Abstract
Background
Patients with pancreatic ductal adenocarcinoma present early postoperative systemic metastases, despite complete oncological resection. The aim of this study was to assess two pancreatoduodenectomy approaches with regard to intraoperative circulating tumour cells and cluster mobilization and their potential association with the development of distant metastasis.
Methods
Patients with periampullary tumours who underwent open pancreatoduodenectomy were randomly allocated to either the no-touch approach or the superior mesenteric artery approach. A total of four intraoperative portal vein samples (at the beginning of the intervention, after portal vein disconnection from the tumour, after tumour resection, and before abdominal closure) were collected to measure circulating tumour cells and cluster numbers. Primary outcomes were the intraoperative number of circulating tumour cells and cluster mobilization. Further, their potential impact on 3-year distant metastasis disease-free survival and overall survival was assessed.
Results
A total of 101 patients with periampullary tumours were randomized (51 in the superior mesenteric artery group and 50 in the no-touch group) and 63 patients with pancreatic ductal adenocarcinoma (34 in the superior mesenteric artery group and 29 in the no-touch group) were analysed. Circulating tumour cells and cluster mobilization were similar in both the no-touch group and the superior mesenteric artery group at all time points. There were no significant differences between surgical groups with regard to the median metastasis disease-free survival (12.4 (interquartile range 6.1–not reached) months in the superior mesenteric artery group and 18.1 (interquartile range 12.1–not reached) months in the no-touch group; P = 0.730). Patients with intraoperative cluster mobilization from the beginning to the end of surgery developed significantly more distant metastases within the first year after surgery (P = 0.023). Two intraoperative factors (the superior mesenteric artery approach (P = 0.025) and vein resection (P < 0.001)) were predictive factors for cluster mobilization.
Conclusion
Patients undergoing pancreatoduodenectomy using either the no-touch approach or the superior mesenteric artery approach had similar circulating tumour cells and cluster mobilization and similar overall survival and metastasis disease-free survival. A high intraoperative cluster dissemination during pancreatoduodenectomy was a predictive factor for early metastases in patients with pancreatic ductal adenocarcinoma.
Registration number
NCT03340844 (http://www.clinicaltrials.gov)—CETUPANC trial.
Circulating tumour cells and cluster spread were observed during pancreatoduodenectomy in patients with pancreatic cancer. There were no significant differences in circulating tumour cells and cluster mobilization by the end of the surgery between the two surgical approaches (the no-touch approach versus the superior mesenteric artery approach). Intraoperative tumour cell cluster mobilization was associated with early metastasis in the first year.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a lethal malignancy with poor overall survival (OS) due to the clinically silent progression of the disease and appearance of metastases at the time of diagnosis or early after surgery1,2.
It has been suggested that patients die, on average, 2 years after the pancreatic tumour cells acquire the ability to metastasize3. This cancer cell capacity is enhanced by the heterogeneous pancreatic cancer microenvironment, which consists of fibrotic stromal cells with different subtypes of extracellular matrix and infiltrating inflammatory cells4–7.
However, even in cases with early diagnosis, many patients develop metastases shortly after complete surgical resection8,9. This could be explained by the presence of non-detected micrometastases at the time of diagnosis. On the other hand, it has been suggested that cancers may be disseminated through the bloodstream by the physiological stress associated with surgical trauma and manipulation of the tumour10,11. Some techniques used to remove pancreatic head cancer, such as the superior mesenteric artery (SMA) approach, require more mobilization and manipulation of the tumour before dissection of the venous drainage. This can potentially promote tumour cell dissemination through the portal venous system12,13. In contrast, the no-touch (NT) approach, which disconnects the tumour vasculature before manipulation, could avoid intraoperative tumour spread14–16.
Liquid biopsy using different modalities, such as circulating tumour cells (CTCs), cell-free circulating tumour DNA, and circulating tumour extracellular vesicles, represents an interesting novel tool for monitoring of disease17,18. Using this technique, a breakthrough has recently been reached in predicting the survival of patients with PDAC by detecting CTCs and clusters in preoperative liquid biopsy analysis19,20. Likewise, intraoperative tumour dissemination assessment can be performed; Hirota et al.15 and Gall et al.16 evaluated the detection of CEA mRNA and CTCs respectively in the portal vein blood after tumour resection. These pilot studies were conducted on a limited number of patients; however, both studies showed increased mRNA and CTC mobilization in conventional pancreatoduodenectomy (PD) when compared with the NT approach. However, no association was observed between tumour marker mobilization and the development of postoperative metastases. Currently, there is limited evidence on the impact of the surgical approach on intraoperative neoplastic cell dissemination and subsequent metastatic spread in patients with PDAC.
Consequently, the CETUPANC trial was designed to compare two surgical approaches for PD (the SMA approach versus the NT approach) with regard to the number of intraoperative CTCs and cluster mobilization (determined using intraoperative liquid biopsy) and their effect on distant metastatic development in patients with PDAC.
Methods
Trial design
This randomized clinical trial (RCT) was a multicentre, individually randomized, patient-blinded trial conducted in two parallel groups at ten university hospitals with specialized hepatopancreatobiliary surgery units (Virgen del Rocío University Hospital of Seville (the main centre), Badajoz University Hospital, University Hospital of Salamanca, University Hospital October 12 in Madrid, Terrassa Mutual University Hospital, Hospital Clinico of Barcelona, Valencia Clinical Hospital, Miguel Servet University Hospital of Zaragoza, Princess University Hospital of Madrid, and Hospital Clinico of Madrid). These hospitals and units were selected on the basis of quality criteria published by the Pancreatoduodenectomy Multicentric Spanish Group, requiring a minimum of 31 PD in each centre per year21. All surgeons had previous experience with the surgical techniques investigated in this study (at least 15 procedures performed for each approach).
Approval from the Ethical Committee of Hospitals was obtained in accordance with the Declaration of Helsinki (date: 21 December 2016; identification number: 1510-M1-17). The study was registered in ClinicalTrials.gov (NCT03340844) and followed the CONSORT guidelines22. The original and translated study protocol and additional amendments are included in the Supplementary material.
Patients
The inclusion criteria were patients over 18 years of age with radiologically resectable PDAC of the head of the pancreas, with less than 180° contact with the portal vein23 who signed the informed consent form. Histological confirmation was carried out after surgery. Preoperative staging consisted of triphasic CT and PET-CT when necessary. Patients were selected for upfront surgery based on the National Comprehensive Cancer Network criteria23 independent of the level of carbohydrate antigen 19-9 (CA 19-9) present.
The exclusion criteria were histology other than PDAC, high-risk patients with severe disease (ASA grade IV24), neoadjuvant chemotherapy, liver metastases or peritoneal carcinomatosis detected during surgery, unresectable tumour due to arterial infiltration, and macroscopic residual tumour.
Trial procedures
To evaluate the potential role of tumour manipulation in tumour cell mobilization, patients were allocated in a 1 : 1 ratio to either the SMA group or the NT group by random assignment and stratified by participating centres to balance the groups. To ensure standardization of the techniques and steps in the determinations, a consensus meeting of surgeons was held before the start of the study.
In the SMA group, the pancreatic head was exposed early by lowering the angle of the colon and a Kocher manoeuvre until exposure of the left renal vein. The SMA was then located and dissected at its origin from the aorta, surrounding it with a vessel loop. Next, at the inframesocolic level, the superior mesenteric vein and SMA were located, dissected, and surrounded with loops. Once the SMA had been identified and marked, at the proximal level and at the level of the mesenteric root, most of the connective, lymphatic, and nervous tissue that forms the lateral portion of the retroportal process was very carefully cut. Following the axis formed by the SMA, gently pulling it through the previously placed loop, it was separated from the pancreatic tissue and the portal vein, identifying and sectioning both pancreatoduodenal arteries. After these manoeuvres, the hepatic pedicle was dissected and the stomach, jejunum, and mesojejunal area were sectioned. Then the jejunum was uncrossed behind the mesenteric vessels and the pancreas was sectioned at the level of the neck. Next, the posterolateral aspect of the portal vein was dissected, sectioning its tributary branches to access the retroperitoneal tissue lateral to the axis of the SMA, the area that had already been practically divided and of which usually at this point of the intervention only a small part remains. It was sectioned in a cephalic direction, always on the right lateral edge of the artery, until finally completing the excision of the piece.
In the NT group, dissection of the greater omentum was carried out, separating it from the thin peritoneal sheath of the transverse mesocolon, advancing in a cephalic direction until the gastrocolic trunk was identified, the gateway to the superior mesenteric vein below the neck of the pancreas. The gastrocolic trunk was sectioned and the dissection of the superior mesenteric vein was continued below the pancreas. After this manoeuvre, the greater omentum was removed and the area of the hepatoduodenal ligament and gallbladder was dissected, allowing identification and dissection of the left and right hepatic arteries, with continuation towards the gastroduodenal artery for subsequent sectioning. Subsequently, lymphadenectomy was continued on the common hepatic artery without reaching the exit of the coeliac trunk. Once the bile duct was sectioned, the portal vein was dissected, removing all lympho-fatty tissue circumferentially. This leads to the entrance of the portal vein into the pancreas from its upper part. The stomach was then prepared and cut using a mechanical stapler. After these steps, Treitz’s angle was opened, sectioning the jejunum at 10–15 cm from it after sectioning its mesentery. It was then time to complete the dissection of the anterior side of the portal mesenteric axis below the neck of the pancreas and, after this, the pancreas was sectioned at the level of the neck. Once all these steps were completed, the small vessels that run from the mesenteric-portal axis to the head of the pancreas were delicately dissected and sectioned, achieving the disconnection of venous drainage from the head of the pancreas to the portal vein. After the pancreatic head was completely disconnected from the portal vein, the wide Kocher manoeuvre was performed and the sectioned jejunum was uncrossed behind the mesenteric vessels. Finally, the excision of the surgical specimen was completed by sectioning the retroperitoneal edge on the right lateral wall of the SMA. Standardization of the technique was especially agreed upon in patients who underwent the NT technique requiring venous resection. In these cases, complete and wide dissection of the superior mesenteric vein and portal vein axis (SMV-PV) in the upper and lower parts of the pancreas was performed and taped. Once the pancreas was sectioned at the level of the pancreatic neck, by lifting the vascular tap, the thin pancreatic veins that drain to the SMV-PV were identified, ligated, and divided until the tumour invaded the axis. Then, the involved SMV-PV was clamped, after which wedge or segmental resection of the vein was performed, followed by the completion of the Kocher manoeuvre and removal of the specimen. Subsequently, the SMV-PV was reconstructed.
Adherence of surgeons to the protocol was assessed every 6 months by means of a video call. A review of patient report forms was carried out by the quality department of the clinical assay department of the main centre. In addition, a follow-up meeting was held halfway through recruitment.
Randomization and blinding
A randomization sequence was created at the Clinical Trial Unit of the main centre by the trial monitoring team using the open source software OxMaR (Oxford Minimization and Randomization)25. Patients were allocated in a 1 : 1 ratio to either the SMA group or the NT group by random assignment in four blocks and stratified by participating centres to balance the groups. The sequence was hidden from researchers and surgeons until the moment of the surgery. The main centre was advised on the date of surgery and direct telephone communication was established in the operating room. Randomization was done intraoperatively once the surgeon confirmed the absence of metastasis or carcinomatosis. The patients were blinded after assignment to the intervention. Data quality control was performed by external outcome assessors who were not blinded to the group assignment.
Intraoperative sampling for determining circulating tumour cells and clusters
A total of four intraoperative portal vein samples (S0 to S3) were obtained for each patient in both groups for CTC and cluster measurements using intraoperative liquid biopsy. A total of 7 ml of whole blood was obtained for each sample through direct portal vein puncture with a hypodermic needle (25G × 1 inch). The puncture hole was covered with moist gauze after the blood was drawn to reduce potential bleeding.
S0 was obtained before tumour manipulation after minimal bile duct dissection in both of the groups. S1 was obtained in the NT group after ligation of the pancreatic vessels that drained into the portal vein to avoid tumour manipulation. In the SMA group, S1 was obtained after extensive SMA dissection before retropancreatic portal vein dissection. Finally, S2 was obtained after resection and S3 just before abdominal closure in both of the groups.
Circulating tumour cell isolation, detection, and enumeration protocol
The first blood sample was discarded to exclude epithelial cells that were dislodged via vein puncture. Samples were collected in K2-EDTA Vacutainer tubes. To achieve sample stability, the samples were transported by a company experienced in transporting biological samples. Biospecimens were stored at 4°C during transportation and processed within 24 h of collection.
All CTC and cluster determinations were performed at the main centre. Blood samples were enriched in peripheral mononuclear blood cells using gradient centrifugation with Histopaque®-1119 and CTCs were isolated using the IsoFlux™ platform. The IsoFlux™ Epithelial-to-Mesenchymal Transition CTC Enrichment Kit (EMT Enrichment Kit, Fluxion, CA, USA; Catalogue No. 910-0106) was used to perform CTC enrichment and the enriched CTCs were fixed and stained with fluorescent reagents (IsoFlux™ Circulating Tumor Cell Enumeration Kit, Fluxion, CA, USA; Catalogue No. 910-0093). The fluorescent reagents included anti-CK-fluorescein isothiocyanate (FITC), anti-CD45-indocarbocyanine (Cy3), and Hoechst 33342. CTC detection and enumeration were performed using fluorescence microscopy. A cluster has been defined as the aggregation of two or more CTCs26.
Finally, the Hough transform algorithm was used to count CTCs and clusters (89% sensitivity and 91% accuracy)20,27.
Follow-up
Follow-up was initiated on the day of surgery and continued throughout the ensuing 3 years. Follow-up visits included CT and CA 19-9 testing every 6 months. A patient could undergo CT or MRI outside of standard surveillance because of symptoms or when required according to clinical criteria. Patients with non-standard CT were not excluded from the study. Review of the radiological scans was not centralized.
Primary outcomes
Primary outcomes were the intraoperative number of CTCs (cells/ml) and cluster (clusters/ml) mobilization during PD. Their association with the appearance of distant metastases was also assessed.
Secondary outcomes
Metastasis disease-free survival (MDFS) was defined as the time from surgery to the appearance of metastases during the 3 years of follow-up. Distant metastases included liver or lung metastases. Local/lymph node recurrence and OS were also assessed.
The pathological study was carried out according to a specific protocol based on the approach of Verbeke et al.28. R0/R1 rates, tumour grade differentiation (G1–G3), and TNM classification of malignant tumours were defined according to the guideline criteria29–31. Surgical complications were classified according to the Clavien–Dindo classification32 and the International Study Group of Pancreatic Surgery33–35.
Statistical analysis
In the context of the CETUPANC trial, an additional specific sample size was calculated for PDAC evaluation according to previous studies15,16. Smaller differences in the mobilization of tumour cell markers were considered. Accepting an α risk of 0.05 and a β risk of 0.01 in a two-sided test, at least 27 subjects were necessary in each group to find a statistically significant proportion difference of tumour cell mobilization expected to be 0.13 in the NT group and 0.50 in the SMA group. During follow-up, a dropout rate of 15% was anticipated.
The cohort characteristics were compared between the surgical approaches using a t test, a Wilcoxon rank sum test, or a chi-squared test. The analyses were performed in accordance with the modified intention-to-treat principle, where patients stayed in their allocated group, but were excluded from analyses after randomization when PD was not performed or the final pathology was not PDAC.
The intraoperative samples were evaluated not only independently (S0, S1, S2, and S3) but also as change (Δ) compared with baseline: S1−S0 (Δ mobilization-to-baseline), S2−S0 (Δ resection-to-baseline), and S3−S0 (Δ end-to-baseline). A more positive difference in Δ values indicated higher CTCs and/or cluster dissemination at each sampling time.
Patient follow-up was tested using an independent Cox proportional hazards regression model for MDFS as the outcome. The model was implemented using a backward elimination process, leaving only the significant variables in the model. Significant variables that fulfilled the proportional hazard assumption were univariately substudied using Kaplan–Meier (K-M) curves with the log rank test, categorical variable strata, or descriptive statistical metrics for continuous variables. Only the K–M curves with the lowest P values were studied together with the surgical approaches in conjunction with the curve strata. Finally, an additional regression logistic model was used to study the impact of categorical variables on candidate K–M curve strata. All tests, except those noted, were two-sided, considering a significance level of α = 0.05, and were analysed using R version 4.0.5.
Results
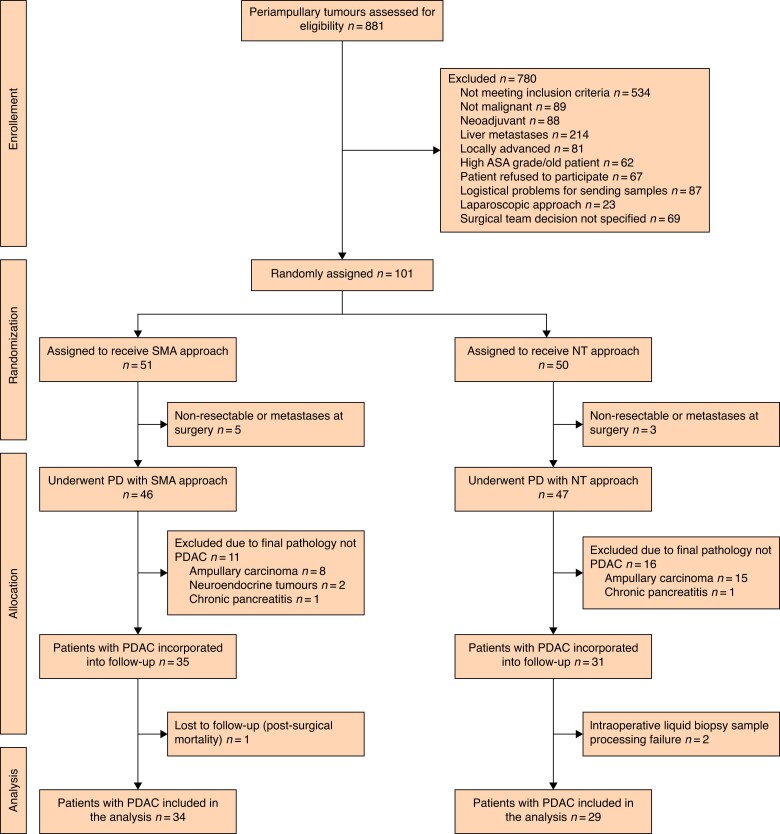
Of the 881 patients with periampullary tumours evaluated by the tumour boards, 101 were randomized, 93 patients who met the preoperative and intraoperative inclusion criteria were allocated to a surgical approach, and 63 patients with PDAC were included for the final analysis. Finally, 34 patients were included in the SMA group and 29 patients were included in the NT group (Fig. 1). Recruitment was performed for 31 months from 17 January 2018 to 24 July 2020. Table 1 shows the demographic data for both groups.
Fig. 1.
CONSORT flow diagram
The diagram shows the flow of participants in the two arms of the clinical trial. SMA, superior mesenteric artery; NT, no-touch; PD, pancreatoduodenectomy; PDAC, pancreatic ductal adenocarcinoma.
Table 1.
Cohort description (comparison of baseline, intraoperative, and surgical parameters according to surgical approach)
| Variable | Surgical approach | |
|---|---|---|
| No-touch (n = 29) |
Superior mesenteric artery (n = 34) | |
| Baseline parameters | ||
| Male | 18 (62) | 14 (41.1) |
| Female | 11 (38) | 20 (58.8) |
| Age (years), median (i.q.r.) | 65 (54–73) | 64 (57–74) |
| Diabetes mellitus | 7 (24) | 6 (18) |
| Arterial hypertension | 9 (31) | 18 (53) |
| Dyslipidaemia | 16 (55) | 9 (26) |
| Preoperative biliary stent | 17 (59) | 26 (76) |
| CA 19-9 (U/ml), median (i.q.r.) | 149 (52–357) | 143 (38–551) |
| Baseline intraoperative liquid biopsy | ||
| CTCs in S0 (cells/ml), median (i.q.r.) | 199 (118–428) | 228 (70–383) |
| Clusters in S0 (cells/ml), median (i.q.r.) | 13 (4–34) | 9 (2–19) |
| Surgical parameters | ||
| Surgery time (min), median (i.q.r.) | 295 (270–360) | 300 (270–357) |
| Vein resection | 9 (31) | 8 (23) |
| Blood loss (ml), median (i.q.r.) | 275 (112–600) | 243.8 (175–550) |
| Blood transfusion | 6 (21) | 6 (18) |
| Number of harvested lymph nodes, median (i.q.r.) | 16 (12–24) | 17 (12–24) |
| Histological characteristics | ||
| Tumour size (mm), median (i.q.r) | 2.5 (2.0–3.5) | 2.9 (2–3.5) |
| Tumour stage | ||
| I (IA/IB) | 6 (21) (1 (3)/5 (17)) | 10 (29) (2 (6)/8 (23)) |
| II (IIA/IIB) | 19 (65) (3 (10)/16 (55)) | 13 (38) (3 (9)/10 (29)) |
| III | 4 (14) | 11 (32) |
| Tumour grade | ||
| G1 | 11 (38) | 6 (18) |
| G2 | 15 (52) | 19 (56) |
| G3 | 3 (10) | 9 (25) |
| Vascular invasion | 16 (55) | 22 (65) |
| Lymphatic invasion | 14 (48) | 16 (47) |
| Neural invasion | 19 (65) | 30 (88) |
| R0/R1 resection | 18 (62)/11 (38) | 23 (68)/11 (32) |
| Postoperative parameters | ||
| Clavien–Dindo grade III/IV complications32 | 7 (24) | 5 (15) |
| Biliary fistula | 2 (7) | 3 (9) |
| Pancreatic fistula | 8 (27) | 3 (9) |
| Delayed gastric emptying | 2 (7) | 8 (23) |
| Haemorrhage | 3 (10) | 3 (9) |
| Readmission | 5 (17) | 3 (9) |
| Time until chemotherapy (days), median (i.q.r) | 94 (84–103) | 98 (92–108) |
| Adjuvant chemotherapy | ||
| No treatment | 5 (17) | 9 (26) |
| Gemcitabine | 10 (34) | 10 (29) |
| Capecitabine | 5 (17) | 3 (9) |
| Folfirinox | 9 (31) | 10 (29) |
| Oxaliplatin + irinotecan + 5-fluorouracil | 0 (0) | 1 (3) |
| Abraxane | 0 (0) | 1 (3) |
| Postoperative CA 19-9 (U/ml), median (i.q.r.) | ||
| 3 months | 27 (11–294) | 34 (12–540) |
| 6 months | 30 (10–93) | 64 (10–354) |
| 12 months | 28 (8–159) | 27 (10–591) |
| 18 months | 25 (9–353) | 42 (7–5671) |
| 24 months | 18 (8–242) | 12 (2–944) |
| 36 months | 10 (6–25) | 9 (2–214) |
Values are n (%) unless otherwise indicated. i.q.r., interquartile range; CA 19-9, carbohydrate antigen 19-9; CTCs, circulating tumour cells; S0, intraoperative portal vein baseline sample.
Primary outcomes
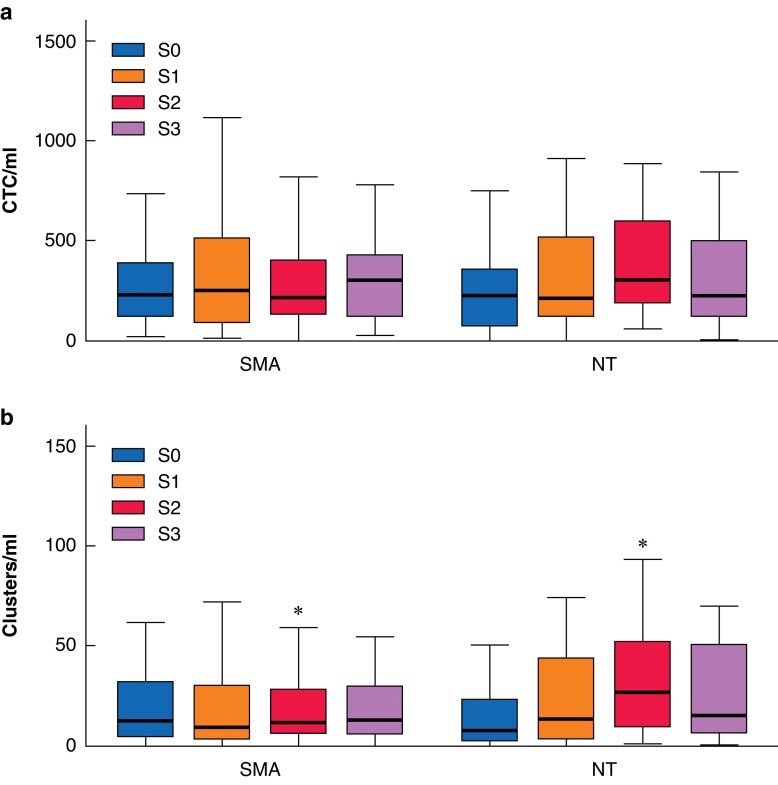
The distributions of the number of CTCs and clusters in each intraoperative sample according to the NT and SMA approach are depicted in Fig. 2. The determination carried out after manipulation of the tumour until portal venous disconnection (S1) showed no differences between the surgical groups with regard to either the CTCs or the cluster values. The main peaks of CTCs and cluster mobilization were observed after complete resection (S2) (Fig. 2). At this sample point, the NT group showed a higher mobilization of clusters (median of 27 (interquartile range (i.q.r.) 9–53) versus 12 (i.q.r. 6–29); P = 0.042). CTCs also showed a higher mobilization at this point in the NT group, although the difference was not statistically significant (median of 306 (i.q.r. 185–604) versus 217 (i.q.r. 129–409)). Finally, before abdominal closure (S3), there were no differences in CTCs and cluster measurements between the NT approach and the SMA approach.
Fig. 2.
Circulating tumour cells and clusters over time
a Circulating tumour cells. b Clusters. Box plots depicting the distribution of circulating tumour cells and clusters in each intraoperative portal vein sample from S0 to S3 by surgical technique. *Statistically significant difference between the two groups (P = 0.042). SMA, superior mesenteric artery; NT, no-touch; S0, sample obtained before tumour manipulation after minimal bile duct dissection in both of the groups; S1, sample obtained after ligation of the pancreatic vessels that drained into the portal vein to avoid tumour manipulation in the no-touch group and sample obtained after extensive superior mesenteric artery dissection before retropancreatic portal vein dissection in the superior mesenteric artery group; S2, sample obtained after resection in both of the groups; S3, sample obtained just before abdominal closure in both of the groups.
Secondary outcomes
There were no significant differences between surgical groups with regard to R0/R1 rate and surgical complications (Table 1). No complications were associated with the portal vein puncture. Bleeding stopped spontaneously in all patients with no other haemostatic approach except moist gauze application.
The median OS was 19 (i.q.r. 10–not reached) months. There were no significant differences between groups (18 (i.q.r. 9–not reached) months in the SMA group versus 23 (i.q.r. 11–not reached) months in the NT group; P = 0.440).
A total of 21 patients (33%) presented with local recurrence during follow-up, without differences between surgical groups (median not reached (i.q.r. 9–not reached) months in the SMA group versus median not reached (i.q.r. 12–not reached) months in the NT group; P = 0.900).
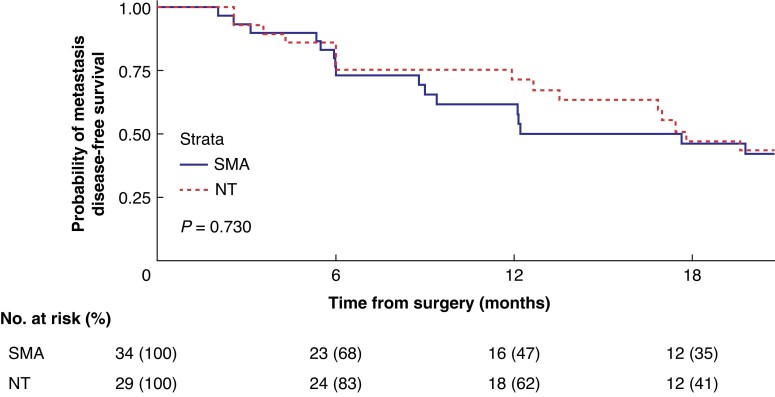
Related to the metastasis analysis, 33 (56%) patients had a systemic recurrence during the 3 years of follow-up. Figure 3 shows the MDFS comparison between groups. The median MDFS was 12 (i.q.r. 6.1–not reached) months and 18 (i.q.r. 12.1–not reached) months in the SMA group and in the NT group respectively (P = 0.730). Regarding the development of early metastases during the first year, 53% of patients in the SMA group presented with distant metastases versus 38% of patients in the NT group.
Fig. 3.
Metastasis disease-free survival analysis
An at-risk table is shown beneath the graph. SMA, superior mesenteric artery; NT, no-touch.
Multivariate analysis
To evaluate the potential factors determining MDFS, both groups were combined in the multivariate analysis. The logistic regression showed that MDFS was associated with two preoperative factors (CA 19.9 (HR 1.00 (95% c.i. 1.0001 to 1.0003); P < 0.001) and vascular invasion presence (HR 2.98 (95% c.i. 1.34 to 6.65); P = 0.007)) and one intraoperative factor (intraoperative cluster Δ end-to-baseline (HR 1.01 per cluster/ml (95% c.i. 1.00 to 1.03); P = 0.031)). The full model is included in the Supplementary material.
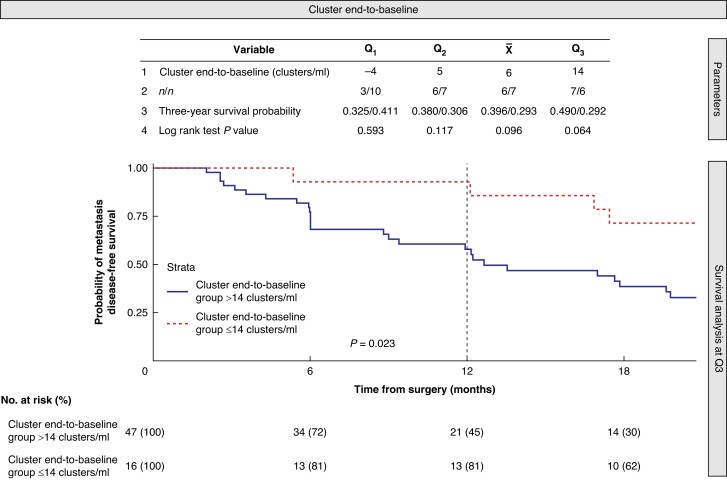
Due to the finding in the previous additional analysis, related to the association of cluster mobilization with distant metastasis, a univariate K–M analysis (using the cluster Δ end-to-baseline quartiles to detect the best cut-off for predicting MDFS) was performed. The additional analysis showed that the cluster Δ end-to-baseline cut-off that best separated the cohort for MDFS was 14 clusters/ml in the third quartile (Fig. 4). Table 2 shows the cohort characteristics for cluster end-to-baseline groups according to this cut-off. Patients with higher cluster dissemination during surgery (Δ end-to-baseline greater than 14 clusters/ml) had significantly higher metastases within the first year (P = 0.023). This association disappeared at 2 and 3 years (P = 0.052 and P = 0.064 respectively) (Fig. 4).
Fig. 4.
Spread of intraoperative tumour cell clusters as a factor associated with early metastasis
Descriptive statistics for cluster Δ end-to-baseline (S3−S0) to detect the best predictive cut-off for metastasis disease-free survival. The descriptive statistics include the first, second, and third quartiles (Q1, Q2, and Q3) and the mean value () for the cluster end-to-baseline (clusters/ml). The cohort was divided into two groups (over and under equal the cut-off value) and obtained the following metrics at 3-year follow-up: i) the number of patients at risk in each group (n/n); ii) the probability of metastasis disease-free survival (probability/probability), and iii) the log-rank test P value obtained between the two groups. Kaplan–Meier curves are presented for the descriptive statistical value with the most significant metastasis disease-free survival curve separation (Q3 with a cluster Δ end-to-baseline cut-off value of 14 clusters/ml). In addition, the 12-month log-rank test P = 0.023 is also presented.
Table 2.
Cohort characteristics for cluster end-to-baseline groups
| Variable | Cluster end-to-baseline group (clusters/ml) | P | |
|---|---|---|---|
| >14 (n = 47) | ≤14 (n = 16) | ||
| Baseline parameters | |||
| Male | 23 (49) | 9 (56) | 0.773 |
| Female | 24 (51) | 7 (44) | |
| Age (years), median (i.q.r.) | 63 (57–73) | 66 (59–75) | 0.472 |
| Diabetes mellitus | 9 (19) | 4 (25) | 0.723 |
| Arterial hypertension | 19 (40) | 8 (50) | 0.566 |
| Dyslipidaemia | 17 (36) | 8 (50) | 0.383 |
| Preoperative biliary stent | 34 (72) | 9 (56) | 0.351 |
| CA 19-9 (U/ml), median (i.q.r.) | 149 (63–366) | 93 (29–950) | 0.069 |
| Baseline intraoperative liquid biopsy | |||
| CTCs in S0 (cells/ml), median (i.q.r.) | 227 (115–350) | 310 (126–443) | 0.731 |
| Clusters in S0 (cells/ml), median (i.q.r.) | 10 (4–29) | 9 (5–21) | 0.433 |
| Surgical parameters | |||
| Surgery time (min), median (i.q.r.) | 300 (270–360) | 285 (265–302) | 0.118 |
| Vein resection | 17 (36) | 0 (0) | 0.003 |
| Blood transfusion | 10 (21) | 2 (12) | 0.714 |
| Blood loss (ml), median (i.q.r.) | 300 (200–600) | 350 (200–650) | 0.572 |
| Number of harvested lymph nodes, median (i.q.r.) | 17 (12–24) | 16 (12–24) | 0.821 |
| Histological characteristics | |||
| Tumour size (mm), median (i.q.r.) | 3.0 (2.0–3.5) | 3.1 (2.5–3.5) | 0.364 |
| Tumour stage | 0.261 | ||
| I (IA/IB) | 13 (28) (3 (6)/10 (21)) | 3 (19) (0 (0)/3 (19)) | |
| II (IIA/IIB) | 21 (45) (4 (8)/17 (36)) | 11 (69) (2 (12)/9 (56)) | |
| III | 13 (28) | 2 (12) | |
| Tumour grade | 0.583 | ||
| G1 | 12 (25) | 5 (31) | |
| G2 | 27 (57) | 7 (44) | |
| G3 | 8 (17) | 4 (25) | |
| Vascular invasion | 29 (62) | 9 (56) | 0.771 |
| Lymphatic invasion | 22 (47) | 8 (50) | 1.000 |
| Neural invasion | 38 (81) | 11 (69) | 0.319 |
| R0/R1 resection | 31 (66)/16 (34) | 10 (62)/6 (38) | 1.000 |
| Postoperative parameters | |||
| Clavien–Dindo grade III/IV complications32 | 8 (17) | 4 (25) | 0.481 |
| Readmission | 6 (13) | 2 (12) | 1.000 |
Values are n (%) unless otherwise indicated. i.q.r., interquartile range; CA 19-9, carbohydrate antigen 19-9; CTCs, circulating tumour cells; S0, intraoperative portal vein baseline sample.
Finally, to determine the factors associated with intraoperative cluster mobilization, a logistic regression was performed using cluster Δ end-to-baseline as the dependent variable, considering cluster Δ end-to-baseline greater than 14 clusters as the reference category. The main factors associated with cluster mobilization greater than 14 clusters/ml were preoperative CA 19-9 (OR 1.0005 per CA 19-9 U/ml (95% c.i. 1.0004 to 1.0006); P = 0.032) and two intraoperative factors: the SMA approach (OR 4.4457 (95% c.i. 4.3653 to 4.5206); P = 0.025) and portal/superior mesenteric vein resection (OR 10.2467 (95% c.i. 6.5224 to 13.9410); P < 0.001). The full model is included in the Supplementary material.
Discussion
The present trial investigated intraoperative CTCs and cluster mobilization during PD in patients with PDAC, comparing two surgical approaches; both the NT approach and the SMA approach had similar tumour cells and cluster mobilization by the end of the surgery and no differences between surgical techniques were observed with regard to MDFS and OS. The study showed that a high intraoperative cluster dissemination during PD was a predictive factor for early metastasis within the first year in patients with PDAC of the pancreatic head.
To determine whether tumour manipulation could influence tumour cell dissemination, only two prospective pilot studies have analysed the mobilization of tumour cell markers during PD, comparing conventional PD with the NT approach15,16. Whereas Hirota et al.15 evaluated the detection of CEA mRNA, Gall et al.16 determined the CTC levels in the portal vein after tumour resection.
The key point of the NT approach in PD is to perform the resection after complete disconnection of tumour vein drainage into the portal vein without tumour mobilization. Theoretically, this approach could avoid tumour cell dissemination. In the present study, this technique was compared with the standard SMA approach for PD in which a Kocher manoeuvre and posterior SMA combined with mesenteric root dissection were performed before the ligation and sectioning of the tumour venous drainage into the portal vein. Theoretically this could increase tumour cell dissemination.
For the evaluation of intraoperative tumour cell mobilization during PD, not only free CTCs, as in the Gall et al.16 study, but also CTC clusters were determined in the portal vein in the present study. Whereas the previous studies analysed tumour markers in two intraoperative portal vein samples (at the beginning of resection and after resection), the present study determined CTCs and clusters at four strategic points (at the beginning of the intervention, after portal vein disconnection from the tumour, after tumour resection, and before abdominal closure). The findings of the present study are partially consistent with those of Gall et al.16 and Hirota et al.15 with a main peak of both CTCs and clusters observed after specimen resection. However, whereas Gall et al.16 and Hirota et al.15 showed fewer CTCs and less mRNA mobilization in patients with the NT approach after resection, in the present study, both CTCs and clusters were higher in the NT group in this sample. This was an unexpected finding. Unfortunately, there is a lack of evidence in this setting because most studies that recommend the use of the NT approach are retrospective with several biases36,37.
Regarding the potential role of intraoperative tumour cell dissemination in the appearance of distant metastases, broadly speaking, surgical resection has long been linked to increased metastasis via tumour cell spread during surgery38,39. However, to date, no studies have demonstrated the potential role of the intraoperative dissemination of tumour cells in the development of metastases in pancreatic cancer. The study by Gall et al.16 failed to demonstrate any correlation between isolated CTCs and OS or disease-free survival.
The present RCT studied the mobilization of not only CTCs but also the CTC clusters and included tumour cell dissemination by the end of surgery. These have been highly interesting when analysing MDFS. In fact, multivariate analysis showed that the metastatic phenomenon was associated with preoperative CA 19-9 levels, the presence of vascular invasion, and increased intraoperative cluster mobilization from the beginning to the end of surgery. CTC and cluster determination after mobilization and resection failed to show any correlation with metastasis.
Whereas both CA 19-9 levels and vascular invasion are well-known predictive factors at baseline40,41, cluster Δ end-to-baseline measurement is a new dynamic predictive marker in the field of oncology, leading to the new concept of intraoperative liquid biopsy. According to the results of the present study, the highest impact of cluster dissemination during surgery on metastasis development occurs during the first year, decreasing gradually through the first 3 years. In patients with high cluster mobilization, the OS prognosis decreased dramatically.
An additional logistic regression analysis was performed to determine the intraoperative factors related to cluster mobilization. Interestingly, using the SMA technique with the Kocher manoeuvre and tumour mobilization prior to tumour venous drainage ligation, the risk of cluster mobilization greater than 14 clusters/ml increased 4.5 times compared with the NT approach. Moreover, when the surgery required portal or superior mesenteric vein resection, the risk of higher cluster dissemination increased 10 times.
Despite this additional finding, surgical technique was not a factor determining the appearance of metastases and both NT and SMA approaches presented similar results in terms of MDFS and OS, similar to what was observed in a recent large randomized trial performed in patients with colon cancer42.
Finally, regarding portal or superior mesenteric vein resection, in patients with resectable pancreatic cancer undergoing upfront surgery, the venous resection rate is approximately 20–27%13,43. This is similar to that observed in the present study (26.9%). Venous resection has been associated with a worse prognosis, which is attributed to a more advanced neoplastic process44. The association between venous resection and intraoperative cluster mobilization observed for the first time in the present study could also partially explain the evolution of these patients.
The main limitations of the present study are related to the small sample size and the high proportion of patients without PDAC. Therefore, the main finding regarding the association between intraoperative cluster spread and early metastasis should be validated in a large prospective study.
A future implication of the findings of the present study could be that patients with greater intraoperative tumour cell mobilization will require an individualized strategy due to the higher risk of early distant metastasis (closer follow-up, early adjuvant treatment, and patient information). Special studies could be performed about locally advanced PDAC, for which extended surgical resection has demonstrated increased survival45. Avoidance of surgical tumour manipulation when extended dissection and vascular resection are necessary is complicated46.
Finally, although there is no evidence for a role of neoadjuvant therapy in resectable patients47, some studies have demonstrated better results in clinical stage IB–III PDAC48 and, in particular, in poorly differentiated resectable PDAC for which the risk of dissemination could be higher49. Thus, additional studies analysing the role of neoadjuvant therapy in the prevention of CTCs and cluster mobilization could be interesting.
Supplementary Material
Acknowledgements
The authors thank Ms Landy Menzies for her assistance with revising the English version of the manuscript. J.P.-R. and C.F. share first authorship and V.D. and L.S. share senior authorship.
Contributor Information
Javier Padillo-Ruiz, Department of Surgery, Virgen del Rocío University Hospital, IBIS, University of Seville, Seville, Spain.
Cristóbal Fresno, Health and Sciences Research Centre, Health and Sciences Faculty, Anahuac University, Huixquilucan, Mexico.
Gonzalo Suarez, Department of Surgery, Virgen del Rocío University Hospital, IBIS, University of Seville, Seville, Spain.
Gerardo Blanco, Department of Surgery, Badajoz University Hospital, University of Extremadura, Badajoz, Spain.
Luis Muñoz-Bellvis, Department of Surgery, University Hospital of Salamanca, Salamanca Biosanitary Institute, University of Salamanca, Salamanca, Spain.
Iago Justo, Department of Surgery, University Hospital October 12 in Madrid, Madrid, Spain.
Maria I García-Domingo, Department of Surgery, Terrassa Mutual University Hospital, Terrassa, Spain.
Fabio Ausania, Hospital-Clinic, August Pi i Sunyer Biomedical Research Institute, University of Barcelona, Barcelona, Spain.
Elena Muñoz-Forner, Department of Surgery, Valencia Clinical Hospital, University of Valencia, Biomedical Research Institute, INCLIVA, Valencia, Spain.
Alejandro Serrablo, Department of Surgery, Miguel Servet University Hospital, Zaragoza, Spain.
Elena Martin, Department of Surgery, Princess University Hospital, Madrid, Spain.
Luis Díez, Department of Surgery, Clinical Hospital, Madrid, Spain.
Carmen Cepeda, Department of Surgery, Virgen del Rocío University Hospital, IBIS, University of Seville, Seville, Spain.
Luis Marin, Department of Surgery, Virgen del Rocío University Hospital, IBIS, University of Seville, Seville, Spain.
Jose Alamo, Department of Surgery, Virgen del Rocío University Hospital, IBIS, University of Seville, Seville, Spain.
Carmen Bernal, Department of Surgery, Virgen del Rocío University Hospital, IBIS, University of Seville, Seville, Spain.
Sheila Pereira, Department of Surgery, Virgen del Rocío University Hospital, IBIS, University of Seville, Seville, Spain.
Francisco Calero, Department of Surgery, Virgen del Rocío University Hospital, IBIS, University of Seville, Seville, Spain.
Jose Tinoco, Department of Surgery, Virgen del Rocío University Hospital, IBIS, University of Seville, Seville, Spain.
Sandra Paterna, Department of Surgery, Miguel Servet University Hospital, Zaragoza, Spain.
Esteban Cugat, Department of Surgery, Terrassa Mutual University Hospital, Terrassa, Spain.
Constantino Fondevila, Hospital-Clinic, August Pi i Sunyer Biomedical Research Institute, University of Barcelona, Barcelona, Spain.
Elisa Diego-Alonso, Department of Surgery, University Hospital of Salamanca, Salamanca Biosanitary Institute, University of Salamanca, Salamanca, Spain.
Diego López-Guerra, Department of Surgery, Badajoz University Hospital, University of Extremadura, Badajoz, Spain.
Miguel Gomez, Department of Surgery, Virgen del Rocío University Hospital, IBIS, University of Seville, Seville, Spain.
Valeria Denninghoff, Department of Surgery, Virgen del Rocío University Hospital, IBIS, University of Seville, Seville, Spain.
Luis Sabater, Department of Surgery, Valencia Clinical Hospital, University of Valencia, Biomedical Research Institute, INCLIVA, Valencia, Spain.
Funding
This work was supported by grants from the Instituto de Salud Carlos III (ISCIII - PI #16/1465) of the Ministry of Health of Spain. Main Investigator: Javier Padillo-Ruiz. The Instituto de Salud Carlos III of the Ministry of Health of Spain had no role in: the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Author contributions
Javier Padillo-Ruiz (Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing—original draft, Writing—review & editing), Cristóbal Fresno (Data curation, Formal analysis, Methodology, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), Gonzalo Suarez (Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Writing—original draft, Writing—review & editing), Gerardo Blanco (Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Writing—original draft, Writing—review & editing), Luis Muñoz-Bellvis (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing—original draft, Writing—review & editing), Iago Justo (Data curation, Funding acquisition, Investigation, Methodology, Resources, Writing—original draft, Writing—review & editing), Maria I. García-Domingo (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Validation, Writing—original draft, Writing—review & editing), Fabio Ausania (Funding acquisition, Investigation, Methodology), Elena Muñoz-Forner (Formal analysis, Funding acquisition, Investigation, Methodology, Writing—original draft, Writing—review & editing), Alejandro Serrablo (Formal analysis, Funding acquisition, Investigation, Methodology, Writing—original draft, Writing—review & editing), Elena Martin (Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing—original draft), Luis Díez (Data curation, Formal analysis, Investigation, Methodology, Writing—original draft), Carmen Cepeda (Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing—original draft, Writing—review & editing), Luis Marin (Formal analysis, Funding acquisition, Investigation, Methodology, Writing—original draft), Jose Alamo (Formal analysis, Funding acquisition, Investigation, Methodology, Writing—original draft), Carmen Bernal (Data curation, Funding acquisition, Investigation, Methodology, Writing—original draft), Sheila Pereira (Data curation, Funding acquisition, Investigation, Methodology, Supervision, Writing—original draft, Writing—review & editing), Francisco Calero (Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Validation, Writing—original draft), Jose Tinoco (Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing—original draft), Sandra Paterna (Formal analysis, Funding acquisition, Investigation, Methodology, Validation), Esteban Cugat (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Validation, Writing—original draft), Constantino Fondevila (Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Writing—original draft, Writing—review & editing), Elisa Diego-Alonso (Formal analysis, Funding acquisition, Investigation, Methodology, Visualization, Writing—original draft), Diego López-Guerra (Formal analysis, Funding acquisition, Investigation, Methodology, Validation, Writing—original draft), Miguel Gomez (Funding acquisition, Investigation, Methodology, Validation, Writing—original draft), Valeria Denninghoff (Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), and Luis Sabater (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing)
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
Research data supporting this publication are not available from an open repository, although they can be shared by the author in selected cases.
References
- 1. Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021;19:439–457 [DOI] [PubMed] [Google Scholar]
- 2. Park W, Chawla A, O’Reilly EM. Pancreatic cancer: a review. JAMA 2021;326:851–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467:1114–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grünwald BT, Devisme A, Andrieux G, Vyas F, Aliar K, McCloskey CW et al. Spatially confined sub-tumor microenvironments in pancreatic cancer. Cell 2021;184:5577–5592.e18 [DOI] [PubMed] [Google Scholar]
- 5. Gardian K, Janczewska S, Olszewski WL, Durlik M. Analysis of pancreatic cancer microenvironment: role of macrophage infiltrates and growth factors expression. J Cancer 2012;3:285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ren B, Cui M, Yang G, Wang H, Feng M, You L et al. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer 2018;17:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chronopoulos A, Robinson B, Sarper M, Cortes E, Auernheimer V, Lachowski D et al. ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat Commun 2016;7:12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200–1210 [DOI] [PubMed] [Google Scholar]
- 9. Sommerville CA, Limongelli P, Pai M, Ahmad R, Stamp G, Habib NA et al. Survival analysis after pancreatic resection for ampullary and pancreatic head carcinoma: an analysis of clinicopathological factors. J Surg Oncol 2009;100:651–656 [DOI] [PubMed] [Google Scholar]
- 10. Market M, Tennakoon G, Auer RC. Postoperative natural killer cell dysfunction: the prime suspect in the case of metastasis following curative cancer surgery. Int J Mol Sci 2021;22:11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuroki T, Eguchi S. No-touch isolation techniques for pancreatic cancer. Surg Today 2017;47:8–13 [DOI] [PubMed] [Google Scholar]
- 12. Nakao A. The mesenteric approach in pancreatoduodenectomy. Dig Surg 2016;33:308–313 [DOI] [PubMed] [Google Scholar]
- 13. Sabater L, Cugat E, Serrablo A, Suarez-Artacho G, Diez-Valladares L, Santoyo-Santoyo J et al. Does the artery-first approach improve the rate of R0 resection in pancreatoduodenectomy?: a multicenter, randomized, controlled trial. Ann Surg 2019;270:738–746 [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi S, Asano T, Ochiai T. A proposal of no-touch isolation technique in pancreatoduodenectomy for periampullary carcinomas. Hepatogastroenterology 2001;48:372–374 [PubMed] [Google Scholar]
- 15. Hirota M, Shimada S, Yamamoto K, Tanaka E, Sugita H, Egami H. Pancreatectomy using the no-touch isolation technique followed by extensive intraoperative peritoneal lavage to prevent cancer cell dissemination: a pilot study. JOP 2005;6:143–151 [PubMed] [Google Scholar]
- 16. Gall TM, Jacob J, Frampton AE, Krell J, Kyriakides C, Castellano L et al. Reduced dissemination of circulating tumor cells with no-touch isolation surgical technique in patients with pancreatic cancer. JAMA Surg 2014;149:482–485 [DOI] [PubMed] [Google Scholar]
- 17. Heredia-Soto V, Rodríguez-Salas N, Feliu J. Liquid biopsy in pancreatic cancer: are we ready to apply it in the clinical practice? Cancers (Basel) 2021;13:1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tie J, Cohen JD, Lahouel K, Lo SN, Wang Y, Kosmider S et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med 2022;386:2261–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poruk KE, Valero V 3rd, Saunders T, Blackford AL, Griffin JF, Poling J et al. Circulating tumor cell phenotype predicts recurrence and survival in pancreatic adenocarcinoma. Ann Surg 2016;264:1073–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Padillo-Ruiz J, Suarez G, Pereira S, Calero-Castro FJ, Tinoco J, Marin L et al. Circulating tumor cells enumeration from the portal vein for risk stratification in early pancreatic cancer patients. Cancers (Basel) 2021;13:6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moya-Herraiz AA, Dorcaratto D, Martin-Perez E, Escrig-Sos J, Poves-Prim I, Fabregat-Prous J et al. Non-arbitrary minimum threshold of yearly performed pancreatoduodenectomies: national multicentric study. Surgery 2021;170:910–916 [DOI] [PubMed] [Google Scholar]
- 22. Schulz KF, Altman DG, Moher D; CONSORT Group . CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials 2010;11:3220334632 [Google Scholar]
- 23. Isaji S, Mizuno S, Windsor JA, Bassi C, Fernández-del Castillo C, Hackert T et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018;18:2–11 [DOI] [PubMed] [Google Scholar]
- 24. ASA . Statement on ASA Physical Status Classification System. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system (accessed 26 August 2022)
- 25. O’Callaghan CA. OxMaR: open source free software for online minimization and randomization for clinical trials. PLoS One 2014;9:e110761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fidler IJ. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer 1973;9:223–227 [DOI] [PubMed] [Google Scholar]
- 27. Calero-Castro FJ, Pereira S, Laga I, Villanueva P, Suárez-Artacho G, Cepeda-Franco C et al. Quantification and characterization of CTCs and clusters in pancreatic cancer by means of the Hough transform algorithm. Int J Mol Sci 2023;24:4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg 2006;93:1232–1237 [DOI] [PubMed] [Google Scholar]
- 29. Verbeke CS. Resection margins and R1 rates in pancreatic cancer–are we there yet? Histopathology 2008;52:787–796 [DOI] [PubMed] [Google Scholar]
- 30. Tanase CP, Neagu M, Albulescu R, Codorean E, Dima SO. Biomarkers in the diagnosis and early detection of pancreatic cancer. Expert Opin Med Diagn 2009;3:533–546 [DOI] [PubMed] [Google Scholar]
- 31. Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours (8th edn). Oxford: Wiley-Blackwell, 2016. (ISBN: 978-1-119-26357-9) [Google Scholar]
- 32. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8–13 [DOI] [PubMed] [Google Scholar]
- 34. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761–768 [DOI] [PubMed] [Google Scholar]
- 35. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20–25 [DOI] [PubMed] [Google Scholar]
- 36. Mou Y, Song Y, Liu J, Song H, Liu X, Li J et al. Long term outcomes of no-touch isolation principles applied in pancreaticoduodenectomy for treatment of pancreatic adenocarcinoma: a multicenter retrospective study with propensity score matching. J Clin Med 2023;12:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mazzotta AD, VAN Bodegraven EA, Petrucciani N, Usai S, Carneiro AC, Tribillon E et al. Oncological outcome after laparoscopic ‘no-touch’ RAMPS versus ‘touch’ left pancreatectomy for pancreatic adenocarcinoma. Anticancer Res 2023;43:4983–4991 [DOI] [PubMed] [Google Scholar]
- 38. Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol 2008;19:1821–1828 [DOI] [PubMed] [Google Scholar]
- 39. Muñoz M, Coveñas R. The neurokinin-1 receptor antagonist aprepitant: an intelligent bullet against cancer? Cancers (Basel) 2020;12:2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Santucci N, Facy O, Ortega-Deballon P, Lequeu JB, Rat P, Rat P. CA 19-9 predicts resectability of pancreatic cancer even in jaundiced patients. Pancreatology 2018;18:666–670 [DOI] [PubMed] [Google Scholar]
- 41. Diaz CL, Cinar P, Hwang J, Ko AH, Tempero MA. CA 19-9 response: a surrogate to predict survival in patients with metastatic pancreatic adenocarcinoma. Am J Clin Oncol 2019;42:898–902 [DOI] [PubMed] [Google Scholar]
- 42. Takii Y, Mizusawa J, Kanemitsu Y, Komori K, Shiozawa M, Ohue M et al. The conventional technique versus the no-touch isolation technique for primary tumor resection in patients with colon cancer (JCOG1006). Ann Surg 2022;275:849–855 [DOI] [PubMed] [Google Scholar]
- 43. Groen J, Michiels N, van Roesseland S, Besselink MG, Bosscha K, Busch OR et al. Venous wedge and segment resection during pancreatoduodenectomy for pancreatic cancer: impact on short- and long-term outcomes in a nationwide cohort analysis. Br J Surg 2022;109:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giovinazzo F, Turri G, Katz MH, Heaton N, Ahmed I. Meta-analysis of benefits of portal-superior mesenteric vein resection in pancreatic resection for ductal adenocarcinoma. Br J Surg 2016;103:179–191 [DOI] [PubMed] [Google Scholar]
- 45. Farnes I, Kleive D, Verbeke CS, Aabakken L, Issa-Epe A, Småstuen MC et al. Resection rates and intention-to-treat outcomes in borderline and locally advanced pancreatic cancer: real-world data from a population-based, prospective cohort study (NORPACT-2). BJS Open 2023;7:zrad137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jang J-Y, Kang MJ, Heo JS, Choi SH, Choi DW, Park SJ et al. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann Surg 2014;259:656–664 [DOI] [PubMed] [Google Scholar]
- 47. van Dam JL, Janssen QP, Besselink MG, Homs MYV, van Santvoort HC, van Tienhoven G et al. Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomised controlled trials. Eur J Cancer 2022;160:140–149 [DOI] [PubMed] [Google Scholar]
- 48. Zou Y, Gao S, Yu X, Zhou T, Xie Y, Guo X et al. Survival outcomes of neoadjuvant therapy followed by radical resection versus upfront surgery for stage I–III pancreatic ductal adenocarcinoma: a retrospective cohort study. Int J Surg 2023;109:1573–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Crippa S, Partelli S, Zamboni G, Barugola G, Capelli P, Inama M et al. Poorly differentiated resectable pancreatic cancer: is upfront resection worthwhile? Surgery 2012; 152(Suppl 1): S112–S119 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data supporting this publication are not available from an open repository, although they can be shared by the author in selected cases.
References
- 1. Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021;19:439–457 [DOI] [PubMed] [Google Scholar]
- 2. Park W, Chawla A, O’Reilly EM. Pancreatic cancer: a review. JAMA 2021;326:851–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467:1114–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grünwald BT, Devisme A, Andrieux G, Vyas F, Aliar K, McCloskey CW et al. Spatially confined sub-tumor microenvironments in pancreatic cancer. Cell 2021;184:5577–5592.e18 [DOI] [PubMed] [Google Scholar]
- 5. Gardian K, Janczewska S, Olszewski WL, Durlik M. Analysis of pancreatic cancer microenvironment: role of macrophage infiltrates and growth factors expression. J Cancer 2012;3:285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ren B, Cui M, Yang G, Wang H, Feng M, You L et al. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer 2018;17:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chronopoulos A, Robinson B, Sarper M, Cortes E, Auernheimer V, Lachowski D et al. ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat Commun 2016;7:12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200–1210 [DOI] [PubMed] [Google Scholar]
- 9. Sommerville CA, Limongelli P, Pai M, Ahmad R, Stamp G, Habib NA et al. Survival analysis after pancreatic resection for ampullary and pancreatic head carcinoma: an analysis of clinicopathological factors. J Surg Oncol 2009;100:651–656 [DOI] [PubMed] [Google Scholar]
- 10. Market M, Tennakoon G, Auer RC. Postoperative natural killer cell dysfunction: the prime suspect in the case of metastasis following curative cancer surgery. Int J Mol Sci 2021;22:11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuroki T, Eguchi S. No-touch isolation techniques for pancreatic cancer. Surg Today 2017;47:8–13 [DOI] [PubMed] [Google Scholar]
- 12. Nakao A. The mesenteric approach in pancreatoduodenectomy. Dig Surg 2016;33:308–313 [DOI] [PubMed] [Google Scholar]
- 13. Sabater L, Cugat E, Serrablo A, Suarez-Artacho G, Diez-Valladares L, Santoyo-Santoyo J et al. Does the artery-first approach improve the rate of R0 resection in pancreatoduodenectomy?: a multicenter, randomized, controlled trial. Ann Surg 2019;270:738–746 [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi S, Asano T, Ochiai T. A proposal of no-touch isolation technique in pancreatoduodenectomy for periampullary carcinomas. Hepatogastroenterology 2001;48:372–374 [PubMed] [Google Scholar]
- 15. Hirota M, Shimada S, Yamamoto K, Tanaka E, Sugita H, Egami H. Pancreatectomy using the no-touch isolation technique followed by extensive intraoperative peritoneal lavage to prevent cancer cell dissemination: a pilot study. JOP 2005;6:143–151 [PubMed] [Google Scholar]
- 16. Gall TM, Jacob J, Frampton AE, Krell J, Kyriakides C, Castellano L et al. Reduced dissemination of circulating tumor cells with no-touch isolation surgical technique in patients with pancreatic cancer. JAMA Surg 2014;149:482–485 [DOI] [PubMed] [Google Scholar]
- 17. Heredia-Soto V, Rodríguez-Salas N, Feliu J. Liquid biopsy in pancreatic cancer: are we ready to apply it in the clinical practice? Cancers (Basel) 2021;13:1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tie J, Cohen JD, Lahouel K, Lo SN, Wang Y, Kosmider S et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med 2022;386:2261–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poruk KE, Valero V 3rd, Saunders T, Blackford AL, Griffin JF, Poling J et al. Circulating tumor cell phenotype predicts recurrence and survival in pancreatic adenocarcinoma. Ann Surg 2016;264:1073–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Padillo-Ruiz J, Suarez G, Pereira S, Calero-Castro FJ, Tinoco J, Marin L et al. Circulating tumor cells enumeration from the portal vein for risk stratification in early pancreatic cancer patients. Cancers (Basel) 2021;13:6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moya-Herraiz AA, Dorcaratto D, Martin-Perez E, Escrig-Sos J, Poves-Prim I, Fabregat-Prous J et al. Non-arbitrary minimum threshold of yearly performed pancreatoduodenectomies: national multicentric study. Surgery 2021;170:910–916 [DOI] [PubMed] [Google Scholar]
- 22. Schulz KF, Altman DG, Moher D; CONSORT Group . CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials 2010;11:3220334632 [Google Scholar]
- 23. Isaji S, Mizuno S, Windsor JA, Bassi C, Fernández-del Castillo C, Hackert T et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018;18:2–11 [DOI] [PubMed] [Google Scholar]
- 24. ASA . Statement on ASA Physical Status Classification System. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system (accessed 26 August 2022)
- 25. O’Callaghan CA. OxMaR: open source free software for online minimization and randomization for clinical trials. PLoS One 2014;9:e110761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fidler IJ. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer 1973;9:223–227 [DOI] [PubMed] [Google Scholar]
- 27. Calero-Castro FJ, Pereira S, Laga I, Villanueva P, Suárez-Artacho G, Cepeda-Franco C et al. Quantification and characterization of CTCs and clusters in pancreatic cancer by means of the Hough transform algorithm. Int J Mol Sci 2023;24:4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg 2006;93:1232–1237 [DOI] [PubMed] [Google Scholar]
- 29. Verbeke CS. Resection margins and R1 rates in pancreatic cancer–are we there yet? Histopathology 2008;52:787–796 [DOI] [PubMed] [Google Scholar]
- 30. Tanase CP, Neagu M, Albulescu R, Codorean E, Dima SO. Biomarkers in the diagnosis and early detection of pancreatic cancer. Expert Opin Med Diagn 2009;3:533–546 [DOI] [PubMed] [Google Scholar]
- 31. Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours (8th edn). Oxford: Wiley-Blackwell, 2016. (ISBN: 978-1-119-26357-9) [Google Scholar]
- 32. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8–13 [DOI] [PubMed] [Google Scholar]
- 34. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761–768 [DOI] [PubMed] [Google Scholar]
- 35. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20–25 [DOI] [PubMed] [Google Scholar]
- 36. Mou Y, Song Y, Liu J, Song H, Liu X, Li J et al. Long term outcomes of no-touch isolation principles applied in pancreaticoduodenectomy for treatment of pancreatic adenocarcinoma: a multicenter retrospective study with propensity score matching. J Clin Med 2023;12:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mazzotta AD, VAN Bodegraven EA, Petrucciani N, Usai S, Carneiro AC, Tribillon E et al. Oncological outcome after laparoscopic ‘no-touch’ RAMPS versus ‘touch’ left pancreatectomy for pancreatic adenocarcinoma. Anticancer Res 2023;43:4983–4991 [DOI] [PubMed] [Google Scholar]
- 38. Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol 2008;19:1821–1828 [DOI] [PubMed] [Google Scholar]
- 39. Muñoz M, Coveñas R. The neurokinin-1 receptor antagonist aprepitant: an intelligent bullet against cancer? Cancers (Basel) 2020;12:2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Santucci N, Facy O, Ortega-Deballon P, Lequeu JB, Rat P, Rat P. CA 19-9 predicts resectability of pancreatic cancer even in jaundiced patients. Pancreatology 2018;18:666–670 [DOI] [PubMed] [Google Scholar]
- 41. Diaz CL, Cinar P, Hwang J, Ko AH, Tempero MA. CA 19-9 response: a surrogate to predict survival in patients with metastatic pancreatic adenocarcinoma. Am J Clin Oncol 2019;42:898–902 [DOI] [PubMed] [Google Scholar]
- 42. Takii Y, Mizusawa J, Kanemitsu Y, Komori K, Shiozawa M, Ohue M et al. The conventional technique versus the no-touch isolation technique for primary tumor resection in patients with colon cancer (JCOG1006). Ann Surg 2022;275:849–855 [DOI] [PubMed] [Google Scholar]
- 43. Groen J, Michiels N, van Roesseland S, Besselink MG, Bosscha K, Busch OR et al. Venous wedge and segment resection during pancreatoduodenectomy for pancreatic cancer: impact on short- and long-term outcomes in a nationwide cohort analysis. Br J Surg 2022;109:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giovinazzo F, Turri G, Katz MH, Heaton N, Ahmed I. Meta-analysis of benefits of portal-superior mesenteric vein resection in pancreatic resection for ductal adenocarcinoma. Br J Surg 2016;103:179–191 [DOI] [PubMed] [Google Scholar]
- 45. Farnes I, Kleive D, Verbeke CS, Aabakken L, Issa-Epe A, Småstuen MC et al. Resection rates and intention-to-treat outcomes in borderline and locally advanced pancreatic cancer: real-world data from a population-based, prospective cohort study (NORPACT-2). BJS Open 2023;7:zrad137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jang J-Y, Kang MJ, Heo JS, Choi SH, Choi DW, Park SJ et al. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann Surg 2014;259:656–664 [DOI] [PubMed] [Google Scholar]
- 47. van Dam JL, Janssen QP, Besselink MG, Homs MYV, van Santvoort HC, van Tienhoven G et al. Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomised controlled trials. Eur J Cancer 2022;160:140–149 [DOI] [PubMed] [Google Scholar]
- 48. Zou Y, Gao S, Yu X, Zhou T, Xie Y, Guo X et al. Survival outcomes of neoadjuvant therapy followed by radical resection versus upfront surgery for stage I–III pancreatic ductal adenocarcinoma: a retrospective cohort study. Int J Surg 2023;109:1573–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Crippa S, Partelli S, Zamboni G, Barugola G, Capelli P, Inama M et al. Poorly differentiated resectable pancreatic cancer: is upfront resection worthwhile? Surgery 2012; 152(Suppl 1): S112–S119 [DOI] [PubMed] [Google Scholar]