Abstract
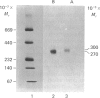
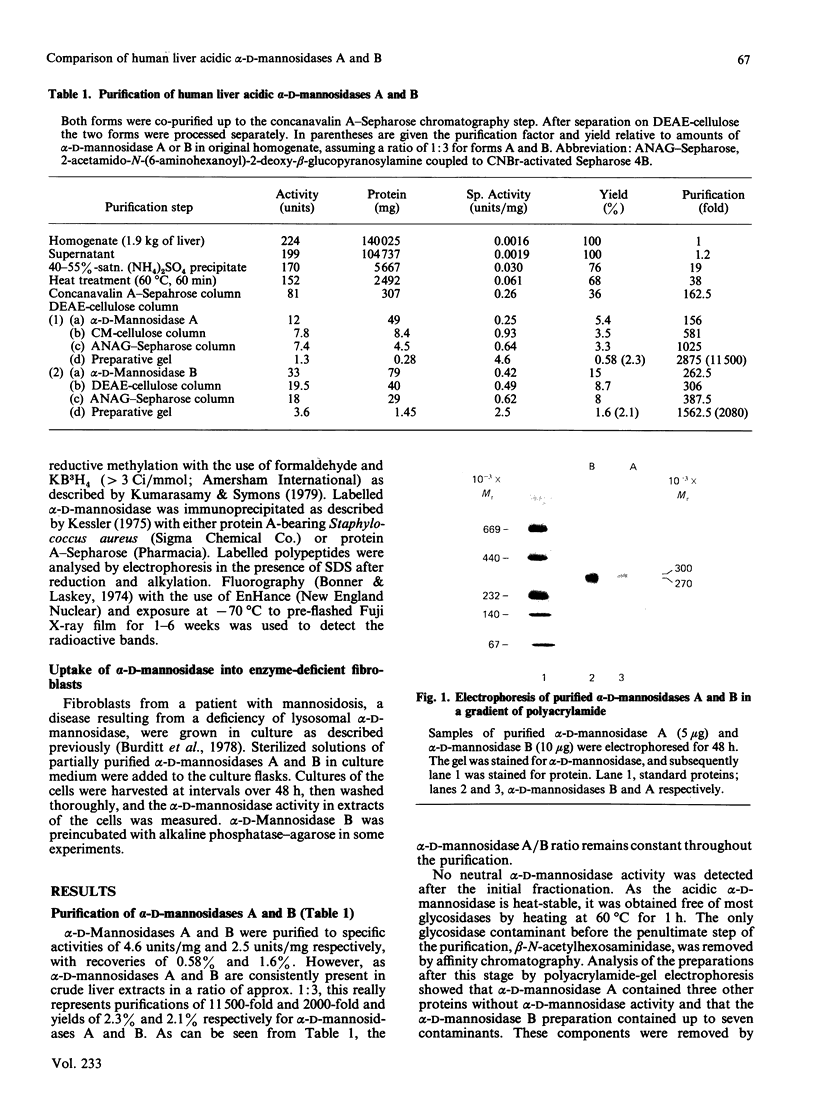
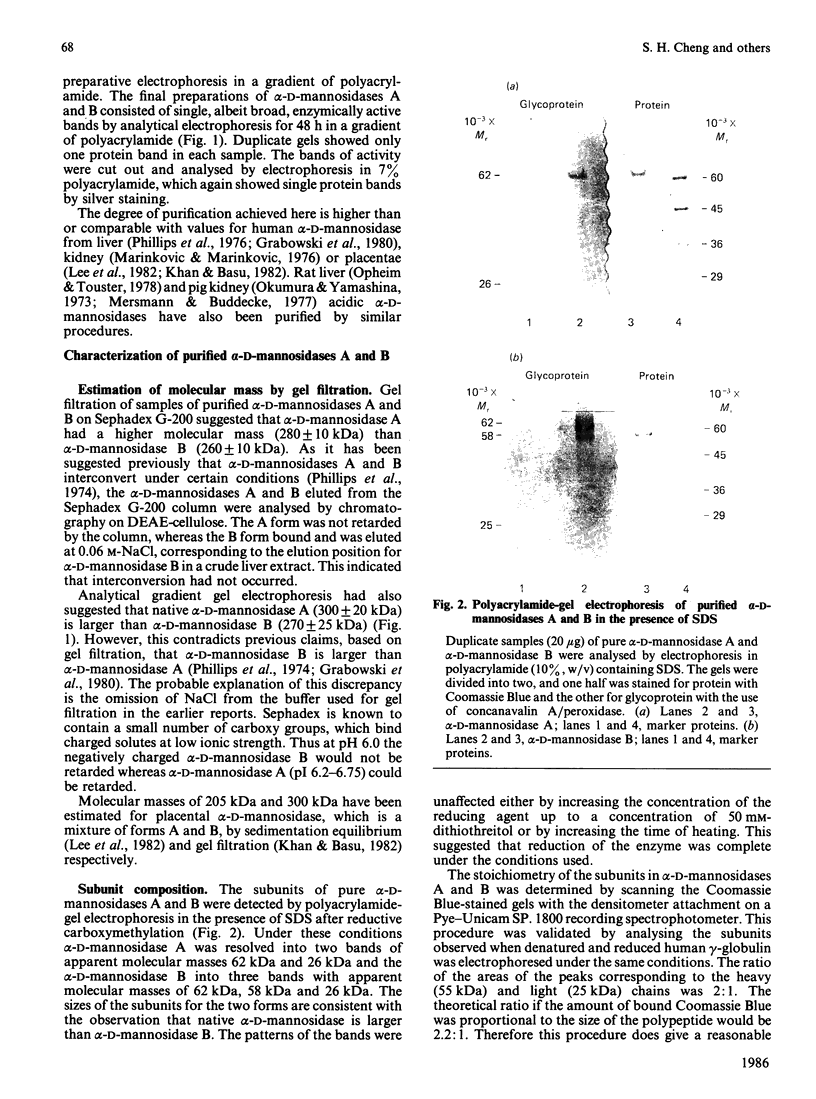
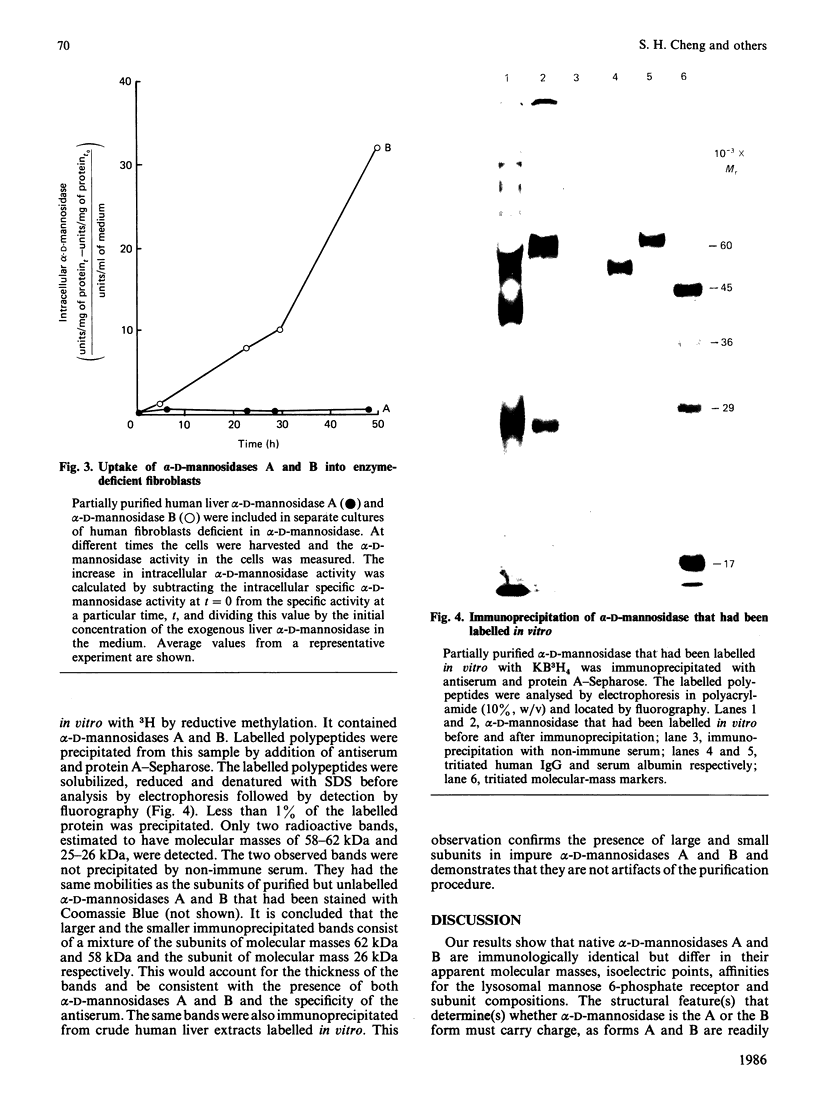
Human liver alpha-D-mannosidases A and B were purified 11 500-fold and 2000-fold respectively. Both showed microheterogeneity when analysed by isoelectric focusing. Alpha-D-Mannosidases A and B are immunologically identical but differ in their range of pI values, molecular masses, uptake into fibroblasts and subunit compositions. Alpha-D-Mannosidase A consists of equimolar proportions of subunits of molecular masses 62 kDa and 26 kDa, which are linked by disulphide bridges in the intact enzyme. Alpha-D-Mannosidase B also contains a small subunit, of molecular mass 26 kDa, and a variable mixture of larger subunits, of molecular masses 58 kDa and 62 kDa. The 62 kDa and 58 kDa subunits, but not the 26 kDa one, contain concanavalin A-recognizing glycans. The 58 kDa subunit has a lower pI, contains less high-mannose glycans but probably contains more mannose 6-phosphate than the 62 kDa subunit. It is postulated that the differences in structure and properties of alpha-D-mannosidases A and B are due to differences in the state of processing of the large subunit. This suggestion is consistent with a single locus on chromosome 19 for lysosomal alpha-D-mannosidase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burditt L. J., Chotai K. A., Winchester B. G. Evidence that the mutant enzyme in fibroblasts of a patient with mannosidosis does not crossreact with antiserum raised against normal acidic alpha-D-mannosidase. FEBS Lett. 1978 Jul 15;91(2):186–189. doi: 10.1016/0014-5793(78)81168-5. [DOI] [PubMed] [Google Scholar]
- Carroll M., Dance N., Masson P. K., Robinson D., Winchester B. G. Human mannosidosis--the enzyme defect. Biochem Biophys Res Commun. 1972 Oct 17;49(2):579–583. doi: 10.1016/0006-291x(72)90450-0. [DOI] [PubMed] [Google Scholar]
- Champion M. J., Shows T. B. Mannosidosis: assignment of the lysosomal alpha-mannosidase B gene to chromosome 19 in man. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2968–2972. doi: 10.1073/pnas.74.7.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester M. A., Lundblad A., Masson P. K. The relationship between different forms of human alpha-mannosidase. Biochim Biophys Acta. 1975 Jun 24;391(2):341–348. doi: 10.1016/0005-2744(75)90258-2. [DOI] [PubMed] [Google Scholar]
- Grabowski G. A., Ikonne J. U., Desnick R. J. Comparative physical, kinetic and immunologic properties of the acidic and neutral alpha-D-mannosidase isozymes from human liver. Enzyme. 1980;25(1):13–25. doi: 10.1159/000459210. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hasilik A., Von Figura K. Oligosaccharides in lysosomal enzymes. Distribution of high-mannose and complex oligosaccharides in cathepsin D and beta-hexosaminidase. Eur J Biochem. 1981 Dec;121(1):125–129. doi: 10.1111/j.1432-1033.1981.tb06440.x. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Koshy A., Robinson K., Stirling J. L. An attempt to purify N-acetyl-beta-hexosaminidases from crude extracts of human liver by affinity chromatography. Biochem Soc Trans. 1975;3(2):244–246. doi: 10.1042/bst0030244. [DOI] [PubMed] [Google Scholar]
- Kumarasamy R., Symons R. H. The tritium labeling of small amounts of protein for analysis by electrophoresis on sodium dodecyl sulfate--polyacrylamide slab gels. Anal Biochem. 1979 Jun;95(2):359–363. doi: 10.1016/0003-2697(79)90739-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. E., Little T. E., Yoshida A. Purification and chemical characterization of human acidic alpha-D-mannosidase. Enzyme. 1982;28(1):33–40. doi: 10.1159/000459082. [DOI] [PubMed] [Google Scholar]
- Marinkovic D. V., Marinkovic J. N. Isolation and properties of alpha-D-mannosidase from human kidney. Biochem J. 1976 May 1;155(2):217–223. doi: 10.1042/bj1550217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur R., Alvares K., Balasubramanian A. S. Two forms of acid alpha-D-mannosidase in monkey brain: evidence for the co-existence of high mannose and complex oligosaccharides in one form. Biochem Biophys Res Commun. 1984 Sep 28;123(3):1185–1193. doi: 10.1016/s0006-291x(84)80258-2. [DOI] [PubMed] [Google Scholar]
- Mersmann G., Becker R., Buddecke E. Amount and properties of uptake forms in preparations of alpha-mannosidase from pig kidney. Biochim Biophys Acta. 1978 Jul 7;525(1):154–161. doi: 10.1016/0005-2744(78)90209-7. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Okumura T., Yamashina I. Further purification and characterization of -mannosidase from hog kidney. J Biochem. 1973 Jan;73(1):131–138. [PubMed] [Google Scholar]
- Opheim D. J., Touster O. Lysosomal alpha-D-mannosidase of rat liver. Purification and comparison with the golgi and cytosolic alpha-D-mannosidases. J Biol Chem. 1978 Feb 25;253(4):1017–1023. [PubMed] [Google Scholar]
- Parish R. W., Schmidlin S., Müller U. The effects of proteases on proteins and glycoproteins of Dictyostelium discoideum plasma membranes. Exp Cell Res. 1977 Dec;110(2):267–276. doi: 10.1016/0014-4827(77)90292-0. [DOI] [PubMed] [Google Scholar]
- Phillips N. C., Robinson D., Winchester B. G. Characterization of human liver alpha-D-mannosidase purified by affinity chromatography. Biochem J. 1976 Mar 1;153(3):579–587. doi: 10.1042/bj1530579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N. C., Robinson D., Winchester B. G. Human liver alpha-D-mannosidase activity. Clin Chim Acta. 1974 Aug 30;55(1):11–19. doi: 10.1016/0009-8981(74)90328-3. [DOI] [PubMed] [Google Scholar]
- Phillips N., Robinson D., Winchester B. Immunological characterization of human liver alpha-D-mannosidase. Biochem J. 1975 Dec;151(3):469–475. doi: 10.1042/bj1510469a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann R., Hasilik A., Cheng S., Pemble S., Winchester B., von Figura K. Synthesis of lysosomal alpha-mannosidase in normal and mannosidosis fibroblasts. Biochem Biophys Res Commun. 1983 Sep 30;115(3):1083–1089. doi: 10.1016/s0006-291x(83)80046-1. [DOI] [PubMed] [Google Scholar]
- Roberts L. M., Lord J. M. The synthesis of Ricinus communis agglutinin, cotranslational and posttranslational modification of agglutinin polypeptides. Eur J Biochem. 1981 Sep;119(1):31–41. doi: 10.1111/j.1432-1033.1981.tb05573.x. [DOI] [PubMed] [Google Scholar]