Abstract
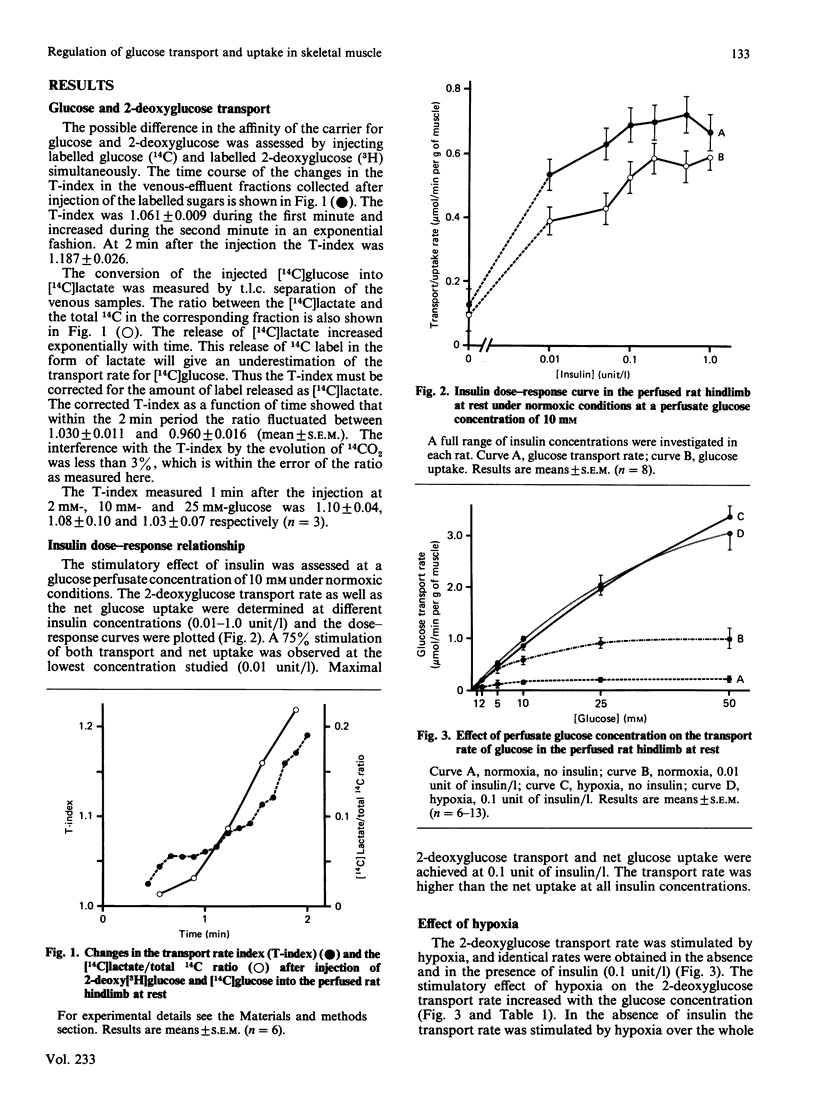
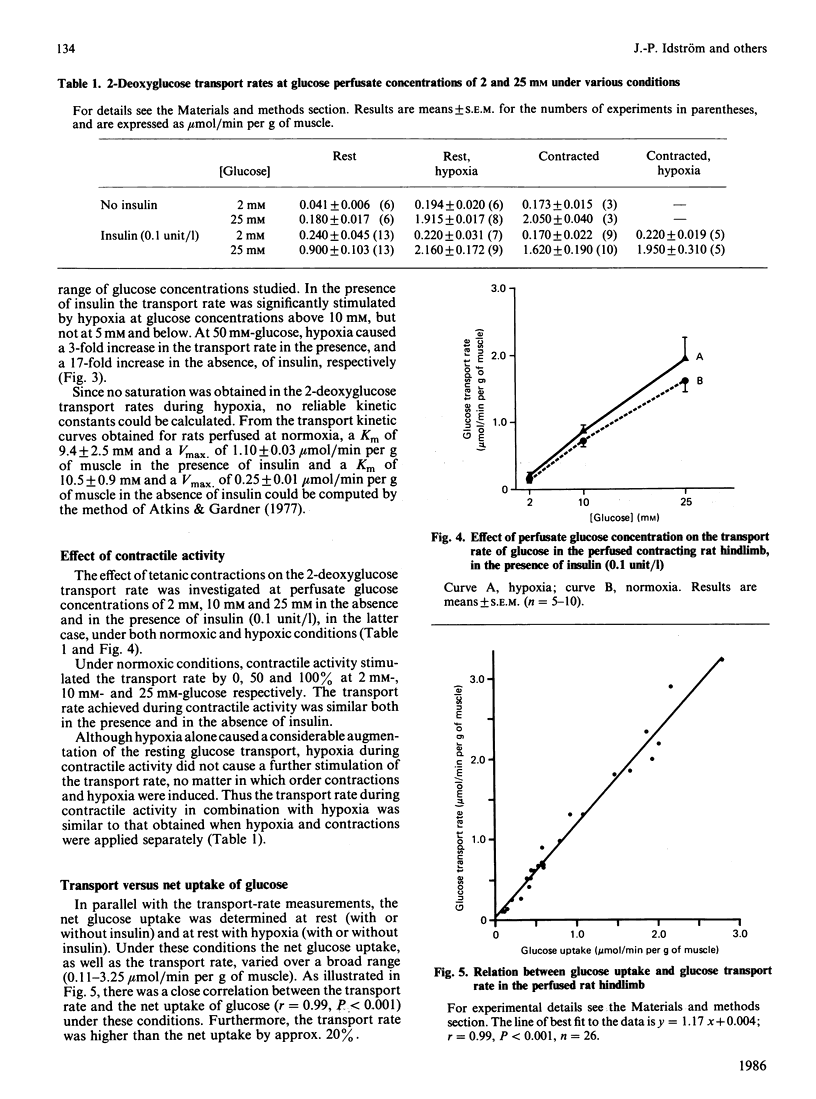
The paired-tracer dilution method applied to the perfused rat hindlimb model was used to study glucose transport in relation to net glucose uptake in skeletal muscle tissue. 2-deoxyglucose was used as an analogue for glucose, since this eliminates the problem with release of labelled metabolites. The affinity of 2-deoxyglucose for the glucose carrier was shown to be indistinguishable from that of glucose. An insulin dose-response study showed maximal stimulation of glucose uptake and transport at 0.1 unit/l, and 75% of maximal stimulation at 0.01 unit of insulin/l. Hypoxia and contractile activity stimulated the 2-deoxyglucose transport rate similarly, and the stimuli were not additive, suggesting a common mechanism. The presence of insulin did not increase the effect of hypoxia or contractile activity, indicating no permissive effect of insulin. The 2-deoxyglucose transport rate was closely correlated with and always higher than that of glucose uptake, demonstrating that the transport is never rate-limiting for the net glucose uptake and that both processes are regulated together. Significant correlations between the 2-deoxyglucose transport rate and the intramuscular concentration of phosphocreatine suggest regulation of the glucose utilization by the energy state of the skeletal muscle tissue.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins G. L., Gardner M. L. The computation of saturable and linear components of intestinal and other transport kinetics. Biochim Biophys Acta. 1977 Jul 4;468(1):127–145. doi: 10.1016/0005-2736(77)90156-0. [DOI] [PubMed] [Google Scholar]
- Berger M., Hagg S., Ruderman N. B. Glucose metabolism in perfused skeletal muscle. Interaction of insulin and exercise on glucose uptake. Biochem J. 1975 Jan;146(1):231–238. doi: 10.1042/bj1460231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudry I. H., Gould M. K. Kinetics of glucose uptake in isolated soleus muscle. Biochim Biophys Acta. 1969 May 6;177(3):527–536. doi: 10.1016/0304-4165(69)90315-8. [DOI] [PubMed] [Google Scholar]
- Cheung J. Y., Conover C., Regen D. M., Whitfield C. F., Morgan H. E. Effect of insulin on kinetics of sugar transport in heart muscle. Am J Physiol. 1978 Jan;234(1):E70–E78. doi: 10.1152/ajpendo.1978.234.1.E70. [DOI] [PubMed] [Google Scholar]
- Foley J. E., Huecksteadt T. P. Glucose 6-phosphate effects on deoxyglucose, glucose and methylglucose transport in rat adipocytes. Evidence for intracellular regulation of sugar transport by glucose metabolites. Biochim Biophys Acta. 1984 Nov 13;805(3):313–316. doi: 10.1016/0167-4889(84)90088-0. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., Ruderman N. B. Insulin sensitivity of rat skeletal muscle: effects of starvation and aging. Am J Physiol. 1979 May;236(5):E519–E523. doi: 10.1152/ajpendo.1979.236.5.E519. [DOI] [PubMed] [Google Scholar]
- Hultman E. Muscle glycogen in man determined in needle biopsy specimens: method and normal values. Scand J Clin Lab Invest. 1967;19(3):209–217. doi: 10.3109/00365516709090628. [DOI] [PubMed] [Google Scholar]
- Idström J. P., Subramanian V. H., Chance B., Schersten T., Bylund-Fellenius A. C. Oxygen dependence of energy metabolism in contracting and recovering rat skeletal muscle. Am J Physiol. 1985 Jan;248(1 Pt 2):H40–H48. doi: 10.1152/ajpheart.1985.248.1.H40. [DOI] [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. V. The penetration and phosphorylation of 2-deoxyglucose in the rat diaphragm. J Biol Chem. 1959 Jan;234(1):171–177. [PubMed] [Google Scholar]
- MORGAN H. E., HENDERSON M. J., REGEN D. M., PARK C. R. Regulation of glucose uptake in muscle. I. The effects of insulin and anoxia on glucose transport and phosphorylation in the isolated, perfused heart of normal rats. J Biol Chem. 1961 Feb;236:253–261. [PubMed] [Google Scholar]
- RANDLE P. J., SMITH G. H. Regulation of glucose uptake by muscle. 1. The effects of insulin, anaerobiosis and cell poisons on the uptake of glucose and release of potassium by isolated rat diaphragm. Biochem J. 1958 Nov;70(3):490–500. doi: 10.1042/bj0700490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie M. J., Holloszy J. O. Inhibition of glucose uptake and glycogenolysis by availability of oleate in well-oxygenated perfused skeletal muscle. Biochem J. 1977 Nov 15;168(2):161–170. doi: 10.1042/bj1680161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie M. J., Idström J. P., Mann G. E., Scherstén T., Bylund-Fellenius A. C. A paired-tracer dilution method for characterizing membrane transport in the perfused rat hindlimb. Effects of insulin, feeding and fasting on the kinetics of sugar transport. Biochem J. 1983 Sep 15;214(3):737–743. doi: 10.1042/bj2140737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter E. A., Garetto L. P., Goodman M. N., Ruderman N. B. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982 Apr;69(4):785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo A. J., Mahler R. J., Szabo O. Glucose uptake by the isolated perfused rat hind limb during rest and exercise. Horm Metab Res. 1969 Jul;1(4):156–161. doi: 10.1055/s-0028-1095147. [DOI] [PubMed] [Google Scholar]
- Walker P. M., Idström J. P., Scherstén T., Bylund-Fellenius A. C. Glucose uptake in relation to metabolic state in perfused rat hind limb at rest and during exercise. Eur J Appl Physiol Occup Physiol. 1982;48(2):163–176. doi: 10.1007/BF00422978. [DOI] [PubMed] [Google Scholar]
- Wallberg-Henriksson H., Holloszy J. O. Contractile activity increases glucose uptake by muscle in severely diabetic rats. J Appl Physiol Respir Environ Exerc Physiol. 1984 Oct;57(4):1045–1049. doi: 10.1152/jappl.1984.57.4.1045. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., Mann G. E. Unidirectional uptake of substrates at the blood side of secretory epithelia: stomach, salivary gland, pancreas. Fed Proc. 1982 Dec;41(14):3045–3053. [PubMed] [Google Scholar]


