Abstract
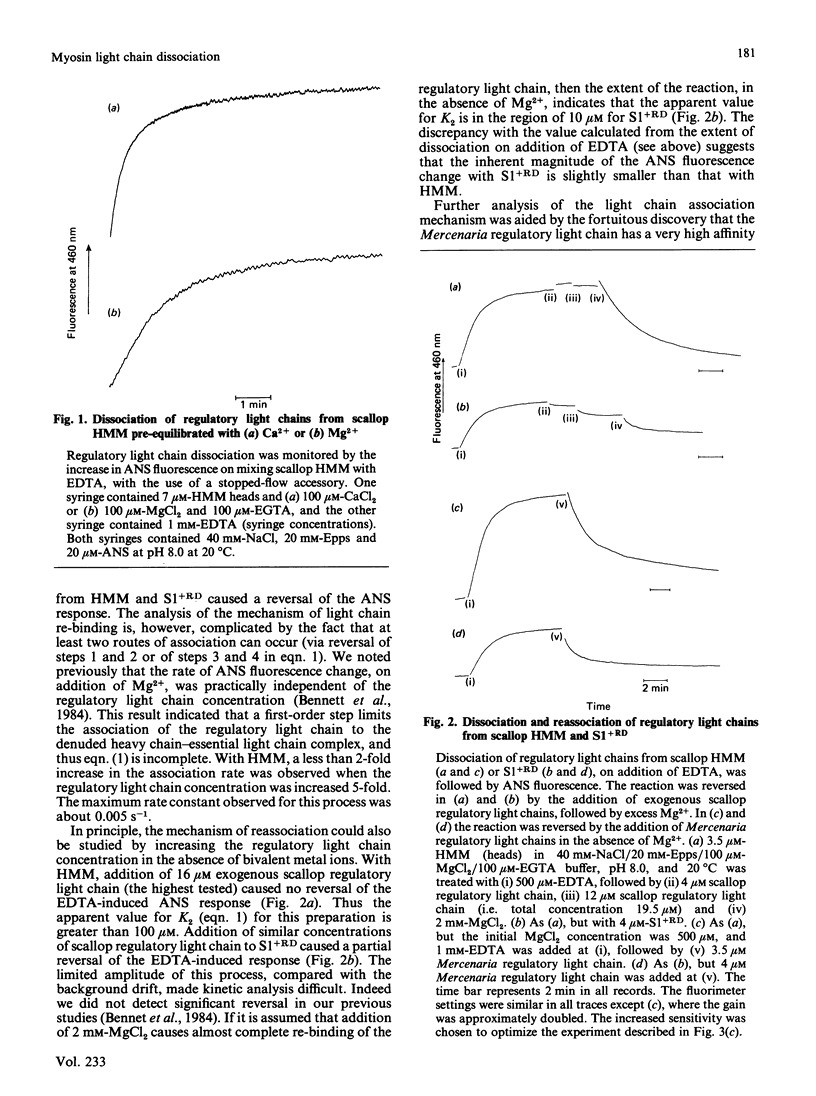
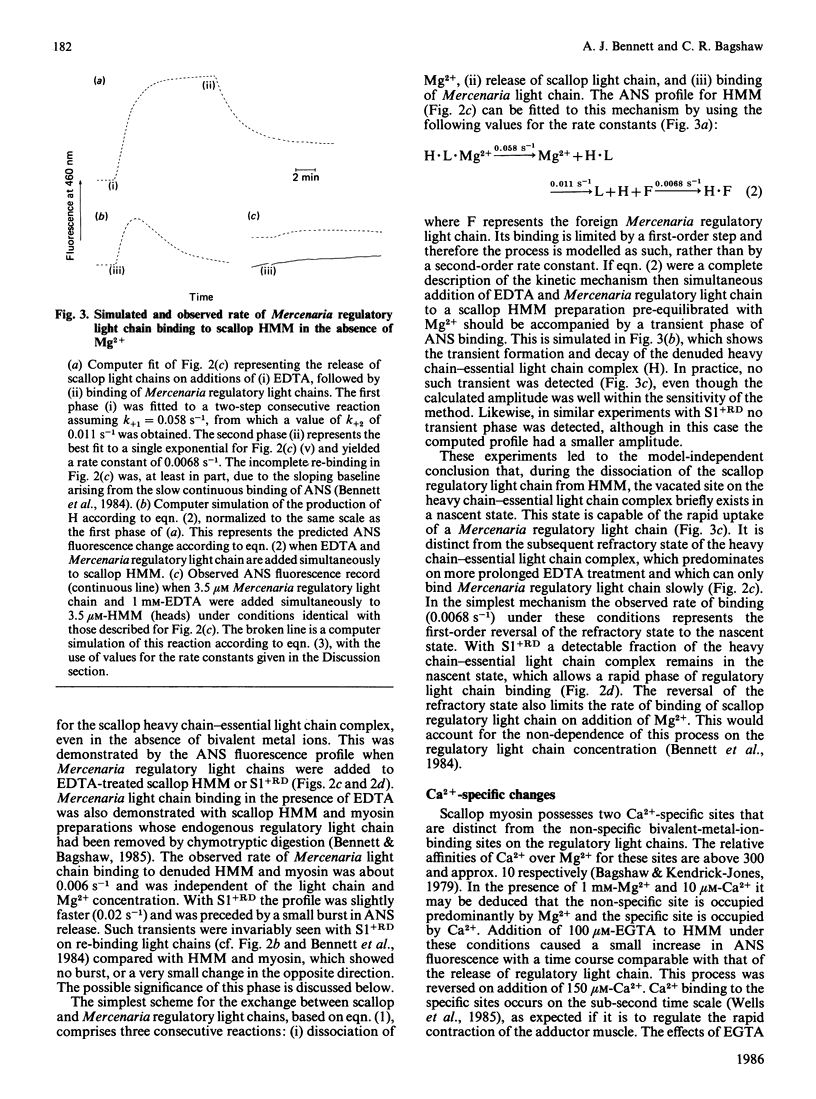
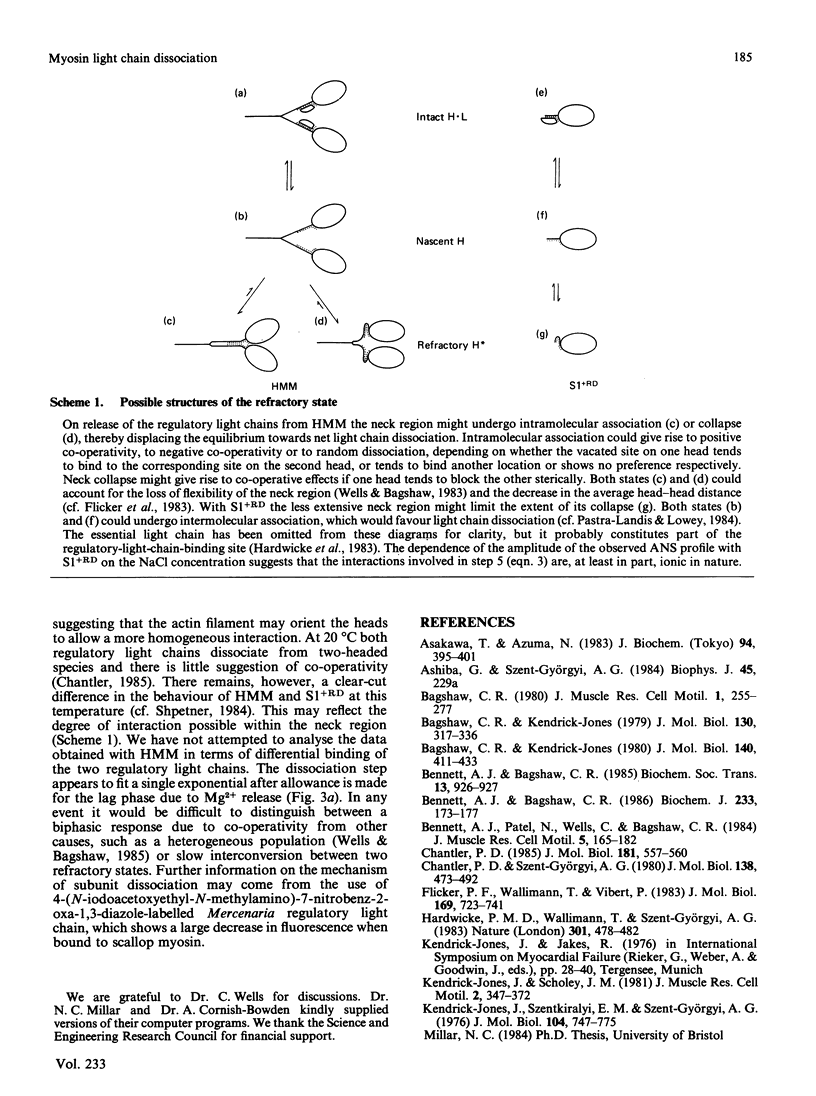
The dissociation of the regulatory light chains from scallop myosin subfragments, on addition of EDTA, was investigated by using the fluorophore 8-anilinonaphthalene-1-sulphonate as a probe. The rate of this process (0.014 s-1) was partially limited by the rate of Mg2+ dissociation (0.058 s-1) from the non-specific high-affinity site. The dissociation of the regulatory light chain subfragment 1 was less extensive than from heavy meromyosin. Reassociation of the scallop regulatory light chain was induced on addition of Mg2+, but it appeared to be limited by a first-order step. The nature of this step was revealed by the kinetics of Mercenaria regulatory light chain association. Scallop heavy meromyosin, denuded of its regulatory light chains, exists in a refractory state, whose reversal to the nascent state limits the rate of light chain association (0.006 s-1). The formation of the refractory state is the driving force for the net dissociation of regulatory light chains from scallop heavy meromyosin. This mechanism is discussed with reference to existing structural information on light-chain-denuded myosin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakawa T., Azuma N. Inhibitory effect of MgATP on the release of regulatory light chain from scallop myosin and light chain composition of scallop myosin hybridized with abalone light chain 2 at 30 degrees C. J Biochem. 1983 Aug;94(2):395–401. doi: 10.1093/oxfordjournals.jbchem.a134368. [DOI] [PubMed] [Google Scholar]
- Bagshaw C. R. Divalent metal ion binding and subunit interactions in myosins: a critical review. J Muscle Res Cell Motil. 1980 Sep;1(3):255–277. doi: 10.1007/BF00711931. [DOI] [PubMed] [Google Scholar]
- Bagshaw C. R., Kendrick-Jones J. Characterization of homologous divalent metal ion binding sites of vertebrate and molluscan myosins using electron paramagnetic resonance spectroscopy. J Mol Biol. 1979 May 25;130(3):317–336. doi: 10.1016/0022-2836(79)90544-8. [DOI] [PubMed] [Google Scholar]
- Bagshaw C. R., Kendrick-Jones J. Identification of the divalent metal ion binding domain of myosin regulatory light chains using spin-labelling techniques. J Mol Biol. 1980 Jul 5;140(3):411–433. doi: 10.1016/0022-2836(80)90392-7. [DOI] [PubMed] [Google Scholar]
- Bennett A. J., Bagshaw C. R. The kinetics of bivalent metal ion dissociation from myosin subfragments. Biochem J. 1986 Jan 1;233(1):173–177. doi: 10.1042/bj2330173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. J., Patel N., Wells C., Bagshaw C. R. 8-Anilino-1-naphthalenesulphonate, a fluorescent probe for the regulatory light chain binding site of scallop myosin. J Muscle Res Cell Motil. 1984 Apr;5(2):165–182. doi: 10.1007/BF00712154. [DOI] [PubMed] [Google Scholar]
- Chantler P. D. Regulatory light chains and scallop myosin. Form of light chain removal or reuptake is dependent on the presence of divalent cations. J Mol Biol. 1985 Feb 20;181(4):557–560. doi: 10.1016/0022-2836(85)90428-0. [DOI] [PubMed] [Google Scholar]
- Chantler P. D., Szent-Györgyi A. G. Regulatory light-chains and scallop myosin. Full dissociation, reversibility and co-operative effects. J Mol Biol. 1980 Apr 15;138(3):473–492. doi: 10.1016/s0022-2836(80)80013-1. [DOI] [PubMed] [Google Scholar]
- Flicker P. F., Wallimann T., Vibert P. Electron microscopy of scallop myosin. Location of regulatory light chains. J Mol Biol. 1983 Sep 25;169(3):723–741. doi: 10.1016/s0022-2836(83)80167-3. [DOI] [PubMed] [Google Scholar]
- Hardwicke P. M., Wallimann T., Szent-Györgyi A. G. Light-chain movement and regulation in scallop myosin. Nature. 1983 Feb 10;301(5900):478–482. doi: 10.1038/301478a0. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Szentkiralyi E. M., Szent-Györgyi A. G. Regulatory light chains in myosins. J Mol Biol. 1976 Jul 15;104(4):747–775. doi: 10.1016/0022-2836(76)90180-7. [DOI] [PubMed] [Google Scholar]
- Sellers J. R., Chantler P. D., Szent-Györgyi A. G. Hybrid formation between scallop myofibrils and foreign regulatory light-chains. J Mol Biol. 1980 Dec 15;144(3):223–245. doi: 10.1016/0022-2836(80)90088-1. [DOI] [PubMed] [Google Scholar]
- Simmons R. M., Szent-Györgyi A. G. A mechanical study of regulation in the striated adductor muscle of the scallop. J Physiol. 1985 Jan;358:47–64. doi: 10.1113/jphysiol.1985.sp015539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford W. F., 3rd, Szentkiralyi E. M., Szent-Györgyi A. G. Regulatory properties of single-headed fragments of scallop myosin. Biochemistry. 1979 Nov 27;18(24):5273–5280. doi: 10.1021/bi00591a002. [DOI] [PubMed] [Google Scholar]
- Stafford W. F., Szent-Györgyi A. G. Physical characterization of myosin light chains. Biochemistry. 1978 Feb 21;17(4):607–614. doi: 10.1021/bi00597a008. [DOI] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szent-Györgyi A. G., Szentkiralyi E. M., Kendrick-Jonas J. The light chains of scallop myosin as regulatory subunits. J Mol Biol. 1973 Feb 25;74(2):179–203. doi: 10.1016/0022-2836(73)90106-x. [DOI] [PubMed] [Google Scholar]
- Szentkiralyi E. M. Tryptic digestion of scallop S1: evidence for a complex between the two light-chains and a heavy-chain peptide. J Muscle Res Cell Motil. 1984 Apr;5(2):147–164. doi: 10.1007/BF00712153. [DOI] [PubMed] [Google Scholar]
- Wells C., Bagshaw C. R. Calcium regulation of molluscan myosin ATPase in the absence of actin. Nature. 1985 Feb 21;313(6004):696–697. doi: 10.1038/313696a0. [DOI] [PubMed] [Google Scholar]
- Wells C., Bagshaw C. R. Segmental flexibility and head-head interaction in scallop myosin. A study using saturation transfer electron paramagnetic resonance spectroscopy. J Mol Biol. 1983 Feb 15;164(1):137–157. doi: 10.1016/0022-2836(83)90090-6. [DOI] [PubMed] [Google Scholar]
- Wells C., Warriner K. E., Bagshaw C. R. Fluorescence studies on the nucleotide- and Ca2+-binding domains of molluscan myosin. Biochem J. 1985 Oct 1;231(1):31–38. doi: 10.1042/bj2310031. [DOI] [PMC free article] [PubMed] [Google Scholar]


