Abstract
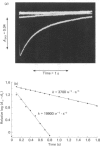
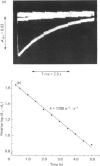
Chymopapain A was isolated from the dried latex of papaya (Carica papaya) by ion-exchange chromatography followed by covalent chromatography by thiol-disulphide interchange. The latter procedure was used to produce fully active enzyme containing one essential thiol group per molecule of protein, to establish that the chymopapain A molecule contains, in addition, one non-essential thiol group per molecule and to recalculate the literature value of epsilon 280 for the enzyme as 36 000 M-1 X cm -1. The Michaelis parameters for the hydrolysis of L-benzoylarginine p-nitroanilide and of benzyloxy-carbonyl-lysine nitrophenyl ester at 25 degrees C, and I 0.1 at several pH values catalysed by chymopapain A, papaya proteinase omega, papain (EC 3.4.22.2) and actinidin (EC 3.4.22.14) were determined. Towards these substrates chymopapain A has kcat./km values similar to those of actinidin and of papaya proteinase omega and significantly lower than those of papain or ficin. The environment of the catalytic site of chymopapain A is markedly different from those of other cysteine proteinases studied to date, as evidenced by the pH-dependence of the second-order rate constant (k) for the reaction of the catalytic-site thiol group with 2,2'-dipyridyl disulphide. The striking bell-shaped component that is a characteristic feature of the reactions of S-/ImH+ (thiolate/imidazolium) ion-pair components of many cysteine-proteinase catalytic sites with the 2,2'-dipyridyl disulphide univalent cation is not present in the pH-k profile for the chymopapain A reaction. The result is consistent with the presence of an additional positive charge in, or near, the catalytic site that repels the cationic form of the probe reagent. Resonance Raman spectra were collected at pH values 2.5, 6.0 and 8.0 for each of the following dithioacyl derivatives of chymopapain A: N-benzoylglycine-, N-(Beta-phenylpropionl)glycine- and N-methoxycarbonylphenylalanylglycine-. The main conclusion of the spectral study is that in each case the acyl group binds as a single population known as conformer B in which the glycinic N atom is in close contact with the thiol S atom of the catalytic-site cysteine residue, as is the case also for papain and other cysteine proteinases studied. Thus the abnormal catalytic-site environment of chymopapain A detected by the reactivity-probe studies, which may have consequences for the acylation step of the catalytic act, does not perturb the conformation of the bound acyl group at the acyl-enzyme-intermediate stage of catalysis.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines B. S., Brocklehurst K. A necessary modification to the preparation of papain from any high-quality latex of Carica papaya and evidence for the structural integrity of the enzyme produced by traditional methods. Biochem J. 1979 Feb 1;177(2):541–548. doi: 10.1042/bj1770541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines B. S., Brocklehurst K. Characterization of papaya peptidase A as a cysteine proteinase of Carica papaya L. with active-centre properties that differ from those of papain by using 2,2'-dipyridyl disulphide and 4-chloro-7-nitrobenzofurazan as reactivity probes. Use of two-protonic-state electrophiles in the identification of catalytic-site thiol groups. Biochem J. 1982 Jul 1;205(1):205–211. doi: 10.1042/bj2050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Buttle D. J. Names and numbers of papaya proteinases. Biochem J. 1985 Jun 1;228(2):527–527. doi: 10.1042/bj2280527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A., Schechter I. Mapping the active site of papain with the aid of peptide substrates and inhibitors. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):249–264. doi: 10.1098/rstb.1970.0024. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Baines B. S., Malthouse J. P. Differences in the interaction of the catalytic groups of the active centres of actinidin and papain. Rapid purification of fully active actinidin by covalent chromatography and characterization of its active centre by use of two-protonic-state reactivity probes. Biochem J. 1981 Sep 1;197(3):739–746. doi: 10.1042/bj1970739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Baines B. S., Mushiri M. S. Evidence that the active centre of chymopapain A is different from the active centres of some other cysteine proteinases and that the Brønsted coefficient (beta nuc.) for the reactions of thiolate anions with 2,2'-dipyridyl disulphide may be decreased by reagent protonation. Biochem J. 1980 Jul 1;189(1):189–129. doi: 10.1042/bj1890189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Baines B. S., Salih E., Hatzoulis C. 'Chymopapain S' is chymopapain A. Biochem J. 1984 Jul 15;221(2):553–554. doi: 10.1042/bj2210553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Carey P. R., Lee H. H., Salih E., Storer A. C. Comparative resonance Raman spectroscopic and kinetic studies of acyl-enzymes involving papain, actinidin and papaya peptidase II. Biochem J. 1984 Nov 1;223(3):649–657. doi: 10.1042/bj2230649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Carlsson J., Kierstan M. P., Crook E. M. Covalent chromatography by thiol-disulfide interchange. Methods Enzymol. 1974;34:531–544. doi: 10.1016/s0076-6879(74)34069-4. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Carlsson J., Kierstan M. P., Crook E. M. Covalent chromatography. Preparation of fully active papain from dried papaya latex. Biochem J. 1973 Jul;133(3):573–584. doi: 10.1042/bj1330573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactions of papain and of low-molecular-weight thiols with some aromatic disulphides. 2,2'-Dipyridyl disulphide as a convenient active-site titrant for papain even in the presence of other thiols. Biochem J. 1973 May;133(1):67–80. doi: 10.1042/bj1330067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Malthouse J. P. Evidence for a two-state transition in papain that may have no close analogue in ficin. Differences in the disposition of cationic sites and hydrophobic binding areas in the active centres of papain and ficin. Biochem J. 1980 Dec 1;191(3):707–718. doi: 10.1042/bj1910707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Malthouse J. P. Evidence that the lack of high catalytic activity of thiolsubtilisin towards specific substrates may be due to an inappropriately located proton-distribution system. Demonstration of highly nucleophilic character of the thiol group of thiolsubtilisin in the catalytically relevant ionization state of the active centre by use of a two-protonic-state reactivity probe. Biochem J. 1981 Mar 1;193(3):819–823. doi: 10.1042/bj1930819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Mushiri S. M., Patel G., Willenbrock F. A marked gradation in active-centre properties in the cysteine proteinases revealed by neutral and anionic reactivity probes. Reactivity characteristics of the thiol groups of actinidin, ficin, papain and papaya peptidase A towards 4,4'-dipyridyl disulphide and 5,5'-dithiobis-(2-nitrobenzoate) dianion. Biochem J. 1983 Mar 1;209(3):873–879. doi: 10.1042/bj2090873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Salih E. A re-evaluation of the nomenclature of the cysteine proteinases of Carica papaya and a rational basis for their identification. Biochem J. 1983 Aug 1;213(2):559–560. doi: 10.1042/bj2130559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Salih E., Lodwig T. S. Differences between the electric fields of the catalytic sites of papain and actinidin detected by using the thiol-located nitrobenzofurazan label as a spectroscopic reporter group. Biochem J. 1984 Jun 1;220(2):609–612. doi: 10.1042/bj2200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Salih E., McKee R., Smith H. Fresh non-fruit latex of Carica papaya contains papain, multiple forms of chymopapain A and papaya proteinase omega. Biochem J. 1985 Jun 1;228(2):525–527. doi: 10.1042/bj2280525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Stuchbury T., Malthouse J. P. Reactivities of neutral and cationic forms of 2,2'-dipyridyl disulphide towards thiolate anions. Detection of differences between the active centres of actinidin, papain and ficin by a three-protonic-state reactivity probe. Biochem J. 1979 Nov 1;183(2):233–238. doi: 10.1042/bj1830233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K. Two-protonic-state electrophiles as probes of enzyme mechanisms. Methods Enzymol. 1982;87:427–469. doi: 10.1016/s0076-6879(82)87026-2. [DOI] [PubMed] [Google Scholar]
- Buttle D. J., Barrett A. J. Chymopapain. Chromatographic purification and immunological characterization. Biochem J. 1984 Oct 1;223(1):81–88. doi: 10.1042/bj2230081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey P. R., Angus R. H., Lee H. H., Storer A. C. Identity of acyl group conformations in the active sites of papain and cathepsin B by resonance Raman spectroscopy. J Biol Chem. 1984 Dec 10;259(23):14357–14360. [PubMed] [Google Scholar]
- Carey P. R., Ozaki Y., Storer A. C. Comparison of the substrate conformations in the active sites of papain, chymopapain, ficin and bromelain by resonance Raman spectroscopy. Biochem Biophys Res Commun. 1983 Dec 28;117(3):725–731. doi: 10.1016/0006-291x(83)91657-1. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Ebata M., Takahashi Y. Proteolytic specificity of chymopapain: hydrolysis of the fraction B of oxidized insulin. Biochim Biophys Acta. 1966 Apr 12;118(1):201–203. doi: 10.1016/s0926-6593(66)80159-5. [DOI] [PubMed] [Google Scholar]
- Kunimitsu D. K., Yasunobu K. T. Chymopapain. IV. The chromatographic fractionation of partially purified chymopapain and the characterization of crystalline chymopapain B. Biochim Biophys Acta. 1967 Jul 11;139(2):405–417. doi: 10.1016/0005-2744(67)90044-7. [DOI] [PubMed] [Google Scholar]
- LOWE G., WILLIAMS A. DIRECT EVIDENCE FOR AN ACYLATED THIOL AS AN INTERMEDIATE IN PAPAIN- AND FICIN-CATALYSED HYDROLYSES. Biochem J. 1965 Jul;96:189–193. doi: 10.1042/bj0960189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G., Yuthavong Y. Kinetic specificity in papain-catalysed hydrolyses. Biochem J. 1971 Aug;124(1):107–115. doi: 10.1042/bj1240107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G., Yuthavong Y. pH-dependence and structure-activity relationships in the papain-catalysed hydrolysis of anilides. Biochem J. 1971 Aug;124(1):117–122. doi: 10.1042/bj1240117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malthouse J. P., Brocklehurst K. Preparation of fully active ficin from Ficus glabrata by covalent chromatography and characterization of its active centre by using 2,2'-depyridyl disulphide as a reactivity probe. Biochem J. 1976 Nov;159(2):221–234. doi: 10.1042/bj1590221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki Y., Pliura D. H., Carey P. R., Storer A. C. Vibrational spectra of scissile bonds in enzyme active sites: a resonance Raman study of dithioacylpapains. Biochemistry. 1982 Jun 22;21(13):3102–3108. doi: 10.1021/bi00256a011. [DOI] [PubMed] [Google Scholar]
- Polgár L. Problems of classification of papaya latex proteinases. Biochem J. 1984 Jul 15;221(2):555–556. doi: 10.1042/bj2210555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G. W. Isolation and characterization of papaya peptidase A from commercial chymopapain. Biochemistry. 1975 Aug 12;14(16):3695–3700. doi: 10.1021/bi00687a028. [DOI] [PubMed] [Google Scholar]
- Salih E., Brocklehurst K. Investigation of the catalytic site of actinidin by using benzofuroxan as a reactivity probe with selectivity for the thiolate-imidazolium ion-pair systems of cysteine proteinases. Evidence that the reaction of the ion-pair of actinidin (pKI 3.0, pKII 9.6) is modulated by the state of ionization of a group associated with a molecular pKa of 5.5. Biochem J. 1983 Sep 1;213(3):713–718. doi: 10.1042/bj2130713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schack P. Fractionation of proteolytic enzymes of dried papaya latex. Isolation and preliminary characterization of a new proteolytic enzyme. C R Trav Lab Carlsberg. 1967;36(4):67–83. [PubMed] [Google Scholar]
- Sluyterman L. A., Wijdenes J. An agarose mercurial column for the separation of mercaptopapain and nonmercaptopapain. Biochim Biophys Acta. 1970 Mar 31;200(3):593–595. doi: 10.1016/0005-2795(70)90122-4. [DOI] [PubMed] [Google Scholar]
- Storer A. C., Lee H., Carey P. R. Relaxed and perturbed substrate conformations in enzyme active sites: evidence from multichannel resonance raman spectra. Biochemistry. 1983 Sep 27;22(20):4789–4796. doi: 10.1021/bi00289a027. [DOI] [PubMed] [Google Scholar]
- Storer A. C., Murphy W. F., Carey P. R. The use of resonance Raman spectroscopy to monitor catalytically important bonds during enzymic catalysis. Application to the hydrolysis of methyl thionohippurate by papain. J Biol Chem. 1979 May 10;254(9):3163–3165. [PubMed] [Google Scholar]
- TSOU C. L. Relation between modification of functional groups of proteins and their biological activity. I.A graphical method for the determination of the number and type of essential groups. Sci Sin. 1962 Nov;11:1535–1558. [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. A general framework of cysteine-proteinase mechanism deduced from studies on enzymes with structurally different analogous catalytic-site residues Asp-158 and -161 (papain and actinidin), Gly-196 (cathepsin B) and Asn-165 (cathepsin H). Kinetic studies up to pH 8 of the hydrolysis of N-alpha-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide catalysed by cathepsin B and of L-arginine 2-naphthylamide catalysed by cathepsin H. Biochem J. 1985 Apr 15;227(2):521–528. doi: 10.1042/bj2270521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. Natural structural variation in enzymes as a tool in the study of mechanism exemplified by a comparison of the catalytic-site structure and characteristics of cathepsin B and papain. pH-dependent kinetics of the reactions of cathepsin B from bovine spleen and from rat liver with a thiol-specific two-protonic-state probe (2,2'-dipyridyl disulphide) and with a specific synthetic substrate (N-alpha-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide). Biochem J. 1984 Sep 15;222(3):805–814. doi: 10.1042/bj2220805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. Preparation of cathepsins B and H by covalent chromatography and characterization of their catalytic sites by reaction with a thiol-specific two-protonic-state reactivity probe. Kinetic study of cathepsins B and H extending into alkaline media and a rapid spectroscopic titration of cathepsin H at pH 3-4. Biochem J. 1985 Apr 15;227(2):511–519. doi: 10.1042/bj2270511. [DOI] [PMC free article] [PubMed] [Google Scholar]