Abstract
Echocardiographic diagnosis of cardiac amyloidosis (CA) is frequently suggested by the presence of a left ventricular (LV) apical sparing pattern (ASP) on longitudinal strain (LS) assessment, the so-called “cherry on top” pattern, defined by strain magnitude preserved exclusively at the apex. However, it is unclear how frequently this strain pattern truly represents CA. This study aimed to evaluate the predictive value of ASP in the diagnosis of CA. We retrospectively identified consecutive adult patients who had the following studies performed within an 18-month period: (1) transthoracic echocardiogram and (2) either (a) cardiac magnetic resonance imaging, (b) Technetium-Pyrophosphate (PYP) imaging, or (c) endomyocardial biopsy. LS was retrospectively measured in the apical 4-, 3-, and 2-chamber views in patients who had adequate noncontrast images (n = 466). An apical sparing ratio (ASR) was calculated as (average apical strain)/[(average basal strain) + (average midventricular strain)]. Patients with ASR ≥1 were evaluated for the presence/absence of CA, using established criteria. Basic LV parameters were also measured. A total of 33 patients (7.1%) had ASP. Nine of these patients (27%) had “confirmed” CA, 2 (6.1%) “highly probable” CA, 1 (3.0%) “possible” CA, and 21 (64%) no evidence of CA. When comparing patients with and without confirmed CA, there were no significant differences in ASR, average global LS, ejection fraction, or LV mass. Patients with confirmed CA were older (76 ± 9 vs 59 ± 18 years, p = 0.01) and had thicker posterior wall (15 ± 3 vs 11 ± 3 mm, p = 0.004) with a trend toward thicker septal wall (15 ± 2 vs 12 ± 4 mm, p = 0.05). In conclusion, the presence of ASP on LS represents confirmed or highly probable CA in only 1/3 of patients and is more likely to indicate true CA in older patients with increased LV wall thickness. Although a larger, prospective study is needed to confirm these findings, 1/3 should be considered as a large diagnostic yield that justifies further testing, given the poor outcomes associated with CA diagnosis.
Cardiac amyloidosis (CA) is an infiltrative restrictive cardiomyopathy caused by extracellular deposition of amyloid fibrils in the myocardium. The most common amyloid sub-types affecting the heart involve misfolded transthyretin (TTR) (either mutant variants or wild-type) or monoclonal immunoglobulin light chains from an abnormal clonal population, resulting in TTR or light chain (AL) CA, respectively.1 Given management strategies for both TTR and AL amyloid have evolved over the past several years, there is now a manifest mortality benefit with current treatment options.2,3 Establishing a diagnosis early in patients is thus paramount; however, identifying patients with suspected CA and confirming the diagnosis remain challenging.4 Transthoracic echocardiography (TTE) is a ubiquitous tool that provides an opportunity to screen for patients who may benefit from further workup for CA. In particular, the ability of 2-dimensional echocardiographic speckle-tracking to calculate longitudinal deformation in individual myocardial segments provides a valuable technique to this effect. Specifically, the presence of an apical sparing pattern (ASP) on longitudinal strain (LS), a pattern colloquially referred to as the “cherry on top,” has been associated with CA. Phelan et al5 first described this pattern in 2012 and reported great sensitivity and specificity for CA when compared with controls with hypertrophic cardiomyopathy or aortic stenosis. However, it is not well known how often ASP exists without CA or what conditions are associated with this finding in the absence of CA. Accordingly, this study sought to determine the positive predictive value (PPV) of ASP for the diagnosis of CA, which we hypothesized was lower than previously described when studying a broader population rather than in a targeted comparison as in the previous report.5
Methods
A subset of consecutive adult patients at the University of Chicago Medical Center who had the following studies performed within an 18-month period (from January 2020 through June 2021) were retrospectively identified: (1) TTE and (2) either (a) cardiac magnetic resonance (CMR) (b) Technetium-Pyrophosphate (PYP) scan, or (c) endomyocardial biopsy. These inclusion criteria were met by 682 patients. Global and regional LS in 18 segments were measured using QLAB Philips Healthcare (Cambridge, MA) in all patients with adequate noncontrast apical 4-chamber, 3-chamber, 2-chamber views that depicted clear endocardial borders in all segments. Patients without sufficient image quality for strain assessment were excluded from the study, resulting in a group of 466 patients. An apical sparing ratio (ASR) was calculated as the ratio of the average apical strain to the sum of the average basal strain and the average midventricular strain.
Patients with an ASR ≥1 were considered to reveal an ASP.5
For all patients who met ASP criteria, demographic information and clinical co-morbidities were assessed on the basis of the electronic health record, and additional echocardiographic parameters including wall thickness and ejection fraction were retrospectively measured. Clinical data for patients with ASP were evaluated by a physician with expertise in CMR and amyloidosis to determine whether a diagnosis of CA was established, with comprehensive review of all available CMR images, PYP scans, biopsy results, genetic analyses, and/or laboratory data (including serum and urine protein electrophoresis/immunofixation and serum light chains).4,6,7 Patients were classified on the basis of likelihood of CA using criteria listed in Table 1.4,6−8 Student’s t tests were used to compare characteristics of patients with ASP with and without CA. Statistical significance was defined as p >0.05.
Table 1.
Classification criteria for cardiac amyloidosis
| Confirmed Cardiac Amyloid | Highly Probable Cardiac Amyloid | Possible Cardiac Amyloid | No Evidence of Cardiac Amyloid |
|---|---|---|---|
| Positive endomyocardial biopsy | Typical CMR findings without other confirmatory data and absence of clear alternate diagnosis | Some typical CMR findings but not diagnostic without additional concerning findings | CMR or biopsy findings not consistent with CA |
| Grade 3 Technetium-Pyrophosphate Imaging (PYP) | Grade 2 PYP without typical CMR findings | ||
| Grade 2 PYP with typical cardiac magnetic resonance imaging (CMR) findings (elevated T1, elevated ECV, patchy circumferential LGE, abnormal gadolinium kinetics) | Evidence of monoclonal protein without available CMR or EMB | ||
| Typical CMR findings with evidence of amyloidosis on other organ biopsy (bone marrow, renal, fat pad) or presence of a monoclonal protein |
Results
Of the 466 patients assessed, 33 patients (7.1%) were found to meet the criteria for ASP (Figure 1). Of the patients with ASP, the mean age at the time of the echocardiogram was 63 ± 17 years; 13 (40%) were female, and 24 (73%) were Black. A total of 27 patients (82%) had hypertension, and 19 (58%) had at least moderate chronic kidney disease.
Figure 1.
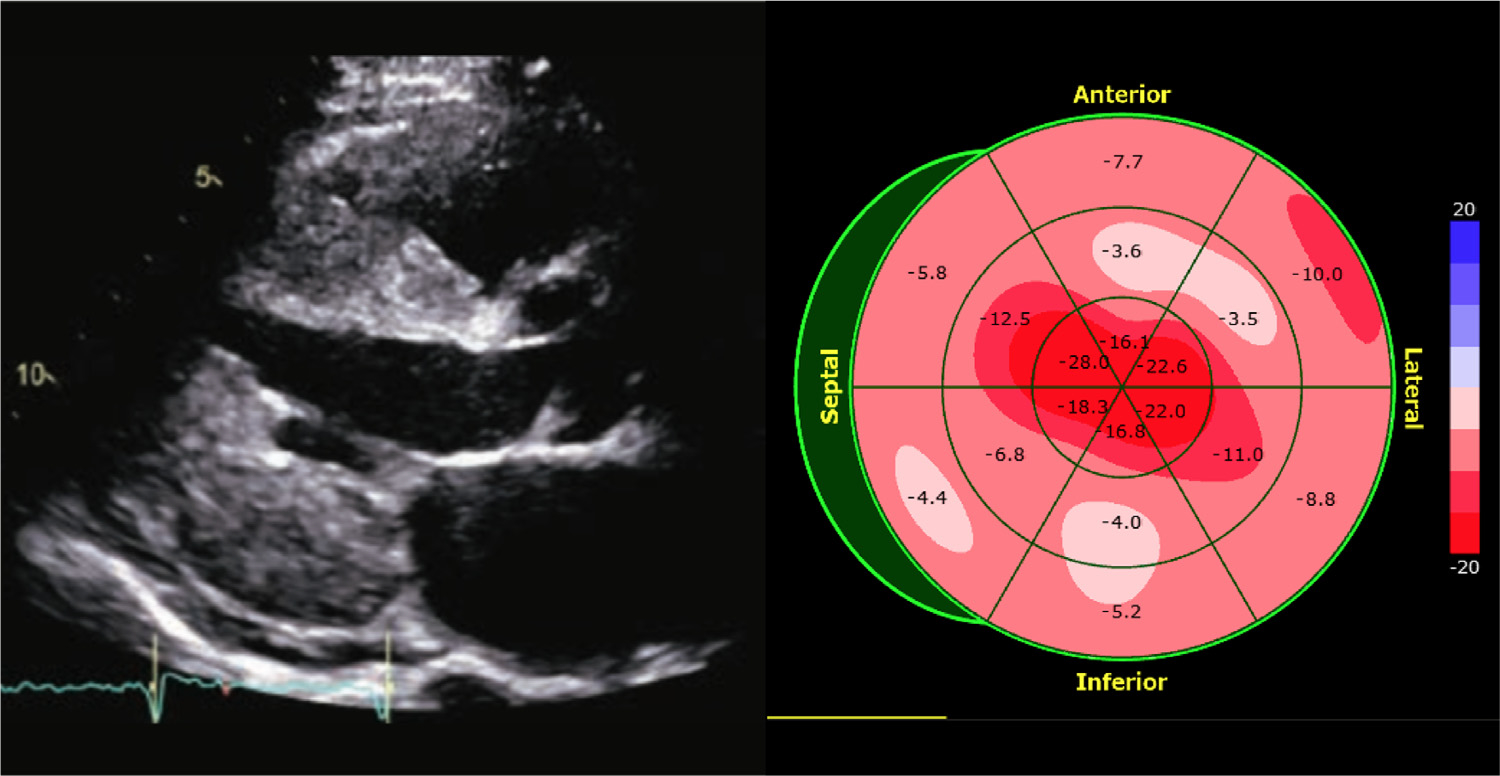
Example of an apical sparing pattern of LV strain on echocardiogram of a patient with suspected cardiac amyloidosis. (Left): parasternal long-axis view; (right): a bull’s eye display of longitudinal strain depicting preserved strain at the apex in the presence of severely reduced strain magnitude in mid- and basal LV segments.
All 33 patients with ASP had additional diagnostic testing performed that could help determine the presence of CA: 29 (87%) had a recent cardiac MRI scan; 10 (30%) had a PYP scan; 15 (45%) had serum and urine electrophoresis/immunofixation and serum light chain assessment; 5 (15%) had an endomyocardial biopsy performed, and 8 (25%) had an extracardiac biopsy performed.
Of the 33 patients with ASP, 9 (27%) had confirmed CA; 2 (6.1%) were classified as highly probable for CA diagnosis; 1 (3.0%) was identified as possible CA, and 21 (64%) received negative test results for CA (Figure 2). The PPV for ASP resulting in a diagnosis of either confirmed or highly probable CA was 33%. Of the 9 patients with CA, 6 had TTR, and 3 had AL amyloidosis.
Figure 2.
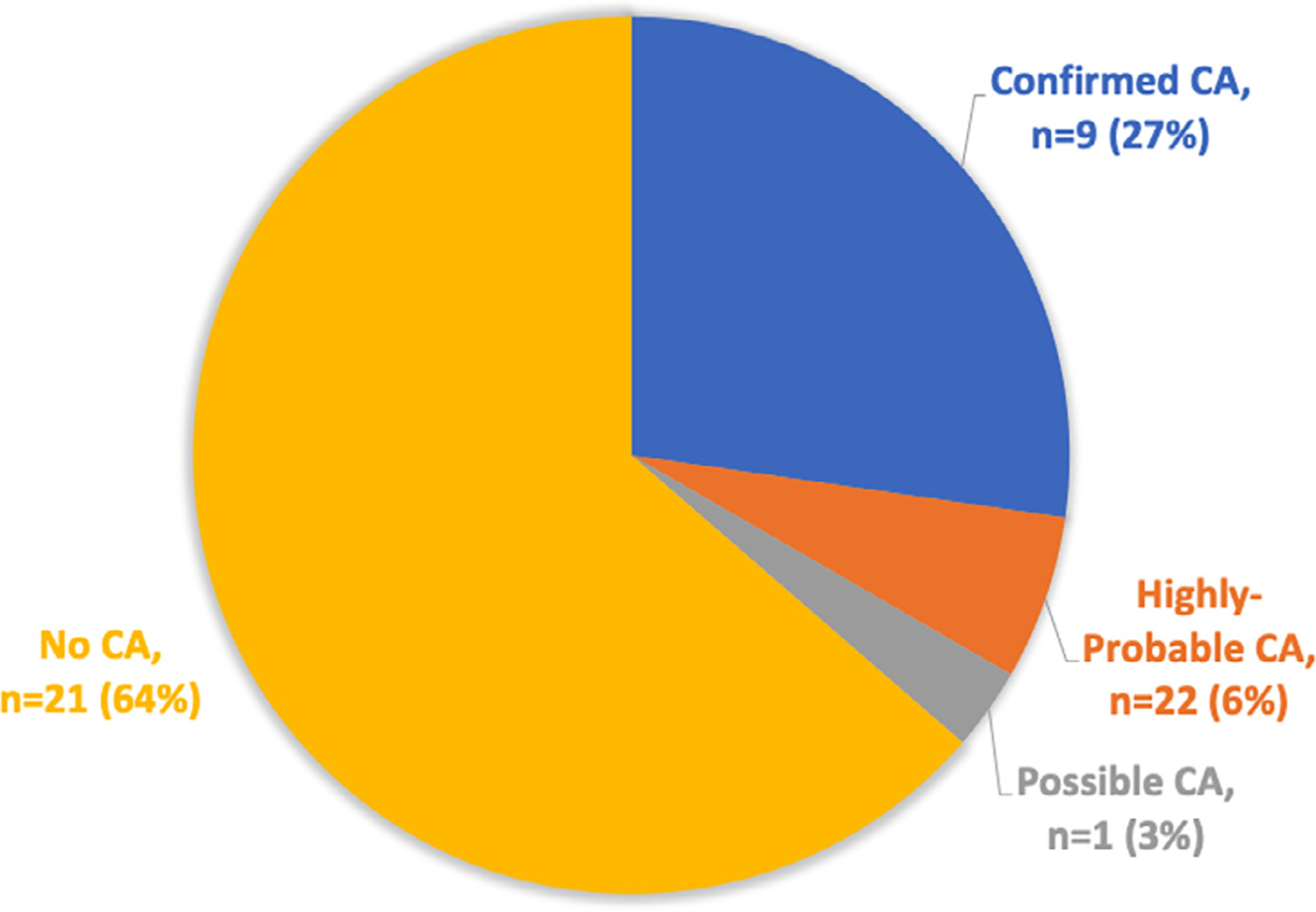
Patients with apical sparing strain pattern, stratified by presence or absence of CA.
Seven of the 9 patients with confirmed CA had a cardiac MRI with suggestive findings of CA; 4 had a grade 3 or 4 PYP scan result; 2 had an endomyocardial biopsy result positive for CA, and 2 had a positive extracardiac biopsy result for amyloid. Three patients had evidence of a paraprotein on electrophoresis/immunofixation and light chain assessment. Two patients had genetic testing consistent with TTR amyloid.
Among patients with ASP, there was no statistically significant difference in global LS or ASR between patients with and those without confirmed CA. Patients with confirmed CA were older and more likely to have increased posterior wall thickness (15 ± 3 vs 11 ± 3 mm, p = 0.004), resulting in an increased relative wall thickness (0.76 ± 0.3 vs 0.49 ± 0.1 mm, p = 0.004). There was also a trend toward increased septal wall thickness (15 ± 2 vs 12 ± 4 mm, p = 0.05). There was no significant difference in ejection fraction or LV mass between the 2 groups (Table 2).
Table 2.
Comparison of positive apical sparing pattern patients with and without cardiac amyloid.
| Age | Total Global Longitudinal Strain (%) | Apical Sparing Ratio | Ejection Fraction (%) | Septal Wall Thickness (mm) | Posterior Wall Thickness (mm) | Relative Wall Thickness (mm) | LV mass (g) | |
|---|---|---|---|---|---|---|---|---|
| Confirmed CA (n=9) | 76±9 | −13±4 | 1.2±0.1 | 50±15 | 15±2 | 15±3 | 0.76±0.3 | 251±41 |
| No CA (n=21) | 59±18 | −12±4 | 1.3±0.3 | 44±15 | 12±4 | 11±3 | 0.49±0.1 | 216±82 |
| P-Value | 0.01 | 0.75 | 0.39 | 0.28 | 0.05 | 0.004 | 0.004 | 0.23 |
Discussion
The finding of ASP of LS presents a potential opportunity to identify patients who should be further screened for CA; however, the value of additional testing in this population has not previously been well studied. The seminal 2012 study indicating the role of LS in patients with suspected CA found that the presence of ASP had a sensitivity 93% and specificity of 82% for distinguishing CA from controls with either known hypertrophic cardiomyopathy or aortic stenosis.5 However, when a patient without differentiation who presents to the echocardiography lab is incidentally found to have ASP, the evidence supporting more testing is less robust. Specifically, it remains not known whether ASP can be attributed to other conditions and how commonly this finding is associated with CA when assessing all-comers. Our aim in this study was to identify a cohort of patients with both ASP and further diagnostic evaluation for CA (including at minimum either a CMR, PYP scan, or endomyocardial biopsy) completed within a short time frame of TTE, to ascertain what the anticipated yield of additional testing may be in this population.
In our study of a broad population of sequential patients referred for TTE, only 1/3 of patients with ASP were found to have either confirmed or highly probable CA. More than 60% of patients with ASP who had additional testing performed were ultimately not found to have CA. Interestingly, the degree of ASP (as assessed by the ASR) was not associated with CA. Another previously unknown finding is that younger patients and those with normal wall thickness were less likely to have CA, despite having ASP.
The concept of ASP without CA is not entirely novel. Although there is significant literature supporting the use of ASP in screening for CA, the prevalence of ASP in patients without CA has been previously described, particularly in patients with aortic stenosis.9−11 A prospective study in 150 patients who underwent surgical aortic valve replacement found that although 23 patients (15%) had ASP on the preprocedural TTE, none of these patients had CA on subsequent CMR or myocardial biopsies.11 A subanalysis of the Impact of AMY-TAVI (Amyloidosis on transcatheter aortic valve implantation Patients) trial also assessed LS patterns in patients with severe AS who did not have evidence of CA on PYP scan or serum protein electrophoresis, and found that 42% of these patients had ASP without having CA.10
It has been recently postulated that the pathophysiologic basis of ASP is likely related to segmental differences in distribution of total amyloid volume.12 There have been a few rare processes described in the literature that may cause a similar pattern of myocardial infiltration and resultant strain pattern. In 1 case report, a patient with primary hyperoxaluria type 1 was found to have ASP, which was believe to be related to regional differences in deposition of oxalate crystals rather than amyloid fibrils within the myocardium.13 Another case report described a patient with end-stage renal disease and ASP, who was evaluated for possible CA, but was ultimately found to have metastatic myocardial calcification on autopsy without evidence of amyloid.14 Danon disease has also been associated with ASP.15 Although these specific scenarios represent extremely uncommon diagnoses that are on the differential for ASP, there are likely additional possible conditions associated with ASP that have not yet been identified.
Although the differential diagnosis for ASP may be broader than previously believed, the percentage of patients with ASP who are ultimately diagnosed with CA is not insignificant. CA has been historically underdiagnosed or mistaken for other disease processes, significantly delaying adequate treatment and affecting associated morbidity and mortality.4,16 The ability to use a readily available noninvasive screening test, such as strain measurement, to identify a population of patients in whom a confirmed or highly likely diagnosis of CA can be made in 33% of patients is extremely valuable, provided the physician and patient understand its limitations and are aware of its predictive value.
Our findings have limitations. Although we sought to determine the prevalence of CA in a broad population of patients with ASP, the retrospective nature of the study limited us to patients who had a diagnostic evaluation for CA owing to either the presence of ASP or additional clinical suspicion. Given this limitation, the PPV of ASP for CA in all-comers may be even lower than reported here. In addition, LS could not be measured in all patients who otherwise met the inclusion criteria, possibly biasing the reported PPV. The small sample size is also a limitation of the study. A larger, prospective study is needed to confirm these findings because variables that were not significantly different among patients with confirmed CA may reach significance in a larger sample. However, the fact that some of the tested parameters reached high levels of statistical significance in our small sample suggests that these particular variables may be more relevant for decision making in individual patients than those that need larger samples to reach significance.
In conclusions, the presence of an ASP of LS reflects confirmed or highly probable CA in only 1/3 of patients. Our results suggest that it is more likely to indicate CA in older patients, in whom the prevalence of amyloidosis is higher, and in patients with increased LV wall thickness. A significant proportion of patients with ASP do not have evidence of CA on further testing. However, 1/3 should be considered a large diagnostic yield that probably justifies further testing, given the poor outcomes associated with CA diagnosis.
Acknowledgments
Dr. Wali was supported by funding from the National Institutes of Health, Bethesda, Maryland, T32 Training Grant 5T32HL7381.
Footnotes
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
References
- 1.Kittleson MM, Maurer MS, Ambardekar AV, Bullock-Palmer RP, Chang PP, Eisen HJ, Nair AP, Nativi-Nicolau J, Ruberg FL, American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association [published correction appears in Circulation. 2021;144: e10] [published correction appears in Circulation. 2021;144:e11]. Circulation 2020;142:e7–e22. [DOI] [PubMed] [Google Scholar]
- 2.Sharpley FA, Petrie A, Mahmood S, Sachchithanantham S, Lachmann HJ, Gillmore JD, Whelan CJ, Fontana M, Martinez-Naharro A, Quarta C, Hawkins PN, Wechalekar AD. A 24-year experience of autologous stem cell transplantation for light chain amyloidosis patients in the United Kingdom. Br J Haematol 2019;187:642–652. [DOI] [PubMed] [Google Scholar]
- 3.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C, ATTR-ACT Study Investigators. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
- 4.Oda S, Kidoh M, Nagayama Y, Takashio S, Usuku H, Ueda M, Yamashita T, Ando Y, Tsujita K, Yamashita Y. Trends in diagnostic imaging of cardiac amyloidosis: emerging knowledge and concepts. Radiographics 2020;40:961–981. [DOI] [PubMed] [Google Scholar]
- 5.Phelan D, Collier P, Thavendiranathan P, Popovi c ZB, Hanna M, Plana JC, Marwick TH, Thomas JD. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012;98:1442–1448. [DOI] [PubMed] [Google Scholar]
- 6.Committee Writing, Kittleson MM, Ruberg FL Ambardekar AV, Brannagan TH Cheng RK, Clarke JO Dember LM, Frantz JG Hershberger RE, Maurer MS, Nativi-Nicolau J, Sanchorawala V, Sheikh FH. 2023 ACC Expert Consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis: A report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol 2023;81:1076–1126. [DOI] [PubMed] [Google Scholar]
- 7.Rapezzi C, Aimo A, Serenelli M, Barison A, Vergaro G, Passino C, Panichella G, Sinagra G, Merlo M, Fontana M, Gillmore J, Quarta CC, Maurer MS, Kittleson MM, Garcia-Pavia P, Emdin M. Critical comparison of documents from scientific societies on cardiac amyloidosis: JACC State-of-the-Art Review. J Am Coll Cardiol 2022;79:1288–1303. [DOI] [PubMed] [Google Scholar]
- 8.Agha AM, Parwani P, Guha A, Durand JB, Iliescu CA, Hassan S, Palaskas NL, Gladish G, Kim PY, Lopez-Mattei J. Role of cardiovascular imaging for the diagnosis and prognosis of cardiac amyloidosis. Open Hear 2018;5:e000881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakao Y, Saito M, Inoue K, Higaki R, Yokomoto Y, Ogimoto A, Suzuki M, Kawakami H, Hiasa G, Okayama H, Ikeda S, Yamaguchi O. Cardiac amyloidosis screening using a relative apical sparing pattern in patients with left ventricular hypertrophy. Cardiovasc Ultra-sound 2021;19:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bastos Fernández M, López Otero D, López Pais J, Pubul Nûñez V, Neiro Rey C, González-Juanatey JR. Left ventricle myocardial deformation pattern in severe aortic valve stenosis without cardiac amyloidosis. The AMY-TAVI trial. Rev Esp Cardiol (Engl Ed) 2020;73:961–964. [DOI] [PubMed] [Google Scholar]
- 11.Abecasis J, Lopes P, Santos RR, Maltês S, Guerreiro S, Ferreira A, Freitas P, Ribeiras R, Andrade MJ, Manso RT, Ramos S, Gil V, Masci PG, Cardim N. Prevalence and significance of relative apical sparing in aortic stenosis: insights from an echo and cardiovascular magnetic resonance study of patients referred for surgical aortic valve replacement [published online February 26, 2023]. Eur Hear J Cardiovasc Imaging doi: 10.1093/ehjci/jead032. [DOI] [PubMed] [Google Scholar]
- 12.Bravo PE, Fujikura K, Kijewski MF, Jerosch-Herold M, Jacob S, El-Sady MS, Sticka W, Dubey S, Belanger A, Park MA, Di Carli MF, Kwong RY, Falk RH, Dorbala S. Relative apical sparing of myocardial longitudinal strain is explained by regional differences in total amyloid mass rather than the proportion of amyloid deposits. JACC Cardiovasc Imaging 2019;12:1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagies R, Beck BB, Hoppe B, Sreeram N, Udink Ten Cate FE. Apical sparing of longitudinal strain, left ventricular rotational abnormalities, and short-axis dysfunction in primary hyperoxaluria Type 1. Circ Heart Fail 2013;6:e45–e47. [DOI] [PubMed] [Google Scholar]
- 14.Zhang KW, Sadhu JS, Miller BW, Brennan DC, Bierhals AJ, Chen JF, Lin CY, Vader JM. Apical sparing pattern of longitudinal strain and positive bone scintigraphy in metastatic myocardial calcification. JACC Case Rep 2020;2:809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bui QM, Hong KN, Kraushaar M, Ma GS, Brambatti M, Kahn AM, Battiha CE, Boynton K, Storm G, Mestroni L, Taylor MRG, DeMaria AN, Adler EA. Myocardial strain and association with clinical outcomes in Danon disease: a model for monitoring progression of genetic cardiomyopathies. J Am Heart Assoc 2021;10: e022544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurer MS, Bokhari S, Damy T, Dorbala S, Drachman BM, Fontana M, Grogan M, Kristen AV, Lousada I, Nativi-Nicolau J, Cristina Quarta C, Rapezzi C, Ruberg FL, Witteles R, Merlini G. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail 2019;12:e006075. [DOI] [PMC free article] [PubMed] [Google Scholar]


