Abstract
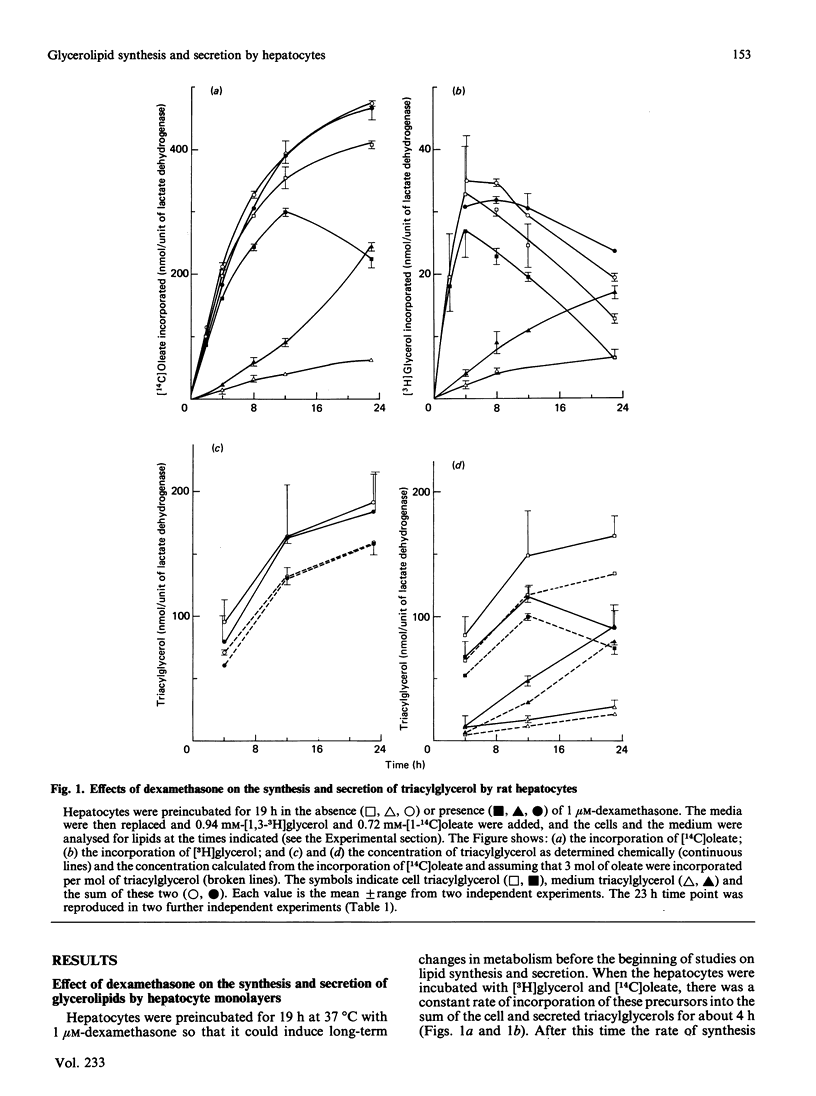
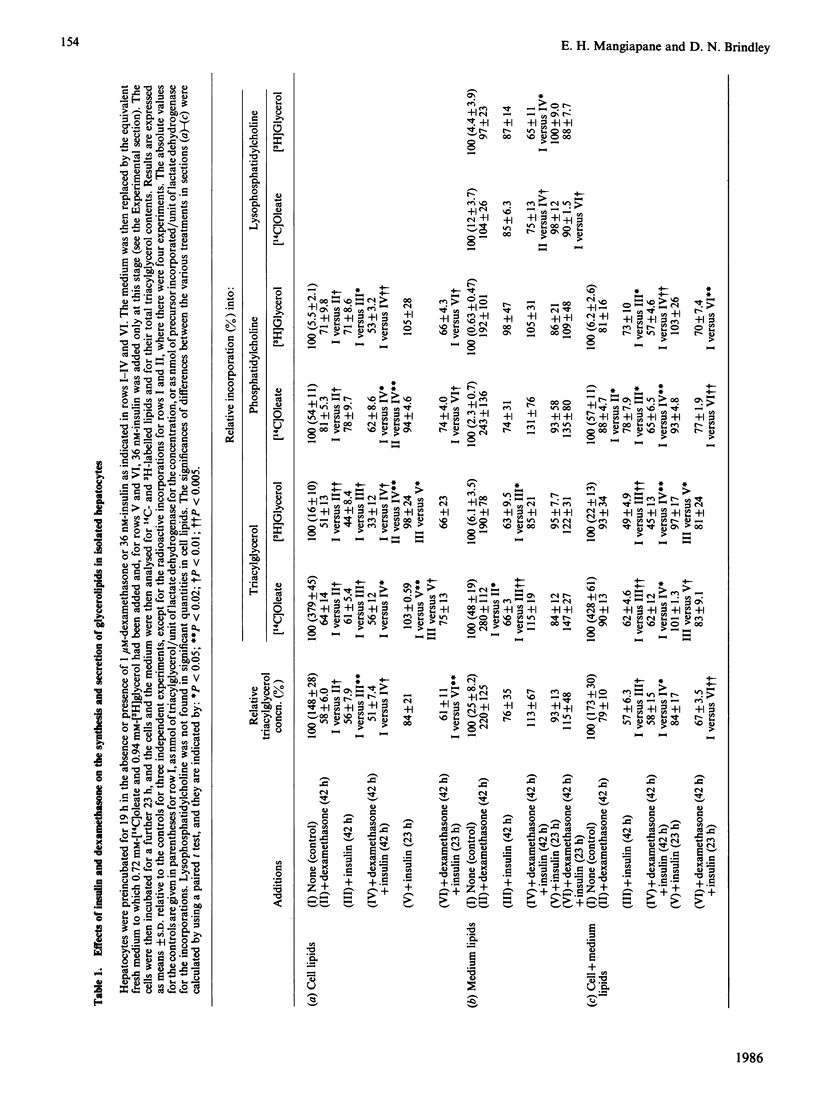
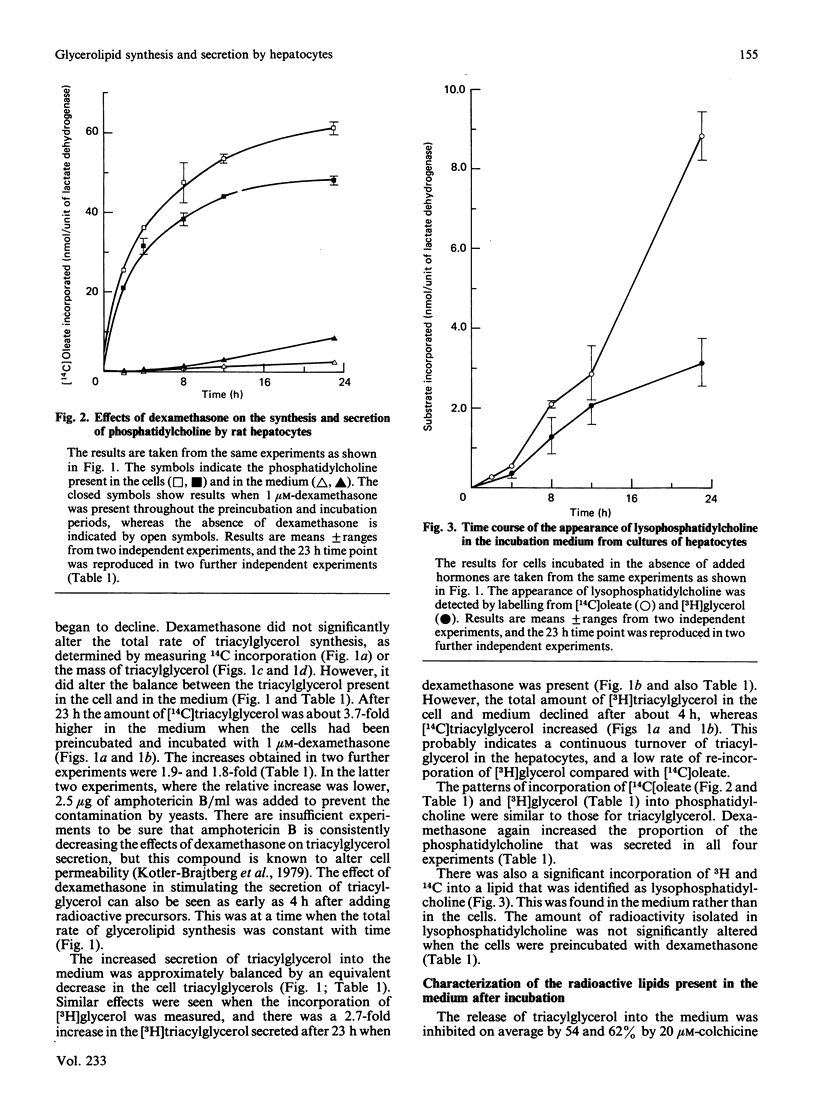
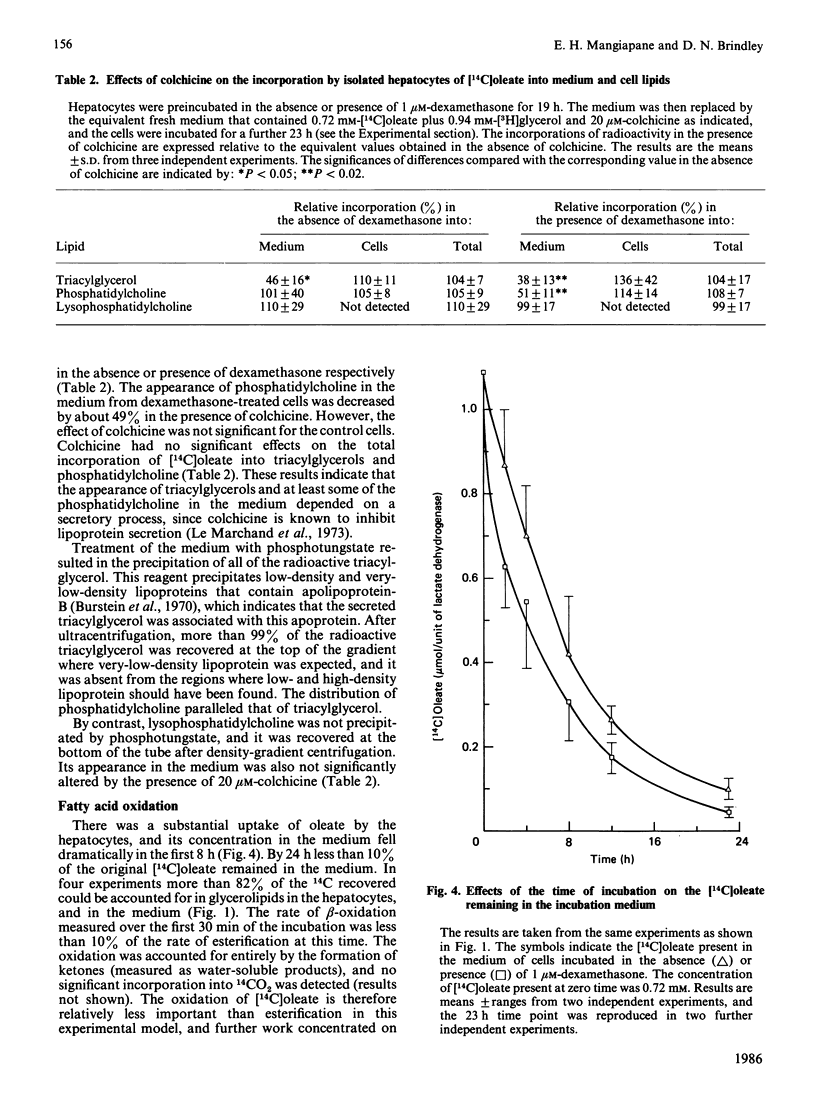
Rat hepatocytes in monolayer culture were preincubated for 19 h with 1 microM-dexamethasone, and the incubation was continued for a further 23 h with [14C]oleate, [3H]glycerol and 1 microM-dexamethasone. Dexamethasone increased the secretion of triacylglycerol into the medium in particles that had the properties of very-low-density lipoproteins. The increased secretion was matched by a decrease in the triacylglycerol and phosphatidylcholine that remained in the hepatocytes. Preincubating the hepatocytes for the total 42 h period with 36 nM-insulin decreased the amount of triacylglycerol in the medium and in the cells after the final incubation for 23 h with radioactive substrates. However, insulin had no significant effect on the triacylglycerol content of the cell and medium when it was present only in the final 23 h incubation. Insulin antagonized the effects of dexamethasone in stimulating the secretion of triacylglycerol from the hepatocytes, especially when it was present throughout the total 42 h period. The labelling of lysophosphatidylcholine in the medium when hepatocytes were incubated with [14C]oleate and [3H]glycerol was greater than that of phosphatidylcholine. The appearance of this lipid in the medium, unlike that of triacylglycerol and phosphatidylcholine, was not stimulated by dexamethasone, or inhibited by colchicine. However, the presence of lysophosphatidylcholine in the medium was decreased when the hepatocytes were incubated with both dexamethasone and insulin. These findings are discussed in relation to the control of the synthesis of glycerolipids and the secretion of very-low-density lipoproteins and lysophosphatidylcholine by the liver, particularly in relation to the interactions of glucocorticoids and insulin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amatruda J. M., Danahy S. A., Chang C. L. The effects of glucocorticoids on insulin-stimulated lipogenesis in primary cultures of rat hepatocytes. Biochem J. 1983 Apr 15;212(1):135–141. doi: 10.1042/bj2120135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur G., Sheltawy A. The presence of lysophosphatidylcholine in chromaffin granules. Biochem J. 1980 Nov 1;191(2):523–532. doi: 10.1042/bj1910523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Beynen A. C., Geelen M. J. Short-term regulation of hepatic triacylglycerol metabolism by insulin and glucagon. Vet Res Commun. 1982 May;5(3):223–236. doi: 10.1007/BF02214989. [DOI] [PubMed] [Google Scholar]
- Beynen A. C., Haagsman H. P., Van Golde L. M., Geelen M. J. The effects of insulin and glucagon on the release of triacylglycerols by isolated rat hepatocytes are mere reflections of the hormonal effects on the rate of triacylglycerol synthesis. Biochim Biophys Acta. 1981 Jul 24;665(1):1–7. doi: 10.1016/0005-2760(81)90224-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brindley D. N. Intracellular translocation of phosphatidate phosphohydrolase and its possible role in the control of glycerolipid synthesis. Prog Lipid Res. 1984;23(3):115–133. doi: 10.1016/0163-7827(84)90001-8. [DOI] [PubMed] [Google Scholar]
- Burstein M., Scholnick H. R., Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res. 1970 Nov;11(6):583–595. [PubMed] [Google Scholar]
- Butterwith S. C., Martin A., Brindley D. N. Can phosphorylation of phosphatidate phosphohydrolase by a cyclic AMP-dependent mechanism regulate its activity and subcellular distribution and control hepatic glycerolipid synthesis? Biochem J. 1984 Sep 1;222(2):487–493. doi: 10.1042/bj2220487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales C., Mangiapane E. H., Brindley D. N. Oleic acid promotes the activation and translocation of phosphatidate phosphohydrolase from the cytosol to particulate fractions of isolated rat hepatocytes. Biochem J. 1984 May 1;219(3):911–916. doi: 10.1042/bj2190911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole T. G., Wilcox H. G., Heimberg M. Effects of adrenalectomy and dexamethasone on hepatic lipid metabolism. J Lipid Res. 1982 Jan;23(1):81–91. [PubMed] [Google Scholar]
- Davis R. A., Boogaerts J. R. Intrahepatic assembly of very low density lipoproteins. Effect of fatty acids on triacylglycerol and apolipoprotein synthesis. J Biol Chem. 1982 Sep 25;257(18):10908–10913. [PubMed] [Google Scholar]
- Diamant S., Shafrir E. Modulation of the activity of insulin-dependent enzymes of lipogenesis by glucocorticoids. Eur J Biochem. 1975 May 6;53(2):541–546. doi: 10.1111/j.1432-1033.1975.tb04097.x. [DOI] [PubMed] [Google Scholar]
- Dich J., Bro B., Grunnet N., Jensen F., Kondrup J. Accumulation of triacylglycerol in cultured rat hepatocytes is increased by ethanol and by insulin and dexamethasone. Biochem J. 1983 Jun 15;212(3):617–623. doi: 10.1042/bj2120617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrington P. N., Newton R. S., Weinstein D. B., Steinberg D. Effects of insulin and glucose on very low density lipoprotein triglyceride secretion by cultured rat hepatocytes. J Clin Invest. 1982 Jul;70(1):63–73. doi: 10.1172/JCI110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko J. D., Matsuoka K. Y. Biosynthesis of phosphatidylcholine from serum phospholipids in Chinese hamster ovary cells deprived of choline. J Biol Chem. 1983 Mar 10;258(5):3051–3057. [PubMed] [Google Scholar]
- Fukuda N., Ontko J. A. Interactions between fatty acid synthesis, oxidation, and esterification in the production of triglyceride-rich lipoproteins by the liver. J Lipid Res. 1984 Aug;25(8):831–842. [PubMed] [Google Scholar]
- Glenny H. P., Brindley D. N. The effects of cortisol, corticotropin and thyroxine on the synthesis of glycerolipids and on the phosphatidate phosphohydrolase activity in rat liver. Biochem J. 1978 Dec 15;176(3):777–784. doi: 10.1042/bj1760777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL R. B., Jr, DROKE W. A. PRODUCTION OF FATTY LIVER IN THE RAT BY CORTISONE. Proc Soc Exp Biol Med. 1963 Dec;114:766–769. doi: 10.3181/00379727-114-28790. [DOI] [PubMed] [Google Scholar]
- Haagsman H. P., van Golde L. M. Regulation of hepatic triacylglycerol synthesis and secretion. Vet Res Commun. 1984 Aug;8(3):157–171. doi: 10.1007/BF02214708. [DOI] [PubMed] [Google Scholar]
- Hopewell R., Martin-Sanz P., Martin A., Saxton J., Brindley D. N. Regulation of the translocation of phosphatidate phosphohydrolase between the cytosol and the endoplasmic reticulum of rat liver. Effects of unsaturated fatty acids, spermine, nucleotides, albumin and chlorpromazine. Biochem J. 1985 Dec 1;232(2):485–491. doi: 10.1042/bj2320485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide T., Ontko J. A. Increased secretion of very low density lipoprotein triglyceride following inhibition of long chain fatty acid oxidation in isolated rat liver. J Biol Chem. 1981 Oct 25;256(20):10247–10255. [PubMed] [Google Scholar]
- Illingworth D. R., Portman O. W. The uptake and metabolism of plasma lysophosphatidylcholine in vivo by the brain of squirrel monkeys. Biochem J. 1972 Nov;130(2):557–567. doi: 10.1042/bj1300557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings R. J., Lawson N., Fears R., Brindley D. N. Stimulation of the activities of phosphatidate phosphohydrolase and tyrosine aminotransferase in rat hepatocytes by glucocorticoids. FEBS Lett. 1981 Oct 12;133(1):119–122. doi: 10.1016/0014-5793(81)80485-1. [DOI] [PubMed] [Google Scholar]
- Kabara J. J., Chen J. S. Microdetermination of lipid classes after thin-layer chromatography. Anal Chem. 1976 May;48(6):814–817. doi: 10.1021/ac60370a019. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Verrinder T. R., Hems D. A. Fatty acid synthesis in the perfused liver of adrenalectomized rats. Biochem J. 1976 Jun 15;156(3):593–602. doi: 10.1042/bj1560593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner H., Heimberg M. Effect of adrenalcortical hormones on release of triglycerides and glucose by liver. Am J Physiol. 1967 Jun;212(6):1236–1246. doi: 10.1152/ajplegacy.1967.212.6.1236. [DOI] [PubMed] [Google Scholar]
- Kotler-Brajtburg J., Medoff G., Kobayashi G. S., Boggs S., Schlessinger D., Pandey R. C., Rinehart K. L., Jr Classification of polyene antibiotics according to chemical structure and biological effects. Antimicrob Agents Chemother. 1979 May;15(5):716–722. doi: 10.1128/aac.15.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausz Y., Bar-On H., Shafrir E. Origin and pattern of glucocorticoid-induced hyperlipidemia in rats. Dose-dependent bimodal changes in serum lipids and lipoproteins in relation to hepatic lipogenesis and tissue lipoprotein lipase activity. Biochim Biophys Acta. 1981 Jan 26;663(1):69–82. doi: 10.1016/0005-2760(81)90195-8. [DOI] [PubMed] [Google Scholar]
- Laker M. E., Mayes P. A. Investigations into the direct effects of insulin on hepatic ketogenesis, lipoprotein secretion and pyruvate dehydrogenase activity. Biochim Biophys Acta. 1984 Sep 12;795(2):427–430. doi: 10.1016/0005-2760(84)90094-8. [DOI] [PubMed] [Google Scholar]
- Lawson N., Jennings R. J., Fears R., Brindley D. N. Antagonistic effects of insulin on the corticosterone-induced increase of phosphatidate phosphohydrolase activity in isolated rat hepatocytes. FEBS Lett. 1982 Jun 21;143(1):9–12. doi: 10.1016/0014-5793(82)80261-5. [DOI] [PubMed] [Google Scholar]
- Lawson N., Pollard A. D., Jennings R. J., Brindley D. N. Effects of corticosterone and insulin on enzymes of triacylglycerol synthesis in isolated rat hepatocytes. FEBS Lett. 1982 Sep 6;146(1):204–208. doi: 10.1016/0014-5793(82)80736-9. [DOI] [PubMed] [Google Scholar]
- Le Marchand Y., Singh A., Assimacopoulos-Jeannet F., Orci L., Rouiller C., Jeanrenaud B. A role for the microtubular system in the release of very low density lipoproteins by perfused mouse livers. J Biol Chem. 1973 Oct 10;248(19):6862–6870. [PubMed] [Google Scholar]
- Martin-Sanz P., Hopewell R., Brindley D. N. Long-chain fatty acids and their acyl-CoA esters cause the translocation of phosphatidate phosphohydrolase from the cytosolic to the microsomal fraction of rat liver. FEBS Lett. 1984 Oct 1;175(2):284–288. doi: 10.1016/0014-5793(84)80752-8. [DOI] [PubMed] [Google Scholar]
- Martin-Sanz P., Hopewell R., Brindley D. N. Spermine promotes the translocation of phosphatidate phosphohydrolase from the cytosol to the microsomal fraction of rat liver and it enhances the effects of oleate in this respect. FEBS Lett. 1985 Jan 7;179(2):262–266. doi: 10.1016/0014-5793(85)80531-7. [DOI] [PubMed] [Google Scholar]
- Murthy V. K., Shipp J. C. Synthesis and accumulation of triglycerides in liver of diabetic rats. Effects of insulin treatment. Diabetes. 1979 May;28(5):472–478. doi: 10.2337/diab.28.5.472. [DOI] [PubMed] [Google Scholar]
- Nelson G. J. The phospholipid composition of plasma in various mammalian species. Lipids. 1967 Jul;2(4):323–328. doi: 10.1007/BF02532119. [DOI] [PubMed] [Google Scholar]
- Nikkilä E. A., Kekki M. Plasma triglyceride transport kinetics in diabetes mellitus. Metabolism. 1973 Jan;22(1):1–22. doi: 10.1016/0026-0495(73)90024-3. [DOI] [PubMed] [Google Scholar]
- Ozegović B., Rodè B., Milkivić S. The role of the adrenal gland in the lipid accumulation process in the liver of rats bearing an acth and prolactin producing tumor. Endokrinologie. 1975 Nov;66(2):128–134. [PubMed] [Google Scholar]
- Patsch W., Franz S., Schonfeld G. Role of insulin in lipoprotein secretion by cultured rat hepatocytes. J Clin Invest. 1983 May;71(5):1161–1174. doi: 10.1172/JCI110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch W., Tamai T., Schonfeld G. Effect of fatty acids on lipid and apoprotein secretion and association in hepatocyte cultures. J Clin Invest. 1983 Jul;72(1):371–378. doi: 10.1172/JCI110977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittner R. A., Fears R., Brindley D. N. Effects of cyclic AMP, glucocorticoids and insulin on the activities of phosphatidate phosphohydrolase, tyrosine aminotransferase and glycerol kinase in isolated rat hepatocytes in relation to the control of triacylglycerol synthesis and gluconeogenesis. Biochem J. 1985 Jan 15;225(2):455–462. doi: 10.1042/bj2250455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittner R. A., Fears R., Brindley D. N. Interactions of insulin, glucagon and dexamethasone in controlling the activity of glycerol phosphate acyltransferase and the activity and subcellular distribution of phosphatidate phosphohydrolase in cultured rat hepatocytes. Biochem J. 1985 Sep 1;230(2):525–534. doi: 10.1042/bj2300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard A. D., Brindley D. N. Effects of vasopressin and corticosterone on fatty acid metabolism and on the activities of glycerol phosphate acyltransferase and phosphatidate phosphohydrolase in rat hepatocytes. Biochem J. 1984 Jan 15;217(2):461–469. doi: 10.1042/bj2170461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullinger C. R., Gibbons G. F. Effects of hormones and pyruvate on the rates of secretion of very-low-density lipoprotein triacylglycerol and cholesterol by rat hepatocytes. Biochim Biophys Acta. 1985 Jan 9;833(1):44–51. doi: 10.1016/0005-2760(85)90251-6. [DOI] [PubMed] [Google Scholar]
- Reaven E. P., Kolterman O. G., Reaven G. M. Ultrastructural and physiological evidence for corticosteroid-induced alterations in hepatic production of very low density lipoprotein particles. J Lipid Res. 1974 Jan;15(1):74–83. [PubMed] [Google Scholar]
- Reaven G. M., Mondon C. E. Effect of in vivo plasma insulin levels on the relationship between perfusate free fatty acid concentration and triglyceride secretion by perfused rat livers. Horm Metab Res. 1984 May;16(5):230–232. doi: 10.1055/s-2007-1014753. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Greenbaum A. L. The effect of dietary and hormonal conditions on the activities of glycolytic enzymes in rat epididymal adipose tissue. Biochem J. 1969 Nov;115(3):405–417. doi: 10.1042/bj1150405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon D. M., Hems D. A. Plasma lipoproteins and the synthesis and turnover of plasma triglyceride in normal and genetically obese mice. Biochem J. 1973 Nov;136(3):551–563. doi: 10.1042/bj1360551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savard J. D., Choy P. C. Phosphatidylcholine formation from exogenous lysophosphatidylcholine in isolated hamster heart. Biochim Biophys Acta. 1982 Apr 15;711(1):40–48. doi: 10.1016/0005-2760(82)90007-8. [DOI] [PubMed] [Google Scholar]
- Sekas G., Patton G. M., Lincoln E. C., Robins S. J. Origin of plasma lysophosphatidylcholine: evidence for direct hepatic secretion in the rat. J Lab Clin Med. 1985 Feb;105(2):190–194. [PubMed] [Google Scholar]
- Stein Y., Stein O. Metabolism of labeled lysolecithin, lysophosphatidyl ethanolamine and lecithin in the rat. Biochim Biophys Acta. 1966 Feb 1;116(1):95–107. doi: 10.1016/0005-2760(66)90095-6. [DOI] [PubMed] [Google Scholar]
- Steiner G., Haynes F. J., Yoshino G., Vranic M. Hyperinsulinemia and in vivo very-low-density lipoprotein-triglyceride kinetics. Am J Physiol. 1984 Feb;246(2 Pt 1):E187–E192. doi: 10.1152/ajpendo.1984.246.2.E187. [DOI] [PubMed] [Google Scholar]
- Taskinen M. R., Nikkilä E. A., Pelkonen R., Sane T. Plasma lipoproteins, lipolytic enzymes, and very low density lipoprotein triglyceride turnover in Cushing's syndrome. J Clin Endocrinol Metab. 1983 Sep;57(3):619–626. doi: 10.1210/jcem-57-3-619. [DOI] [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. The immediate effects of insulin and fructose on the metabolism of the perfused liver. Changes in lipoprotein secretion, fatty acid oxidation and esterification, lipogenesis and carbohydrate metabolism. Biochem J. 1972 Jan;126(2):295–311. doi: 10.1042/bj1260295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D. E., Weinstein D. B., Steinberg D. Isolation and analysis of lipoproteins secreted by rat liver hepatocytes. Biochim Biophys Acta. 1984 Jan 17;792(1):39–47. doi: 10.1016/0005-2760(84)90280-7. [DOI] [PubMed] [Google Scholar]
- Whitton P. D., Hems D. A. Glycogen synthesis in the perfused liver of adrenalectomized rats. Biochem J. 1976 Jun 15;156(3):585–592. doi: 10.1042/bj1560585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. A., Knauer T. E., Lamb R. G. The acute effects of streptozotocin-induced diabetes on rat liver glycerolipid biosynthesis. Biochim Biophys Acta. 1981 Dec 23;666(3):482–492. doi: 10.1016/0005-2760(81)90310-6. [DOI] [PubMed] [Google Scholar]
- Woodside W. F., Heimberg M. Effects of anti-insulin serum, insulin, and glucose on output of triglycerides and on ketogenesis by the perfused rat liver. J Biol Chem. 1976 Jan 10;251(1):13–23. [PubMed] [Google Scholar]


