Abstract
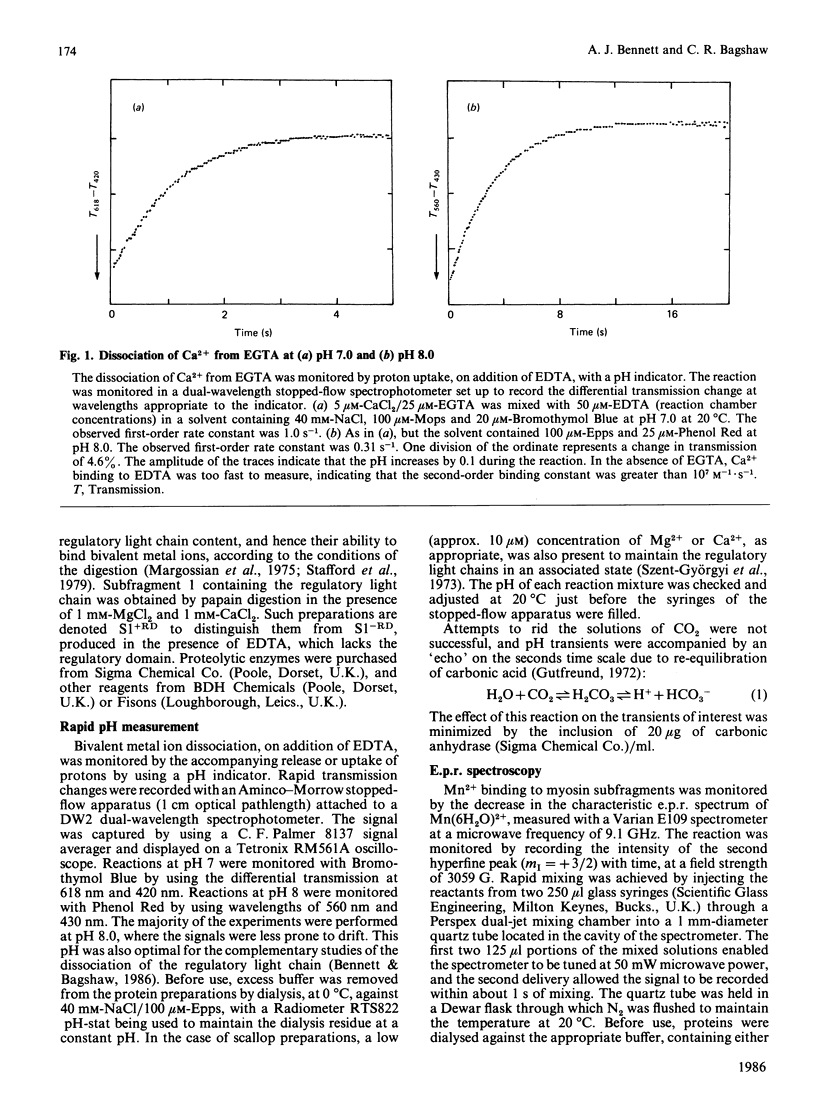
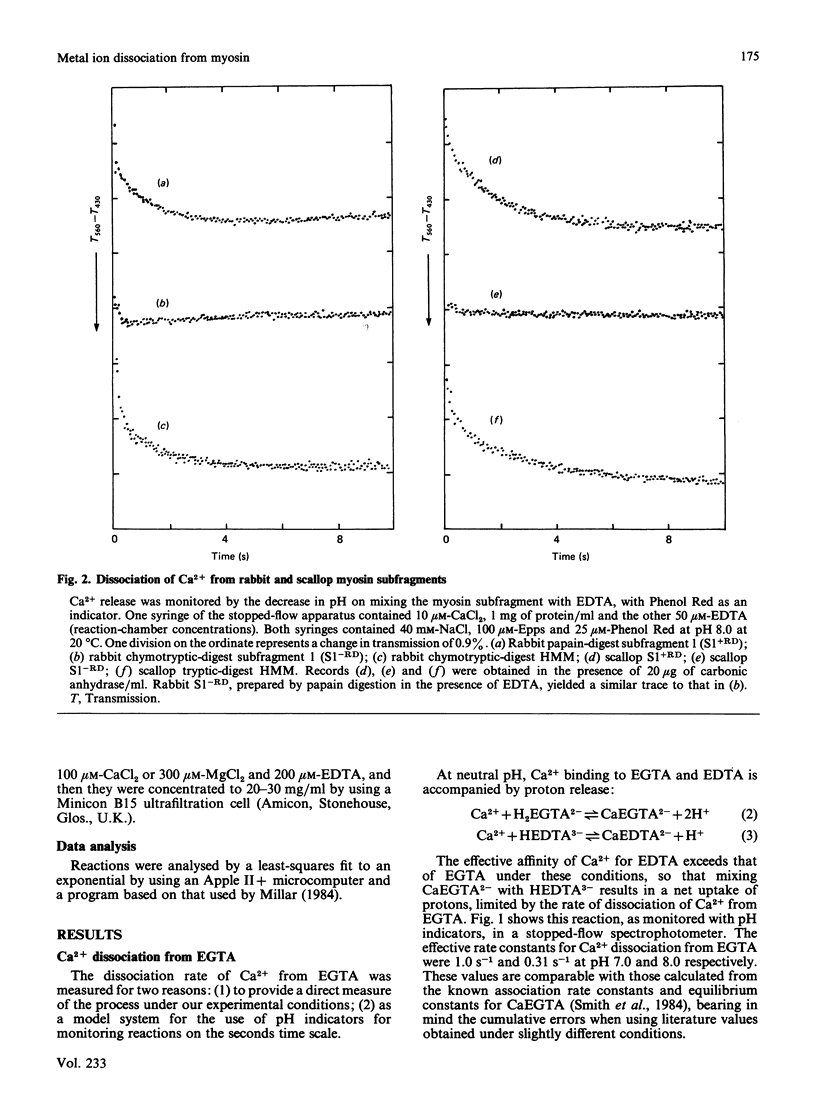
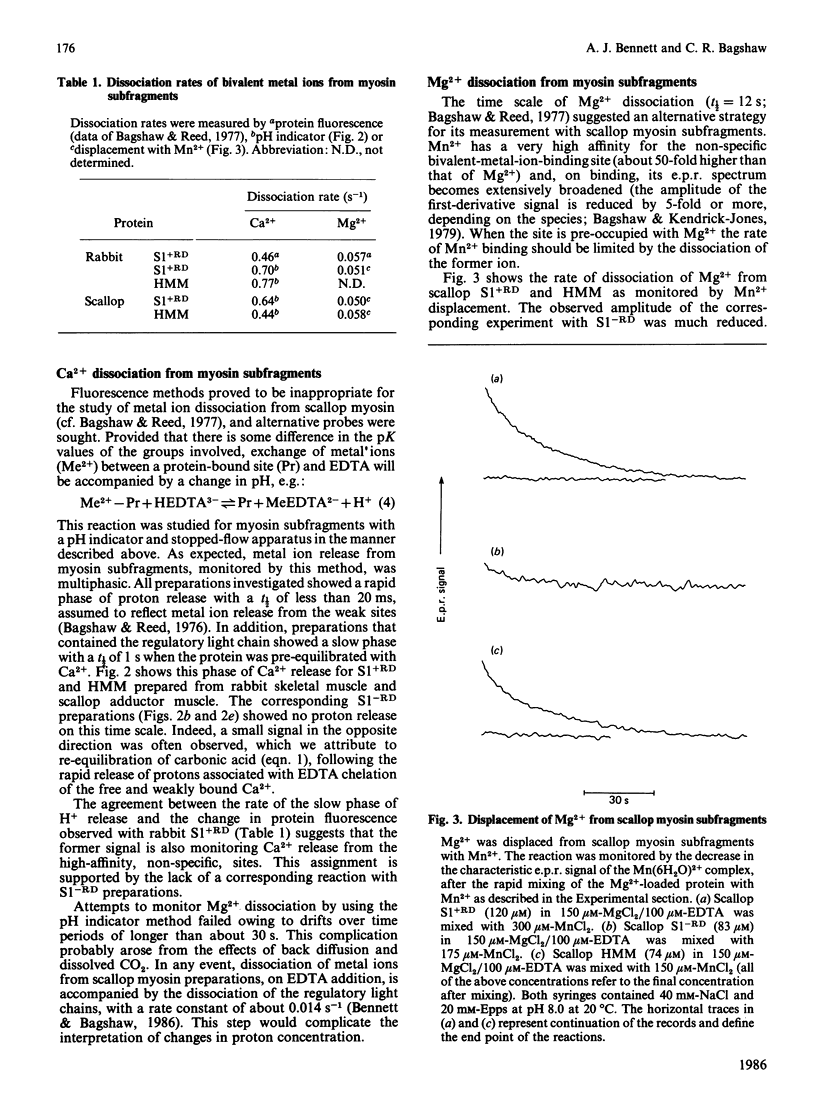
Bivalent metal ions have multiple roles in subunit association and ATPase regulation in scallop adductor-muscle myosin. To help elucidate these functions, the rates of Ca2+ and Mg2+ dissociation from the non-specific high-affinity sites on the regulatory light chains were measured and compared with those of rabbit skeletal-muscle myosin subfragments. Ca2+ dissociation had a rate constant of about 0.7 s-1 in both species, as measured by the time course of the pH change on EDTA addition. Mg2+ dissociation had a rate constant of 0.05 s-1, as monitored by its displacement with the paramagnetic Mn2+ ion. It is concluded that the exchange between Ca2+ and Mg2+ at the non-specific site, on excitation of both skeletal and adductor muscles, is too slow to contribute to the activation itself. The release of bivalent metal ions from the non-specific site is, however, the first step in release of the scallop regulatory light chain (Bennett & Bagshaw (1986) Biochem. J. 233, 179-186). In scallop myosin additional specific sites are present, which can bind Ca2+ rapidly, to effect activation of the ATPase. In the course of this work, Ca2+ dissociation from EGTA was studied as a model system. This gave rates of 1 s-1 and 0.3 s-1 at pH 7.0 and pH 8.0 respectively.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagshaw C. R. Divalent metal ion binding and subunit interactions in myosins: a critical review. J Muscle Res Cell Motil. 1980 Sep;1(3):255–277. doi: 10.1007/BF00711931. [DOI] [PubMed] [Google Scholar]
- Bagshaw C. R., Kendrick-Jones J. Characterization of homologous divalent metal ion binding sites of vertebrate and molluscan myosins using electron paramagnetic resonance spectroscopy. J Mol Biol. 1979 May 25;130(3):317–336. doi: 10.1016/0022-2836(79)90544-8. [DOI] [PubMed] [Google Scholar]
- Bagshaw C. R., Kendrick-Jones J. Identification of the divalent metal ion binding domain of myosin regulatory light chains using spin-labelling techniques. J Mol Biol. 1980 Jul 5;140(3):411–433. doi: 10.1016/0022-2836(80)90392-7. [DOI] [PubMed] [Google Scholar]
- Bagshaw C. R. On the location of the divalent metal binding sites and the light chain subunits of vertebrate myosin. Biochemistry. 1977 Jan 11;16(1):59–67. doi: 10.1021/bi00620a010. [DOI] [PubMed] [Google Scholar]
- Bagshaw C. R., Reed G. H. The significance of the slow dissociation of divalent metal ions from myosin 'regulatory' light chains. FEBS Lett. 1977 Sep 15;81(2):386–390. doi: 10.1016/0014-5793(77)80560-7. [DOI] [PubMed] [Google Scholar]
- Bagshow C. R., Reed G. H. Investigations of equilibrium complexes of myoxin subfragment 1 with the manganous ion and adenosine diphosphate using magnetic resonance techniques. J Biol Chem. 1976 Apr 10;251(7):1975–1983. [PubMed] [Google Scholar]
- Bennett A. J., Bagshaw C. R. The mechanism of regulatory light chain dissociation from scallop myosin. Biochem J. 1986 Jan 1;233(1):179–186. doi: 10.1042/bj2330179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler P. D., Szent-Györgyi A. G. Regulatory light-chains and scallop myosin. Full dissociation, reversibility and co-operative effects. J Mol Biol. 1980 Apr 15;138(3):473–492. doi: 10.1016/s0022-2836(80)80013-1. [DOI] [PubMed] [Google Scholar]
- Konno K., Arai K., Watanabe S. Fluorescence intensity and UV absorption changes accompanying dissociation and association of regulatory light chain of scallop adductor myosin. J Biochem. 1983 Oct;94(4):1061–1066. doi: 10.1093/oxfordjournals.jbchem.a134448. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S., Barshop B. Effect of DTNB light chain on the interaction of vertebrate skeletal myosin with actin. Nature. 1975 Nov 13;258(5531):163–166. doi: 10.1038/258163a0. [DOI] [PubMed] [Google Scholar]
- Persechini A., Rowe A. J. Modulation of myosin filament conformation by physiological levels of divalent cation. J Mol Biol. 1984 Jan 5;172(1):23–39. doi: 10.1016/0022-2836(84)90412-1. [DOI] [PubMed] [Google Scholar]
- Smith P. D., Liesegang G. W., Berger R. L., Czerlinski G., Podolsky R. J. A stopped-flow investigation of calcium ion binding by ethylene glycol bis(beta-aminoethyl ether)-N,N'-tetraacetic acid. Anal Biochem. 1984 Nov 15;143(1):188–195. doi: 10.1016/0003-2697(84)90575-x. [DOI] [PubMed] [Google Scholar]
- Stafford W. F., 3rd, Szentkiralyi E. M., Szent-Györgyi A. G. Regulatory properties of single-headed fragments of scallop myosin. Biochemistry. 1979 Nov 27;18(24):5273–5280. doi: 10.1021/bi00591a002. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A. G., Szentkiralyi E. M., Kendrick-Jonas J. The light chains of scallop myosin as regulatory subunits. J Mol Biol. 1973 Feb 25;74(2):179–203. doi: 10.1016/0022-2836(73)90106-x. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- Wells C., Bagshaw C. R. Calcium regulation of molluscan myosin ATPase in the absence of actin. Nature. 1985 Feb 21;313(6004):696–697. doi: 10.1038/313696a0. [DOI] [PubMed] [Google Scholar]
- Wells C., Bagshaw C. R. Segmental flexibility and head-head interaction in scallop myosin. A study using saturation transfer electron paramagnetic resonance spectroscopy. J Mol Biol. 1983 Feb 15;164(1):137–157. doi: 10.1016/0022-2836(83)90090-6. [DOI] [PubMed] [Google Scholar]
- Wells C., Warriner K. E., Bagshaw C. R. Fluorescence studies on the nucleotide- and Ca2+-binding domains of molluscan myosin. Biochem J. 1985 Oct 1;231(1):31–38. doi: 10.1042/bj2310031. [DOI] [PMC free article] [PubMed] [Google Scholar]


