
‘Failure time’ is counted if: (a) blinded RASS is outside the target range (ie, less than −4 or greater than −1), (b) rescue sedation is needed, that is, RASS target is not achieved, despite use of study drug or (c) ‘treatment failure’, where treatment failure is defined as when study drug is deemed insufficient to reach or maintain the target RASS range for sedation, and the resulting amount of rescue sedation meets either of the following criteria: there is a clinical need for infusion of dexmedetomidine for >3 hours per 24 hours; and/or there is a clinical need for >3 midazolam boluses per 24 hours.
If a blinded RASS assessment is not performed per study schedule (missed assessment), the missed assessment will not be accounted for in the primary endpoint but will be counted as failure in a sensitivity analysis. When SOC procedures imply significant change to sedation level, blinded assessments will not be performed. Such omitted blinded RASS assessments due to SOC procedures in or outside the ICU are not considered protocol deviations and are not counted as failure time.
The detail of the analysis and the description of the estimand and missing data handling will be provided in the Statistical Analysis Plan (SAP) finalised prior to database lock.
Secondary and exploratory outcomes
Four key secondary outcomes will be evaluated. First, the change in mean fentanyl-equivalent opioid dose during the study drug treatment period compared with the mean opioid dose during the 60 min prior to randomisation. Second, the time from cessation of study drug treatment to RASS≥0 (up to 4 hours) as ascertained by the wake-up test. Third, delirium and delirium severity as assessed by the Confusion Assessment Method for the Intensive Care Unit-7 (CAM-ICU-7)44 at 60±10 min after EOT in patients clinically appropriate for CAM-ICU-7 assessment, as discussed subsequently. Fourth, the proportion of ventilator parameter observations indicating spontaneous breathing during the study drug treatment period.
Additional secondary outcomes comparing the effects of isoflurane versus propofol on time to extubation, days alive and free of mechanical ventilation (through study day 30), days alive and free of the ICU (through study day 30), delirium and coma free days (until 7 days after EOT), mortality (at 30 days, 3 months and 6 months after randomisation), and use of restraints will be examined. Safety outcomes, exploratory outcomes and exploratory long-term outcomes are delineated in online supplemental appendix E.
Observations and measures, data collection and data management
Observations and measures
A listing of key observations and measures and their associated time points during the trial are delineated in online supplemental table and reviewed subsequently.
RASS and CPOT
RASS is used for the assessment of agitation and sedation throughout the trial, and CPOT is used to evaluate the adequacy of analgesia. Unblinded RASS and CPOT assessments are conducted within 30 min prior to study drug administration, serving as a baseline. For the primary endpoint, blinded RASS assessments begin 2 hours after initiation of the study drug and continue every 2 hours until EOT. A supplemental blinded RASS assessment also occurs within 15 min of EOT to establish a baseline value for the wake-up test. Blinded assessors are instructed to observe the patient for at least 30–60 s, score the lightest RASS observed, and use a shoulder shake (and not a sternal rub) if required to discriminate between a score of −4 and −5. Blinded pain assessment using the CPOT occurs every 2 hours until the EOT. Blinded RASS and CPOT assessment determinations are shared with bedside clinical staff after documentation. SOC assessments of agitation and pain by clinical staff to titrate medications are performed in addition to the blinded study assessments.
The extent to which blinded RASS and CPOT assessments reflect the overall depth of sedation may be confounded by clinically appropriate intentional deepening of sedation, lightening of sedation or the need for neuromuscular blocking agents. To that end, the protocol provides criteria for resumption of blinded RASS and CPOT assessments when these interventions are administered (see online supplemental appendix F).
Confusion Assessment Method for the Intensive Care Unit-7
Cognitive recovery at 60±10 min after EOT is evaluated by a blinded assessor using the CAM-ICU-7. This blinded assessor also ascertains the RASS at this time point as the evaluation could be confounded by deeper level of sedation. Patients already re-sedated under the auspices of the SOC are excluded, as well as patients with an RASS of −4 or −5. CAM-ICU-7 is assessed at least daily during the treatment period and through 7 days after EOT or until hospital discharge, whichever comes first.
Physical and neurocognitive function and outcomes
Activities of daily living and cognition at baseline are measured by the Katz Index of Independence in Activity of Daily Living,45 Pfeffer functional activities questionnaire46 and Informant Questionnaire on Cognitive Decline in the Elderly Short Form.47 Long-term psychological and cognitive outcome assessments are conducted by blinded neuropsychology professionals over telephone at 3 months (±4 weeks) and 6 months (±4 weeks) using a comprehensive battery of instruments.
Other parameters
Patient characteristics, concomitant medications (eg, analgesics, sedatives, vasopressors), organ function assessed by Sequential Organ Failure Assessment (SOFA) score, ventilator parameters, laboratory findings (including arterial blood gas analysis), major ICU interventions, other clinical complications, length of stay, disposition and mortality are all assessed at specified intervals throughout the study period.
Data collection and management
Sites record data using an electronic case report form (eCRF), which is verified against source documentation by clinical research associates and reviewed during regularly occurring on-site and remote monitoring visits. Validation checks programmed within the eCRF, as well as supplemental validation performed via review of the downloaded data, is applied to the data to ensure accuracy, consistency and reliability. Audits may be performed at individual sites to ensure data validity. Research staff at sites receive relevant training to support adherence to data collection and management protocols. The eCRF is used to facilitate automatic data validation alongside regular review and ad hoc checks of the entered data. Any known or suspected errors are referred to the relevant site for resolution. All corrections or changes made to any trial data are appropriately tracked in an audit trail in compliance with Title 21 of the Code of Federal Regulations Part 11.
Education and training, study withdrawal, adherence and monitoring
Education and training
Site research staff are trained to support successful protocol implementation, associated study procedures, and data collection and management. Given that inhaled isoflurane for routine sedation during mechanical ventilation in the ICU has been previously unavailable in the USA, a set of complete written, summary and audiovisual educational tools have been developed for the training of physician, nurse and respiratory therapy clinical staff, as well as investigational pharmacy. These educational efforts include multidisciplinary training in the setup, use, maintenance and discontinuation of the Sedaconda ACD-S and related equipment and other relevant protocol elements. Sites are additionally supported by dedicated educational staff.
Early study drug discontinuation and/or withdrawal
In certain instances, investigators may determine that continued study drug treatment is not in the best interest of the patient, warranting early study drug discontinuation. Similarly, clinical or other circumstances may warrant withdrawal from the trial. Criteria for early study drug discontinuation and study withdrawal are delineated in online supplemental appendix G.
Adherence
Investigators are charged with ensuring protocol adherence, and clinical research associates regularly monitor all participating centres to verify adherence. The principal investigators hold regular investigator meetings with the Sponsor to discuss trial updates and monitor trial progress, aid in the monitoring of adherence, provide feedback about quality and safety-related matters, review any site-specific issues and discuss adverse events.
Monitoring
At EOT, vital signs, laboratory assessments, adverse events and the time of extubation (among other variables summarised in online supplemental table are monitored for 24 hours. Patients are then monitored daily for 7 days for adverse events, relevant medications, RASS, CAM-ICU-7 and SOFA score. Additional assessments occur at day 30 (+5) days, including major ICU interventions, adverse events and outcomes. Exploratory long-term outcomes are assessed with phone calls in a consecutive subset of patients still alive at 3 months (±4 weeks) and 6 months (±4 weeks).
An independent data safety monitoring board (DSMB) monitors the safety of trial patients for both INSPiRE-ICU1 and the parallel INSPiRE-ICU2 trial. The DSMB is comprised of four members with appropriate expertise who are independent of the Sponsor. The DSMB reviews all relevant safety data, including adverse events, severe adverse events, serious adverse events and suspected unexpected serious adverse reactions. Adverse events of special interest for this trial are delineated in online supplemental appendix H. DSMB meetings are planned after approximately 25% of randomised patients in the two studies have completed the 30-day follow-up period and again when 50% and 75% of patients have completed 30-day follow-up. Stopping criteria are delineated in online supplemental appendix I. All DSMB recommendations apply to both INSPiRE-ICU1 and INSPiRE-ICU2 given the similarity of the studies.
Protocol amendments
The protocol details described herein are based on INSPiRE-ICU protocol Version 7, dated 6 October 2023. The timing of this protocol publication was informed by prior protocol amendments and a desire for the published protocol to most closely reflect and align with the eventual trial results published.
INSPiRE-ICU1 protocol Version 3 (dated 9 February 2022) was the first under which patients were enrolled, with Versions 1 and 2 having been revised in response to US FDA and IRB reviews.
Compared with Version 3, revisions to protocol Version 4 (dated 31 March 2022) included: expanded exclusion criteria for patients with contraindications to propofol or isoflurane, updated the approach to concomitant medication collection through day 30 for consistency, clarified the timing of day 30 study procedures and revised an original plan to monitor ABCDEF bundle compliance to instead focus on emphasis of ABCDEF compliance.
Protocol Version 5 (dated 30 May 2023) was developed but not submitted as FDA feedback was received on 31 May 2023, requiring further protocol review and revision. As such, no patients were enrolled under Version 5.
Compared with Version 4, revisions to protocol Version 6 (dated 22 June 2023) included: making specific assessments less frequent with wider time windows to enhance trial practicability, disallowing continuation of study drug after a failed extubation attempt to ensure validity of related endpoint measures, allowing more flexible isoflurane dosing and titration guided by clinical response, clarifying the approach to rescue sedation, clarifying circumstances in which blinded RASS and CPOT assessments are excluded, adding a blinded RASS assessment immediately prior to EOT, clarifying exclusion criterion #2 for re-intubated patients, and providing specific examples of severe neurological conditions constituting exclusions under criterion #3.
Compared with Version 6, revisions to protocol Version 7 (dated 6 October 2023 and described herein) included: clarifying that study assessments could be performed more frequently than specified in the protocol as clinically indicated, added meningitis as an additional example to exclusion criterion #3, defining study drug boluses as a rescue sedative and clarifying the analytic approach as outlined subsequently.
Data analysis
Categorical data will generally be summarised with counts and percentages of patients. The denominator used for the percentage calculation will be clearly defined. Continuous data will generally be summarised with descriptive statistics including n (number of non-missing values), mean and SD or median and IQR, minimum, and maximum. The SAP will be finalised before the database lock.
A fixed sequential testing procedure will be implemented. In a hierarchical step-down manner, the primary efficacy endpoint will be tested at the one-sided 0.025 level first (non-inferiority test), followed by testing the key secondary efficacy endpoints at the two-sided 0.05 level (superiority test) in the following hierarchical manner:
Change in mean fentanyl-equivalent opioid dose during the study drug treatment period compared with mean opioid dose during the 60 min prior to randomisation.
Time from stop of study drug treatment to RASS 0, up to 4 hours.
Delirium by CAM-ICU-7 assessments 60 min (±10 min) after EOT in patients not re-sedated with benzodiazepine or propofol infusions.
Proportion of ventilator parameter observations with spontaneous breathing efforts during the study drug treatment period.
Only the primary efficacy endpoint analysis will use non-inferiority testing; the other efficacy endpoints analyses will use superiority testing.
The primary analysis on the primary efficacy endpoint will be performed based on an analysis of variance (ANOVA) model, including treatment group and stratification factor (SAPS III (0 to <40, >40 to <60 and >60) and patient type (medical and surgical)) as fixed effects.
The treatment comparisons will be estimated together with a one-sided 97.5% CI and p value for the hypothesis testing. Least squares mean for each treatment group will also be provided. The primary analysis will be performed on the intention to treat (ITT) Analysis Set. The hypothesis test for primary efficacy endpoint analysis is based on a one-sided significance level of 0.025. The primary efficacy endpoint will be summarised for the ITT Analysis Set by stratification factor. ANOVA model will be used to analyse the primary efficacy endpoint for each subgroup, which will include randomised treatment group as a fixed effect. Sensitivity and supplementary analyses will be specified in the SAP.
The analyses of the key secondary efficacy endpoints will be performed on the ITT Analysis Set (superiority analysis), unless otherwise specified. The hypothesis tests for the key secondary endpoint analyses are based on a two-sided significance level of 0.05.
No interim analysis of outcome data is planned for this trial. All safety analyses will be performed on the Safety Analysis Set. Patients will be analysed by the treatment received. Safety measures will be summarised descriptively. Qualitative variables will be summarised using counts and percentages by treatment group at each trial visit. A separate analysis and study report will be performed for the long-term outcomes (3 and 6 months) once all patients have performed the 6-month follow-up.
Ethics and dissemination
Ethical approvals were obtained from local IRB for each study site prior to patient recruitment. The trial protocol and appropriate documentation was reviewed and approved by the US FDA, as well as central (Advarra SSU00208265) and local IRBs (Cleveland Clinic IRB FWA 00005367, Tufts HS IRB 20221969, Houston Methodist IRB PRO00035247, Mayo Clinic IRB Mod22-001084-08, University of Chicago IRB21-1917-AM011 and Intermountain IRB 033175).
Continuing review processes occur as needed with final packet submission to be sent on trial completion. The trial is conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation guideline for Good Clinical Practice, and applicable local regulatory bodies. Written informed consent is obtained from the patient or patient’s legally authorised representative (LAR) prior to the initiation of any study procedure. Final trial dataset will be available to investigators and provided within FDA submission. Datasets will be stored at Sedana Medical AB for at least 10 years following trial completion.
Trial results for publication will be submitted to peer-reviewed journals and the results will be presented at one or more scientific conferences with an expected timeframe for publication of 2025. The data will also be submitted to the FDA by Sedana Medical AB.
Protocol changes
ClinicalTrials.gov will be updated with any amendments to the protocol as per SPIRIT guidelines.
supplementary material
Footnotes
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-086946).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Christina Boncyk, Email: christina.s.boncyk@vumc.org.
John W Devlin, Email: jwdevlin@bwh.harvard.edu.
Hina Faisal, Email: hfaisal@houstonmethodist.org.
Timothy D Girard, Email: timothy.girard@pitt.edu.
Steven H Hsu, Email: shhsu@mdanderson.org.
Craig S Jabaley, Email: csjabaley@emory.edu.
Ida Sverud, Email: ida.sverud@sedanamedical.com.
Magnus Falkenhav, Email: magnus.falkenhav@sedanamedical.com.
John Kress, Email: jkress@bsd.uchicago.edu.
Karen Sheppard, Email: karen.l.sheppard@vumc.org.
Peter V Sackey, Email: peter.sackey@sedanamedical.com.
Christopher G Hughes, Email: christopher.hughes@vumc.org.
References
- 1.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018;46:e825–73. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 2.Fulton B, Propofol SEM. An overview of its pharmacology and a review of its clinical efficacy in intensive care sedation. Drugs Oct. 1995;50:636–57. doi: 10.2165/00003495-199550040-00006. [DOI] [PubMed] [Google Scholar]
- 3.Barr J, Egan TD, Sandoval NF, et al. Propofol dosing regimensDosing Regimens for ICU sedation based upon an integrated pharmacokinetic– pharmacodynamic modeSedation Based upon an Integrated Pharmacokinetic– Pharmacodynamic Model. Anesthesiology. 2001;95:324–33. doi: 10.1097/00000542-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Krajčová A, Waldauf P, Anděl M, et al. Propofol infusion syndrome: a structured review of experimental studies and 153 published case reports. Crit Care. doi: 10.1186/s13054-015-1112-5. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maze M, Scarfini C, Cavaliere F. New agents for sedation in the intensive care unit. Crit Care Clin. 2001;17:881–97. doi: 10.1016/s0749-0704(05)70185-8. [DOI] [PubMed] [Google Scholar]
- 6.Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: patient and clinician perceptions †. Br J Anaesth. 2001;87:684–90. doi: 10.1093/bja/87.5.684. [DOI] [PubMed] [Google Scholar]
- 7.Weerink MAS, Struys M, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidinePharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin Pharmacokinet. 2017;56:893–913. doi: 10.1007/s40262-017-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shehabi Y, Serpa Neto A, Bellomo R, et al. Dexmedetomidine and propofol sedation in criticallyPropofol Sedation in Critically Ill patients and dose-associated 90-day mortality: a secondary cohort analysis of a randomized controlled tri Patients and Dose-associated 90-Day Mortality: A Secondary Cohort Analysis of a Randomized Controlled Trial (SPICE III) Am J Respir Crit Care Med. doi: 10.1164/rccm.202206-1208OC. n.d. [DOI] [PubMed] [Google Scholar]
- 9.Shelly MP, Sultan MA, Bodenham A, et al. Midazolam infusions in critically ill patients. Eur J Anaesthesiol. 1991;8:21–7. [PubMed] [Google Scholar]
- 10.Carrasco G, Molina R, Costa J, et al. Propofol vs midazolam in short-, medium-, and long-term sedation of critically ill patients. A cost-benefit analysis. Chest. 1993;103:557–64. doi: 10.1378/chest.103.2.557. [DOI] [PubMed] [Google Scholar]
- 11.Chamorro C, Latorre FJ, Montero A, et al. Comparative study of propofol versus midazolam in the sedation of critically ill patients: results of a prospective, randomized, multicenter trial. Crit Care Med Jun. 1996;24:932–9. doi: 10.1097/00003246-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Hall RI, Sandham D, Cardinal P, et al. Propofol vs midazolam for ICU sedation: a Canadian multicenter randomized trial. Chest Apr. 2001;119:1151–9. doi: 10.1378/chest.119.4.1151. [DOI] [PubMed] [Google Scholar]
- 13.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 301:489–99. doi: 10.1001/jama.2009.56. n.d. [DOI] [PubMed] [Google Scholar]
- 14.Lonardo NW, Mone MC, Nirula R, et al. Propofol is associated with favorable outcomes compared with benzodiazepines in ventilated intensive care unit patients. Am J Respir Crit Care Med. 2014;189:1383–94. doi: 10.1164/rccm.201312-2291OC. [DOI] [PubMed] [Google Scholar]
- 15.Zaal IJ, Devlin JW, Hazelbag M, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 2015;41:2130–7. doi: 10.1007/s00134-015-4063-z. [DOI] [PubMed] [Google Scholar]
- 16.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–53. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 17.Kawazoe Y, Miyamoto K, Morimoto T, et al. Effect of dexmedetomidine on mortality and ventilator-free days in patients requiring mechanical ventilation with sepsis: a randomized clinical triaDexmedetomidine on Mortality and Ventilator-Free Days in Patients Requiring Mechanical Ventilation With Sepsis: A Randomized Clinical Trial. JAMA. doi: 10.1001/jama.2017.2088. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shehabi Y, Howe BD, Bellomo R, et al. Early sedation with dexmedetomidine in criticallySedation with Dexmedetomidine in Critically Ill patientsPatients. N Engl J Med n.d. [Google Scholar]
- 19.Hughes CG, Mailloux PT, Devlin JW, et al. Dexmedetomidine or propofol for sedation in mechanically ventilated adults with sepsisPropofol for Sedation in Mechanically Ventilated Adults with Sepsis. N Engl J Med. doi: 10.1056/NEJMoa2024922. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically Critically Ill patients with thePatients with the ABCDEF bundle: results of theBundle: Results of the ICU liberation collaborative in over 15,000 adulLiberation Collaborative in Over 15,000 Adults. Crit Care Med. 2019;47:3–14. doi: 10.1097/CCM.0000000000003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pun BT, Badenes R, Heras La Calle G, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. 2021;9:239–50. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephens RJ, Evans EM, Pajor MJ, et al. A dual-center cohort study on the association between early deep sedation and clinical outcomes in mechanically ventilated patients during the COVID-19 pandemic: The COVID-SED study. Crit Care. 2022;26:179. doi: 10.1186/s13054-022-04042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wongtangman K, Santer P, Wachtendorf LJ, et al. Association of sedation, coma, and in-hospital mortality in mechanically ventilated patients with coronavirus disease 2019-related acute respiratory distress syndrome: a retrospective cohort studySedation, Coma, and In-Hospital Mortality in Mechanically Ventilated Patients With Coronavirus Disease 2019-Related Acute Respiratory Distress Syndrome: A Retrospective Cohort Study. Crit Care Med. doi: 10.1097/CCM.0000000000005053. n.d. [DOI] [PubMed] [Google Scholar]
- 24.Eger EI. The pharmacology of isoflurane. Br J Anaesth. 1984;56 Suppl 1:71S–99S. [PubMed] [Google Scholar]
- 25.Kong KL, Willatts SM, Prys-Roberts C. Isoflurane compared with midazolam for sedation in the intensive care unit. BMJ. 1989;298:1277–80. doi: 10.1136/bmj.298.6683.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newton DE, Thornton C, Konieczko K, et al. Levels of consciousness in volunteers breathing sub-MAC concentrations of isoflurane. Br J Anaesth. 1990;65:609–15. doi: 10.1093/bja/65.5.609. [DOI] [PubMed] [Google Scholar]
- 27.Zacny JP, Sparacino G, Hoffmann P, et al. The subjective, behavioral and cognitive effects of subanesthetic concentrations of isoflurane and nitrous oxide in healthy volunteers. Psychopharmacology (Berl) 1994;114:409–16. doi: 10.1007/BF02249330. [DOI] [PubMed] [Google Scholar]
- 28.Sackey PV, Martling C-R, Nise G, et al. Ambient isoflurane pollution and isoflurane consumption during intensive care unit sedation with the Anesthetic Conserving Device. Crit Care Med. 2005;33:585–90. doi: 10.1097/01.ccm.0000156294.92415.e2. [DOI] [PubMed] [Google Scholar]
- 29.Sackey PV, Martling C-R, Granath F, et al. Prolonged isoflurane sedation of intensive care unit patients with the Anesthetic Conserving Device. Crit Care Med. 2004;32:2241–6. doi: 10.1097/01.ccm.0000145951.76082.77. [DOI] [PubMed] [Google Scholar]
- 30.Hellström J, Öwall A, Sackey PV. Wake-up times following sedation with sevoflurane versus propofol after cardiac surgery. Scand Cardiovasc J. 2012;46:262–8. doi: 10.3109/14017431.2012.676209. [DOI] [PubMed] [Google Scholar]
- 31.Jabaudon M, Boucher P, Imhoff E, et al. Sevoflurane for sedation in acute respiratory distress syndrome. a randomized controlled pilot studySedation in Acute Respiratory Distress Syndrome. A Randomized Controlled Pilot Study. Am J Respir Crit Care Med. doi: 10.1164/rccm.201604-0686OC. n.d. [DOI] [PubMed] [Google Scholar]
- 32.Meiser A, Volk T, Wallenborn J, et al. Inhaled isoflurane via the anaesthetic conserving device versus propofol for sedation of invasively ventilated patients in intensive care units in Germany and Slovenia: an open-label, phase 3, randomised controlled, non-inferiority trial. Lancet Respir Med. 2021;9:1231–40. doi: 10.1016/S2213-2600(21)00323-4. [DOI] [PubMed] [Google Scholar]
- 33.Bracht H, Meiser A, Wallenborn J, et al. ICU- and ventilator-free days with isoflurane or propofol as a primary sedative – A post- hoc analysis of a randomized controlled trial. J Crit Care. 2023;78:154350. doi: 10.1016/j.jcrc.2023.154350. [DOI] [PubMed] [Google Scholar]
- 34.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–16. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuninghame S, Jerath A, Gorsky K, et al. Corrigendum to “Effect of inhaled anaesthetics on cognitive and psychiatric outcomes in critically ill adults: a systematic review and meta-analysis” (Br J Anaesth 2023; 131: 314-27) Br J Anaesth. 2023;131:788. doi: 10.1016/j.bja.2023.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Prin M, Wunsch H. International comparisons of intensive care: informing outcomes and improving standards. Curr Opin Crit Care. 2012;18:700–6. doi: 10.1097/MCC.0b013e32835914d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burns KEA, Raptis S, Nisenbaum R, et al. International practice variation in weaning criticallyPractice Variation in Weaning Critically Ill adults from invasive mechanical ventilationAdults from Invasive Mechanical Ventilation. Ann ATS. 2018;15:494–502. doi: 10.1513/AnnalsATS.201705-410OC. [DOI] [PubMed] [Google Scholar]
- 38.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–7. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 40.Jakob SM, Ruokonen E, Grounds RM. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilationMidazolam or Propofol for Sedation During Prolonged Mechanical Ventilation. JAMA. doi: 10.1001/jama.2012.304. n.d. [DOI] [PubMed] [Google Scholar]
- 41.Metnitz PGH, Moreno RP, Almeida E, et al. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 1: Objectives, methods and cohort description. Intensive Care Med. 2005;31:1336–44. doi: 10.1007/s00134-005-2762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gélinas C, Fillion L, Puntillo KA, et al. Validation of the critical-care pain observation tool in adult patients. Am J Crit Care. 2006;15:420–7. [PubMed] [Google Scholar]
- 43.Marra A, Ely EW, Pandharipande PP, et al. The ABCDEF bundle in critical careBundle in Critical Care. Crit Care Clin. 2017;33:225–43. doi: 10.1016/j.ccc.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan BA, Perkins AJ, Gao S, et al. The confusion assessment method for thConfusion Assessment Method for the ICU-7 delirium severity scale: a novel delirium severity instrument for use in thDelirium Severity Scale: A Novel Delirium Severity Instrument for Use in the ICU. Crit Care Med. 2017;45:851–7. doi: 10.1097/CCM.0000000000002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of adl: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 46.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–9. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 47.Jorm AF, Korten AE. Assessment of cognitive decline in the elderly by informant interview. Br J Psychiatry. 1988;152:209–13. doi: 10.1192/bjp.152.2.209. [DOI] [PubMed] [Google Scholar]