Abstract
Signaling by the LET-60 Ras GTPase/ MPK-1 Extracellular Regulated Kinase pathway specifies the vulva cell fate in C. elegans . The let-7 miRNA family negatively regulates LET-60 Ras but other miRNAs can also modulate vulva induction. To determine the impact of globally reducing miRNA function on LET-60 Ras-mediated vulva induction we analyzed the effect of loss of the ALG-1 miRNA regulator on vulva development . Contrary to our expectations, we find that ALG-1 promotes vulva induction independently of LET-60 Ras. We found that the reduced vulva cell fate induction of alg-1 deletion mutants could be due to delayed development of the vulva, or a requirement to maintain the competence of the uninduced precursor cells.
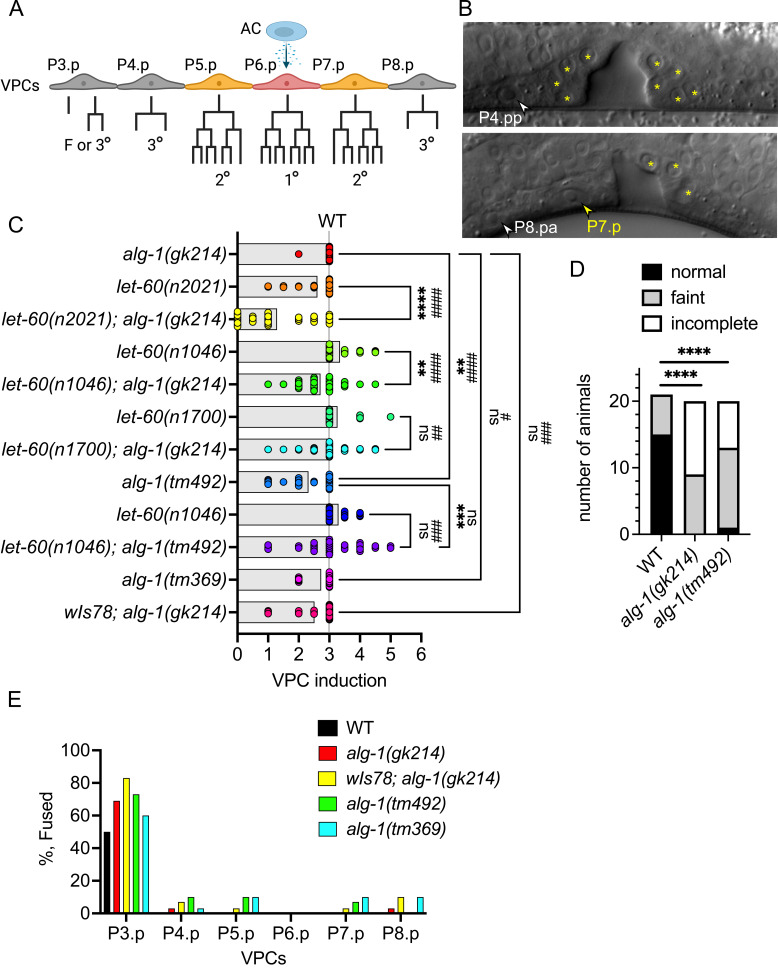
Figure 1. alg-1 deletion alleles cause a partial vulvaless phenotype .
( A ) Schematic of vulva induction. Six vulva precursor cells (VPCs) P3.p-P8.p are competent to be induced to adopt vulval cell fates. However, only the VPCs closest to the anchor cell (AC) are induced to adopt 1° and 2° vulval fates which undergo three rounds of cell division to generate 22 vulval cells. The remaining VPCs adopt the 3° non-vulval fate and divide once with the exception of P3.p, which 50% of the time loses competence and adopts the fused (F) fate without dividing. Created using Biorender ( B ) Differential interference contrast images of normal (top) and partially induced (bottom) vulvas of L4 stage alg-1 ( tm369 ) deletion mutant strain. In the top image (anterior is left, ventral is down) yellow asterisks mark the visible nuclei of P5.p and P7.p descendants. White arrow marks the uninduced posterior daughter of P4.p. In the bottom image (anterior is right) yellow asterisks mark the visible nuclei of the descendants of P5.p and a yellow arrow marks the P7.p cell that failed to divide and appears to have adopted a fused fate. White arrow marks the uninduced anterior daughter of P8.p. ( C ) Bar graph/scatter dot plot depicting vulva induction in various alg-1 and let-60 mutants. Each dot represents the VPC induction score for one animal and the bar represents the average VPC induction score for the strain. A score of 3 is wild type (WT), <3 is vulvaless, and >3 is multivulva. Since the VPCs are still competent to be induced after the first division, half inductions are common when signaling is compromised. A one-way ANOVA with multiple comparisons was used to determine the significance between average VPC inductions scores ** P<0.01 , *** P<0.001 and **** P<0.0001. A Fisher's exact test was used to determine the significance of the vulvaless phenotypes ## P<0.01 , ### P<0.001 and #### P<0.0001. ns, not significant. ( D ) Bar graph depicting the number of animals with normal alae or defective alae that were either faint or incomplete in wild type, alg-1 ( gk214 ) and alg-1 ( tm492 ). A Fisher's exact test was used to determine the difference in normal versus defective (faint + incomplete) alae between wild type and the alg-1 mutants. **** P<0.0001. ( E ) Bar graph depicting the % Fused fate adopted by each VPC in the alg-1 mutants compared to that expected for wild type. Between 30-34 animals were scored for the vulval phenotypes depicted in panels C and E.
Description
LET-60 Ras is an essential component of an Epidermal Growth Factor Receptor/Ras GTPase/Mitogen Activated Protein Kinase signaling pathway that induces three of six epithelial cells to adopt vulva cell fates ( Figure 1A ) (Beitel et al. , 1990; Han et al. , 1990; Han and Sternberg, 1990; Schmid and Hajnal, 2015). During the second larval stage, six epithelial cells (P3.p-P8.p) are maintained in a competent state to be induced by a combination of LET-23 EGFR and LIN-12 Notch signaling pathways in the third larval stage (Greenwald et al. , 1983; Yochem et al. , 1988; Aroian et al. , 1990; Wang and Sternberg, 1999). Three cells, P5.p, P6.p and P7.p, are induced by a graded LIN-3 EGF-like signal from the overlying gonad ( Figure 1A ) (Sternberg and Horvitz, 1986; Hill and Sternberg, 1992; Katz et al. , 1995). P6.p, the closest to the ligand is induced to adopt the primary (1°) vulval fate and lateral LIN-12 Notch signaling from P6.p paired with the graded LIN-3 signaling specifies the secondary (2°) vulval fates in P5.p and P7.p. P5.p, P6.p and P7.p undergo the stereotypical three rounds of division to produce the 22 nuclei that make up the mature vulva ( Figure 1A, B top). The remaining P3.p, P4.p and P5.p cells adopt the tertiary (3°) non-vulval fate in which they divide once and fuse with the surrounding hypodermis, except for P3.p which 50% of the time fuses prior to dividing and adopts a fused (F) fate (Sternberg and Horvitz, 1986; Eisenmann et al. , 1998).
Argonaute proteins are the core component of the miRISC complex that guide miRNAs to the 3'UTR of their mRNA targets to inhibit translation and/or initiate mRNA degradation (Frederick and Simard, 2022; Ambros, 2024). The C. elegans miRNA argonautes, ALG-1 and ALG-2 , are redundantly required for embryogenesis (Grishok et al. , 2001; Vasquez-Rifo et al. , 2012) however, only alg-1 deletions have post-embryonic miRNA associated phenotypes suggesting that ALG-1 has a greater role in regulating miRNA function during larval development.
The 3'UTR of let-60 ras has several let-7 miRNA target sites (Johnson et al. , 2005). Overexpression of let-7 family members mir-48 and mir-84 partly suppress the multivulva phenotype of activating alleles of let-60 (Johnson et al. , 2005; Li et al. , 2005). Furthermore, mir-48 and mir-84 have reciprocal expression patterns with let-60 in the VPCs and this is dependent on the 3'UTR of let-60 (Esquela-Kerscher et al. , 2005; Johnson et al. , 2005). Although, loss of let-7 family members mir-48 , mir-84 and mir-241 independently or in combination, fail to cause a vulval phenotype (Abbott et al. , 2005), and conversely, precocious expression of let-7 results in a multivulva phenotype (Hunter et al. , 2013). Other components of the Ras/MAPK pathway have predicted let-7 miRNA target sites (Johnson et al. , 2005), and other miRNAs can differentially modulate the let-60 ras multivulva phenotype (Brenner et al. , 2012). Thus, multiple miRNAs may regulate LET-60 Ras signaling at multiple targets.
To assess the global requirements of miRNAs in LET-60 Ras-mediated vulva induction, we tested for genetic interactions between a deletion allele of alg-1 , gk214 , with hypomorphic and hypermorphic alleles of let-60 ras (Ferguson and Horvitz, 1985; Beitel et al. , 1990; Ding et al. , 2005) . While the alg-1 ( gk214 ) strain had close to normal vulva development, we found that it enhanced the vulvaless phenotype of let-60 ( n2021 ) hypomorphic allele, opposite what we would expect with a loss of the let-7 miRNA family ( Figure 1C ). Next, we tested if alg-1 ( gk214 ) could suppress the multivulva phenotype of the let-60 ( n1046 ) hypermorphic mutant. Surprisingly, the let-60 ( n1046 ); alg-1 ( gk214 ) multivulva phenotype was not significantly suppressed, but instead displayed a vulvaless phenotype not seen in either single mutant ( Figure 1C ). Suspecting that one of the strains could have a genetic modifier in the background, we crossed alg-1 ( gk214 ) into the let-60 ( n1700 ) hypermorphic mutant that possess the same G13D point mutation found in the let-60 ( n1046 ) mutant (Beitel et al. , 1990). Again, we saw a vulvaless phenotype in let-60 ( n1700 ); alg-1 ( gk214 ) double mutants ( Figure 1C ). We then crossed another deletion of alg-1 , tm492 , with let-60 ( n1046 ) (Yigit et al., 2006) . Here we found that both alg-1 ( tm492 ) and let-60 ( n1046 ); alg-1 ( tm492 ) had similar vulvaless phenotypes suggesting that loss of alg-1 itself results in a previously undescribed vulvaless phenotype ( Figure 1C ). Thus, we looked at a third deletion allele, alg-1 ( tm369 ) (Yigit et al., 2006) , and an alg-1 ( gk214 ) strain which was further crossed with a seam cell marker, wIs78 (Koh and Rothman, 2001) . We found that both strains displayed a vulvaless phenotype that together, were significantly more vulvaless than the starting alg-1 ( gk214 ) strain ( Figure 1B,C ). Thus, the starting strain likely harbors one or more genetic suppressors that were lost in subsequent crosses with the let-60 mutants and wIs78 . These suppressors could be specific to VPC induction as we did not find differences in alae defects between the starting alg-1 ( gk214 ) strain and the alg-1 ( tm492 ) mutant ( Figure 1D ).
All VPCs divide once, whether they are induced or not induced, excluding P3.p which fuses with the surrounding hypodermis prior to induction about 50% of the time in wild-type hermaphrodites ( Figure 1A ). We found that in the alg-1 deletion alleles, a low level of VPCs failed to divide suggesting that either they fused prematurely and lost competence or had a cell cycle defect ( Figure 1E ). While all alg-1 mutants had over 50% of P3.p cells adopting the F fate, only wIs78 ; alg-1 ( gk214 ) reached a statistically significant increase above 50%. However, the low level of fused VPCs does not account for the vulvaless phenotype seen in alg-1 mutants as most uninduced cells divided once. Another phenotype we noted was a low percentage of incompletion lineages in let-60 (gf); alg-1 (-) strains, in which the induced VPCs failed to complete the third cell division by the fourth larval stage, suggesting either a delayed or incomplete execution of the lineages.
Here, we report that alg-1 mutants have a vulvaless phenotype. Mutations in alg-1 and ain-1 GW182 , a component of the miRISC complex, were previously identified as suppressors of the lin-31 multivulva phenotype (Ding et al. , 2005; Morita and Han, 2006); however, the role of alg-1 in regulating vulval cell fate induction was not characterized. While the alg-1 mutant vulvaless phenotype is inconsistent with a simple loss of let-7 family miRNAs antagonizing let-60 ras , the incomplete lineages could reflect a heterochronic phenotype (Euling and Ambros, 1996) . Mutations in the lin-4 miRNA cause a vulvaless phenotype with a failure of division in some VPCs and supernumerary divisions in other VPCs (Chalfie et al. , 1981; Sulston and Horvitz, 1981; Ambros and Horvitz, 1984; Ferguson and Horvitz, 1985). The mir-48 mir-241 ; mir-84 triple mutant in combination with the lin-46 heterochronic mutant has a retarded vulva development phenotype (Abbott et al. , 2005), and alg-1 ( gk214 ) enhances the retarded vulva development phenotype of a lin-66 heterochronic mutant (Morita and Han, 2006) . Thus, some of the alg-1 vulval phenotypes could be a result of heterochronic phenotypes due to a reduction in lin-4 and let-7 miRNA family function. However, the lin-4 and mir-61 miRNAs promote LIN-12 Notch signaling and the 2° vulval fate (Yoo and Greenwald, 2005; Li and Greenwald, 2010) . Notably, we found that P6.p was always induced in the alg-1 deletions, which could be consistent with a specific failure to induce the 2° vulval fates or could reflect a partial loss of LET-60 Ras signaling as P6.p was induced in all hypomorphic let-60 ( n2021 ) mutant animals analyzed. The failure of P6.p to adopt an F fate is also reminiscent of some Wnt signaling mutants (Myers and Greenwald, 2007) . Thus, ALG-1 and various miRNAs could promote vulva cell fate induction via regulation of developmental timing and promoting the LIN-12 Notch, LET-60 Ras, and Wnt signaling pathways. Transcriptomic analysis of the VPCs identified transcripts predicted to be regulated by 114 miRNAs (Zhang et al. , 2022). Further work will be required to delineate the mechanisms and miRNAs promoting vulva induction with ALG-1 . The partial vulvaless phenotype of alg-1 mutants could be leveraged for the further identification and characterization of miRISC regulators.
Methods
Strain construction and maintenance were done as previous described (Stiernagle, 2006; Fay, 2013) . N2 was the wild type strain from which all mutants were derived. HB101 E. coli was used as a food source. Wormbase.org was critical for identifying reagents and designing experiments (Davis et al., 2022). Vulva induction scoring was done as previously described (Gauthier and Rocheleau, 2017) . Differential interference contrast images were acquired on an Axio Imager A1 using the Axiocam 305 mono camera with Zen software (Zeiss, Germany). Graphpad Prism 10 was used to generate graphs and statistical analyses.
Reagents
Acknowledgments
Acknowledgments
We thank Jung Hwa Seo for technical assistance. We thank the Simard (Université Laval) and Ambros (University of Massachusetts, Worcester) for sharing strains. Deletion alleles of alg-1 were generated by the National Bioresource Project for the nematode (Tokyo Women's Medical University School of Medicine, Japan) and the International C. elegans Gene Knockout Consortium (Oklahoma Medical Research Foundation, USA and the University of British Columbia, Canada). Some strains were provided by the Caenorhabditis Genetics Center (CGC), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Funding Statement
This work was funded by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (RGPIN-2018-05673) to CER.
References
- Abbott AL, Alvarez-Saavedra E, Miska EA, Lau NC, Bartel DP, Horvitz HR, Ambros V. The let-7 MicroRNA family members mir-48, mir-84, and mir-241 function together to regulate developmental timing in Caenorhabditis elegans. Dev Cell. 2005 Sep 1;9(3):403–414. doi: 10.1016/j.devcel.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V. MicroRNA-mediated gene regulation and the resilience of multicellular animals. Postepy Biochem. 2024 May 23;70(1):62–70. doi: 10.18388/pb.2021_515. [DOI] [PubMed] [Google Scholar]
- Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984 Oct 26;226(4673):409–416. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- Aroian RV, Koga M, Mendel JE, Ohshima Y, Sternberg PW. The let-23 gene necessary for Caenorhabditis elegans vulval induction encodes a tyrosine kinase of the EGF receptor subfamily. Nature. 1990 Dec 20;348(6303):693–699. doi: 10.1038/348693a0. [DOI] [PubMed] [Google Scholar]
- Beitel GJ, Clark SG, Horvitz HR. Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction. Nature. 1990 Dec 6;348(6301):503–509. doi: 10.1038/348503a0. [DOI] [PubMed] [Google Scholar]
- Brenner JL, Kemp BJ, Abbott AL. The mir-51 family of microRNAs functions in diverse regulatory pathways in Caenorhabditis elegans. PLoS One. 2012 May 16;7(5):e37185–e37185. doi: 10.1371/journal.pone.0037185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Horvitz HR, Sulston JE. Mutations that lead to reiterations in the cell lineages of C. elegans. Cell. 1981 Apr 1;24(1):59–69. doi: 10.1016/0092-8674(81)90501-8. [DOI] [PubMed] [Google Scholar]
- Davis P, Zarowiecki M, Arnaboldi V, Becerra A, Cain S, Chan J, Chen WJ, Cho J, da Veiga Beltrame E, Diamantakis S, Gao S, Grigoriadis D, Grove CA, Harris TW, Kishore R, Le T, Lee RYN, Luypaert M, Müller HM, Nakamura C, Nuin P, Paulini M, Quinton-Tulloch M, Raciti D, Rodgers FH, Russell M, Schindelman G, Singh A, Stickland T, Van Auken K, Wang Q, Williams G, Wright AJ, Yook K, Berriman M, Howe KL, Schedl T, Stein L, Sternberg PW. WormBase in 2022-data, processes, and tools for analyzing Caenorhabditis elegans. Genetics. 2022 Apr 4;220(4) doi: 10.1093/genetics/iyac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Spencer A, Morita K, Han M. The developmental timing regulator AIN-1 interacts with miRISCs and may target the argonaute protein ALG-1 to cytoplasmic P bodies in C. elegans. Mol Cell. 2005 Aug 19;19(4):437–447. doi: 10.1016/j.molcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Eisenmann DM, Maloof JN, Simske JS, Kenyon C, Kim SK. The beta-catenin homolog BAR-1 and LET-60 Ras coordinately regulate the Hox gene lin-39 during Caenorhabditis elegans vulval development. Development. 1998 Sep 1;125(18):3667–3680. doi: 10.1242/dev.125.18.3667. [DOI] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Johnson SM, Bai L, Saito K, Partridge J, Reinert KL, Slack FJ. Post-embryonic expression of C. elegans microRNAs belonging to the lin-4 and let-7 families in the hypodermis and the reproductive system. Dev Dyn. 2005 Dec 1;234(4):868–877. doi: 10.1002/dvdy.20572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euling S, Ambros V. Heterochronic genes control cell cycle progress and developmental competence of C. elegans vulva precursor cells. Cell. 1996 Mar 8;84(5):667–676. doi: 10.1016/s0092-8674(00)81045-4. [DOI] [PubMed] [Google Scholar]
- Fay DS. Classical genetic methods. WormBook. 2013 Dec 30;:1–58. doi: 10.1895/wormbook.1.165.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson EL, Horvitz HR. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. Genetics. 1985 May 1;110(1):17–72. doi: 10.1093/genetics/110.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frédérick PM, Simard MJ. Regulation and different functions of the animal microRNA-induced silencing complex. Wiley Interdiscip Rev RNA. 2021 Nov 1;13(4):e1701–e1701. doi: 10.1002/wrna.1701. [DOI] [PubMed] [Google Scholar]
- Gauthier K, Rocheleau CE. C. elegans Vulva Induction: An In Vivo Model to Study Epidermal Growth Factor Receptor Signaling and Trafficking. Methods Mol Biol. 2017;1652:43–61. doi: 10.1007/978-1-4939-7219-7_3. [DOI] [PubMed] [Google Scholar]
- Greenwald IS, Sternberg PW, Horvitz HR. The lin-12 locus specifies cell fates in Caenorhabditis elegans. Cell. 1983 Sep 1;34(2):435–444. doi: 10.1016/0092-8674(83)90377-x. [DOI] [PubMed] [Google Scholar]
- Grishok A, Pasquinelli AE, Conte D, Li N, Parrish S, Ha I, Baillie DL, Fire A, Ruvkun G, Mello CC. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell. 2001 Jul 13;106(1):23–34. doi: 10.1016/s0092-8674(01)00431-7. [DOI] [PubMed] [Google Scholar]
- Han M, Aroian RV, Sternberg PW. The let-60 locus controls the switch between vulval and nonvulval cell fates in Caenorhabditis elegans. Genetics. 1990 Dec 1;126(4):899–913. doi: 10.1093/genetics/126.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Sternberg PW. let-60, a gene that specifies cell fates during C. elegans vulval induction, encodes a ras protein. Cell. 1990 Nov 30;63(5):921–931. doi: 10.1016/0092-8674(90)90495-z. [DOI] [PubMed] [Google Scholar]
- Hill RJ, Sternberg PW. The gene lin-3 encodes an inductive signal for vulval development in C. elegans. Nature. 1992 Aug 6;358(6386):470–476. doi: 10.1038/358470a0. [DOI] [PubMed] [Google Scholar]
- Hunter SE, Finnegan EF, Zisoulis DG, Lovci MT, Melnik-Martinez KV, Yeo GW, Pasquinelli AE. Functional genomic analysis of the let-7 regulatory network in Caenorhabditis elegans. PLoS Genet. 2013 Mar 14;9(3):e1003353–e1003353. doi: 10.1371/journal.pgen.1003353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005 Mar 11;120(5):635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Katz WS, Hill RJ, Clandinin TR, Sternberg PW. Different levels of the C. elegans growth factor LIN-3 promote distinct vulval precursor fates. Cell. 1995 Jul 28;82(2):297–307. doi: 10.1016/0092-8674(95)90317-8. [DOI] [PubMed] [Google Scholar]
- Koh K, Rothman JH. ELT-5 and ELT-6 are required continuously to regulate epidermal seam cell differentiation and cell fusion in C. elegans. Development. 2001 Aug 1;128(15):2867–2880. doi: 10.1242/dev.128.15.2867. [DOI] [PubMed] [Google Scholar]
- Li J, Greenwald I. LIN-14 inhibition of LIN-12 contributes to precision and timing of C. elegans vulval fate patterning. Curr Biol. 2010 Oct 14;20(20):1875–1879. doi: 10.1016/j.cub.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Jones-Rhoades MW, Lau NC, Bartel DP, Rougvie AE. Regulatory mutations of mir-48, a C. elegans let-7 family MicroRNA, cause developmental timing defects. Dev Cell. 2005 Sep 1;9(3):415–422. doi: 10.1016/j.devcel.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Morita K, Han M. Multiple mechanisms are involved in regulating the expression of the developmental timing regulator lin-28 in Caenorhabditis elegans. EMBO J. 2006 Nov 30;25(24):5794–5804. doi: 10.1038/sj.emboj.7601451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers TR, Greenwald I. Wnt signal from multiple tissues and lin-3/EGF signal from the gonad maintain vulval precursor cell competence in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007 Dec 10;104(51):20368–20373. doi: 10.1073/pnas.0709989104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid T, Hajnal A. Signal transduction during C. elegans vulval development: a NeverEnding story. Curr Opin Genet Dev. 2015 Feb 9;32:1–9. doi: 10.1016/j.gde.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Sternberg PW, Horvitz HR. Pattern formation during vulval development in C. elegans. Cell. 1986 Mar 14;44(5):761–772. doi: 10.1016/0092-8674(86)90842-1. [DOI] [PubMed] [Google Scholar]
- Stiernagle T. Maintenance of C. elegans. WormBook. 2006 Feb 11;:1–11. doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Abnormal cell lineages in mutants of the nematode Caenorhabditis elegans. Dev Biol. 1981 Feb 1;82(1):41–55. doi: 10.1016/0012-1606(81)90427-9. [DOI] [PubMed] [Google Scholar]
- Vasquez-Rifo A, Jannot G, Armisen J, Labouesse M, Bukhari SI, Rondeau EL, Miska EA, Simard MJ. Developmental characterization of the microRNA-specific C. elegans Argonautes alg-1 and alg-2. PLoS One. 2012 Mar 20;7(3):e33750–e33750. doi: 10.1371/journal.pone.0033750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Sternberg PW. Competence and commitment of Caenorhabditis elegans vulval precursor cells. Dev Biol. 1999 Aug 1;212(1):12–24. doi: 10.1006/dbio.1999.9357. [DOI] [PubMed] [Google Scholar]
- Yigit E, Batista PJ, Bei Y, Pang KM, Chen CC, Tolia NH, Joshua-Tor L, Mitani S, Simard MJ, Mello CC. Analysis of the C. elegans Argonaute family reveals that distinct Argonautes act sequentially during RNAi. Cell. 2006 Nov 17;127(4):747–757. doi: 10.1016/j.cell.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Yochem J, Weston K, Greenwald I. The Caenorhabditis elegans lin-12 gene encodes a transmembrane protein with overall similarity to Drosophila Notch. Nature. 1988 Oct 6;335(6190):547–550. doi: 10.1038/335547a0. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Greenwald I. LIN-12/Notch activation leads to microRNA-mediated down-regulation of Vav in C. elegans. Science. 2005 Oct 20;310(5752):1330–1333. doi: 10.1126/science.1119481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Hrach H, Mangone M, Reiner DJ. Identifying the Caenorhabditis elegans vulval transcriptome. G3 (Bethesda) 2022 May 30;12(6) doi: 10.1093/g3journal/jkac091. [DOI] [PMC free article] [PubMed] [Google Scholar]