Abstract
BACKGROUND:
High-risk non-muscle invasive bladder cancer (HR-NMIBC) patients require long-term surveillance with cystoscopies, cytology and upper tract imaging. Previously, we developed a genomic urine assay for surveillance of HR-NMIBC patients with high sensitivity and anticipatory value.
OBJECTIVE:
We aimed to validate the performance of the assay in an unselected prospectively collected cohort of HR-NMIBC patients under surveillance.
METHODS:
We included patients from five centers and collected urine sample pairs (evening and morning urines) prior to cystoscopy. Mutation status (FGFR3/TERT) and methylation status (OTX1) was analyzed on DNA from voided urine specimens. A test was considered positive if≥1 alteration was detected in at least one urine sample. The primary endpoint was tumor recurrence. Sensitivity and specificity were determined. A generalized mixed effects model was used to adjust for within-patient correlation. Cox proportional hazard analyses with time-dependent covariates assessed the anticipatory effect of the urine assay.
RESULTS:
In total, 204 patients and 736 sample pairs were collected. Sixty-three recurrences were diagnosed for which we had concomitant assay results. On cross-sectional analyses, the assay detected 75% (95% CI 62.1% –84.7%) of recurrences, with a specificity of 70% (95% CI 66.4% –73.5%). Furthermore, mixed effects model analyses revealed OTX1 (p = 0.005) and TERT (p = 0.004) as significant predictors for disease recurrence. Median follow-up was 25.3 months (IQR 18.6–30.7). Twenty-nine tumors were diagnosed without concomitant urine samples, which included recurrences detected after urine collection ended. Longitudinal analyses showed that a positive urine assay predicted a recurrence over time (HR 3.5, p < 0.001). Furthermore, a recurrence during the study period was also a predictor for developing future recurrences (HR 2.1, p < 0.001).
CONCLUSIONS:
This study validates the performance of a previously developed urine assay in an unselected cohort of HR-NMIBC patients under surveillance. With a robust sensitivity/specificity and a strong anticipatory effect, this assay proves a useful adjunct ready for evaluation in a future randomized controlled trial.
Keywords: Keywords (MeSH): Bladder cancer, urinary biomarkers, mutation, methylation, prospective
INTRODUCTION
Bladder cancer has a global incidence of 570,000 patients, making it the fourth and tenth most common malignancy in men and women, respectively [1]. Approximately 75% of patients present with non-muscle invasive bladder cancer (NMIBC). The NMIBC guideline of the European Association of Urology (EAU) recommends stratification of NMIBC into risk groups (low, intermediate, and [very] high), each being associated with different probabilities of disease recurrence and progression. It is advised that high-risk NMIBC receive life-long follow-up, which includes cystoscopy, urine cytology and upper tract imaging at regular intervals of 3–12 months [2].
Frequent cystoscopic evaluations are considered the gold standard for diagnosis of recurrent NMIBC. However, cystoscopy is not flawless. Sensitivity of cystoscopy is not 100% and unnecessary cystoscopies and transurethral biopsies of suspect bladder wall aberrations represent a significant burden related to patient discomfort and health economics [3]. For these reasons, many investigators have explored the use of urine markers for detecting recurrent NMIBC. Unfortunately, no biomarker has shown the performance that would justify replacement of cystoscopy and cytology. Despite these findings, current EAU guidelines do acknowledge an adjunctive role for urine tests prior to cystoscopy during follow-up to improve the quality of cystoscopies [2, 4].
Previously, we developed and evaluated the performance of a non-invasive urine assay in a large international multicenter study [5]. This assay included methylation of the OTX1 gene and mutation of the FGFR3 and TERT genes and showed a cross-sectional sensitivity of 72% for recurrent tumor detection in patients with a high-grade primary tumor [5]. In addition, the assay displayed a strong anticipatory effect; a positive test without a concomitant recurrence was more frequently followed by a recurrence in the next 24 months [6]. The aim of the present study was to validate this assay and evaluate its utility in an unselected prospectively collected cohort of high-risk NMIBC patients under surveillance.
MATERIALS & METHODS
Patient population data collection
For the present study, we prospectively included high-risk NMIBC patients, who were under diagnostic follow-up in five Dutch Hospitals (Rotterdam: Erasmus University Medical Center, Franciscus hospital; The Hague: Haga teaching hospital; Breda: Amphia hospital and Leiden: Leiden University Medical Center) between April 2015 and October 2017. Institutional Review Board approval was obtained at Erasmus MC Medical Research Committee (MEC-2014-544) and subsequently approved by all participating centers. High-risk NMIBC was defined as including any of the following: T1 tumor, G3/HG tumor or carcinoma in situ (CIS); very high-risk status was determined in 2022 after guideline changes when possible [2]. Furthermore, urologists were recommended to adhere to the EAU guidelines in NMIBC, which includes cystoscopy, cytology and regular upper tract imaging, which could be via CT-urography or ultrasound. Cytology was assessed according to The Paris System (TPS) in the participating centers. Next, written informed consent was obtained. Notifications with urine containers and return envelopes were sent to the patient by the coordinating center approximately 1 month before their follow-up cystoscopy, so that test results could be available before the cystoscopy. Urine sediment analysis was performed after voided urine specimens were obtained from all patients. To maximize the number of successive urines, we collected two consecutive urine samples (evening and morning), as suggested in previous work [7]. Notably, a case of tumor recurrence was defined as; I) being confirmed histologically/radiologically, or II) being clearly visible at cystoscopy (without any doubt from the consulting urologist). The latter criterion was allowed, because we aimed to assess the accuracy of our diagnostic assay within daily clinical practice.
Sample processing
Sample collection material (mail packages and urine containers) were sent to patients, approximately 4 weeks before their follow-up cystoscopy. Voided urine samples were returned per mail and processed at the Erasmus MC Pathology laboratory. Evening and morning urines were collected in two 80 ml containers, containing a preservation tablet (Sedi-tectTM, Global Scientific inc.). Samples were centrifuged at 2,000 g for 10 minutes. Urine supernatant was discarded; the cell pellet was re-suspended in 900μl PBS and transferred to a 1.5 ml Eppendorf vial. The Eppendorf vials were centrifuged once more at 6,000 rpm for 5 minutes. Excess fluids were removed, and the cell pellets were stored at –20°C until DNA isolation.
DNA isolation and molecular analyses
DNA was isolated using the Puregene DNA Isolation kit (Fischer Scientific). Qubit was used for DNA quality control. Hotspot analyses for FGFR3 (R248C, S249C, G372C, Y375C and A393E), and hTERT promotor mutations (C>T at 1295228, 1295242 and 1295250) were performed, via single-nucleotide extension reaction (SNaPshot™) as described previously [8, 9]. This results in peaks that differ in colour for WT and MT nucleotides. Mutation markers were dichotomized based on previous work. For OTX1, DNA was first converted with bisulfite to modify non-methylated C nucleotides into U’s. Methylated and not converted Cs were then detected using the snapshot analysis. Primer and probe sequences have been reported previously too [5].
Statistical analyses
Statistical analyses were performed using IBM® SPSS® Statistics for Windows, version 24.0, and R statistical software (R foundation for Statistical Computing, Vienna, Austria), version 4.0.3. A positive urine assay was defined as ≥1 positive score for one of the mutation/methylation markers (FGFR3, TERT, OTX1) in at least one out of two (evening/morning) urine samples. A negative urine assay was defined as absence of a positive marker score in both evening and morning urine samples. p-values for subgroup comparison were computed using X2 tests (categorical data) and two-sided Wilcoxon rank-sum tests (continuous data). Our primary aim was to determine the cross-sectional sensitivity/specificity for the urine assay to detect recurrent tumor cases. In addition, positive/negative scores from both urine samples were combined for each marker to analyze the association between a single marker and tumor recurrence. For this analysis, we computed mixed effects logistic regression models with R package lme4 [10]. These models account for within-patient correlation, adjusting for potential random effects that are caused by the different number of follow-up moments per patient.
Secondary aim was to determine the so called ‘anticipatory effect’, for which we conducted longitudinal analyses over time, determining whether a positive urine test without abnormal findings at cystoscopy or imaging is followed by a future tumor recurrence within the duration of the study period. We analyzed the relationship between a positive urine and the associated recurrence risk with a Cox proportional hazard model, where a recurrence yes/no was taken as the outcome. Importantly, we accounted for the fact that most patients had more than one urine measurement. Therefore, consecutive urine assay results were taken as a time-dependent covariate in the Cox model, as dictated by Therneau et al. [11]. The hazard ratio (HR) from the model can then be interpreted as the effect of a positive urine assay on the hazard of a recurrence, regardless of previous negative samples in the same patients. The predictive value of a positive urine test was determined by Kaplan-Meier estimation, coupled with long-rank testing [12]. This was done by analysis of one or more consecutive follow-up visits (series), meaning that series could start with a positive or negative urine test, ending at (a) a recurrence, (b) change in urine test status from negative to positive, or (c) at the end of follow-up. In case of (a) or (b), a new series would start. Curves of recurrence proportions were computed and stratified by urine test results using Kaplan-Meier analysis. Probability of recurrence development was compared using a log-rank statistic. Results were considered statistically significant at p < 0.05. Further aims include test characteristics for morning or evening urines separately.
RESULTS
Baseline patient population, urines and follow-up
Baseline clinicopathological characteristics of patients included in the study cohort are listed in Table 1. In total, 742 urine sample pairs were collected from 204 patients, resulting in a mean of 3,64 urine pairs per patient, with a median follow-up time of 25.3 months (IQR 18.6–30.7) per patient. From these 742 samples, 736 (99.2%) were linked to a corresponding clinical follow-up moment, consisting of cystoscopy in 612, cystoscopy and ultrasound in 19, cystoscopy and computed tomography (CT) in 101, CT only in 3, and cystoscopy and MRI in 1. Table 2 lists further details of the 736 follow-up moments for which urine samples were analyzed. Combining assay results from evening and morning urines (Table 3), resulted in available mutation / methylation status for all 3 biomarker genes in 724 out of 736(98%) samples (Table S1). No association was found between the test’s success rate and the urine sediment analysis.
Table 1.
Baseline clinicopathological characteristics of the study cohort consisting of N = 204 high-risk non-muscle invasive bladder cancer patients under surveillance
| Variables | N (%) | |
| Total | 204 (100%) | |
| Age | Median (IQR) | 71,0 (64,6–77,2) |
| Sex | Female | 36 |
| Male | 168 | |
| Hospital | EMC | 60 |
| Franciscus | 54 | |
| Haga | 45 | |
| Amphia | 30 | |
| LUMC | 15 | |
| Previous intravesical treatment (before study inclusion) | None | 28 |
| Chemo | 23 | |
| BCG | 124 | |
| BCG + Chemo | 27 | |
| KLH | 2 | |
| Stage/Grade index tumor | Tis | 20 |
| TaG2 HG | 20 | |
| TaG3 | 53 | |
| T1G2 | 38 | |
| T1G3 | 73 | |
| Concomitant CIS | Yes | 55 |
| No | 129 | |
| CIS only | 20 | |
| EAU risk category | High-risk | 144 |
| Very high-risk | 17 | |
| Unclear* | 43 |
Abbreviations: Chemo = Chemotherapy, BCG = Bacillus Calmette-Guérin, KLH = Keyhole Limpet Hemocyanin, CIS = Carcinoma-In-Situ; *Unclear due to missing information (tumor focality and/or tumor size).
Table 2.
Clinical follow-up moments linked to 736 collected urine sample pairs from N = 204 high-risk non-muscle invasive bladder cancer patients under surveillance
| Variables | N (%) | |
| Total | 736 (100%) | |
| Bladder visualization | Cystoscopy only | 612 |
| Cystoscopy + CT | 101 | |
| Cystoscopy + Echo | 19 | |
| CT only | 3 | |
| Cystoscopy + MRI | 1 | |
| Cytology | Not done | 286 |
| No abnormalities | 381 | |
| No diagnosis possible | 8 | |
| Atypic | 27 | |
| Suspect (TPS4) | 8 | |
| High Grade (TPS5) | 3 | |
| Low Grade (TPS6) | 23 |
Abbreviations: Cysto = Cystoscopy, CT = Computed Tomography, abn.=abnormalities, diagn.=diagnosis possible
Table 3.
Successfully analyzed urine tests in morning and evening sample in N = 736 collected pairs
| Variables | Evening N (%) | Morning N (%) | |
| Total | 736 (100%) | 736 (100%) | |
| Test result | Positive (+) | 204 | 189 |
| Negative (–) | 501 | 514 | |
| NA | 31 | 33 |
Cross-sectional urine test sensitivity and specificity
In total, 63 recurrences were diagnosed in 724 follow-up moments for which we had concomitant assay results. Table 4A lists the characteristics of these recurrences. Seven (11%) out of 63 recurrences showed disease progression, of which 4 were radiologically confirmed (Table 4A). A total of 63 recurrences were found in 37 patients, of which 3 were female and 34 were male. Fifty three of the 63 (84%) recurrences were confirmed histologically (Table 4A). Cross-sectional analysis revealed that the urine assay detected 75% (95% CI 62.1% –84.7%) of recurrences (47 of 63), with a specificity of 70% (95% CI 66.4% –73.5%) (463 of 661). Results stratified by EAU risk category were similar (Table S2). From the 16 recurrences that were missed by the concomitant urine assay, 6 were TaG3, 3 TaG1, 2 Tis only, 2 TaG2 (HG), 1 cT3, 1 T1G3 and 1 TaG3 with concomitant CIS. Sensitivity-specificity combinations were 66% (41/62) –77% (493/641) when using morning urines only, and 70% (43/61) –75% (483/644) when using evening urines only. Of note, two upper urinary tract malignancies were diagnosed on imaging (Table 4A), and both were detected by the assay. Finally, urologists were recommended to collect urine cytology at every cystoscopy. However, this was only performed in 439 (61%) follow-up moments that included urine test results, which corresponds to 31 recurrences. In line with the literature, cytology had a 14/31 (45%) sensitivity and 365/408 (89%) specificity. Urine test sensitivity in these samples was 87% (27/31), with a 64% (279/408) specificity.
Table 4A.
Characteristics of N = 63 detected tumor recurrences associated with concomitant urine tests during follow-up of N = 204 high-risk non-muscle invasive bladder cancer patients
| Variables | N (%) | |
| Total | 63 (100%) | |
| Histologically confirmed | Tis only | 18 |
| TaG1 | 10 | |
| TaG2 LG | 6 | |
| TaG2 HG | 5 | |
| TaG3 | 7 | |
| TaG3 + CIS | 2 | |
| T1G3 | 2 | |
| T2G2 HG | 1 | |
| T2G3 | 1 | |
| T3G3 | 1 | |
| Radiologically confirmed | cN+ | 1 |
| cM+ | 2 | |
| cT3 (transmural) | 1 | |
| Kidney | 1 | |
| Ureter | 1 | |
| Clinical presence | Watchful Waiting | 3 |
| Pt denied TURBT | 1 |
Abbreviations: Pt = Patient, TURBT = Transurethral Resection of the Bladder Tumor. •Disease progression
Single markers performance of the urine assay
To evaluate the predictive performance of each marker, we conducted a multivariable generalized mixed effects model analyses. Table 5 lists the association between individual assay marker scores and tumor recurrence. For this cohort, mixed effects model analyses revealed that OTX1 (p = 0.005) and TERT (p = 0.004) were significant predictors for disease recurrence in the cross-sectional setting, hence these markers appeared the strongest predictors for detecting a recurrence at the corresponding follow-up moment. In addition, we analyzed if OTX1 and TERT were associated with baseline clinical information. OTX1 was associated with female gender (p = 0.041), but other parameters had no association.
Table 5.
Multivariable generalized mixed effects model analysis for the association between assay marker scores and tumor recurrence at the corresponding follow-up moment
| Fixed Effects | Coefficient | P Value | OR (95% CI) |
| OTX1 | –8.999 (intercept) | *0.005 | 5.36 (1.64–17.51) |
| FGFR3 | 1.679 | 0.079 | 10.24 (0.76–137.44) |
| TERT | 2.327 | *0.004 | 5.72 (1.77–18.50) |
| Male sex | 1.744 | 0.382 | 3.60 (0.20–63.84) |
| 1.281 |
Longitudinal analyses evaluating the anticipatory effect
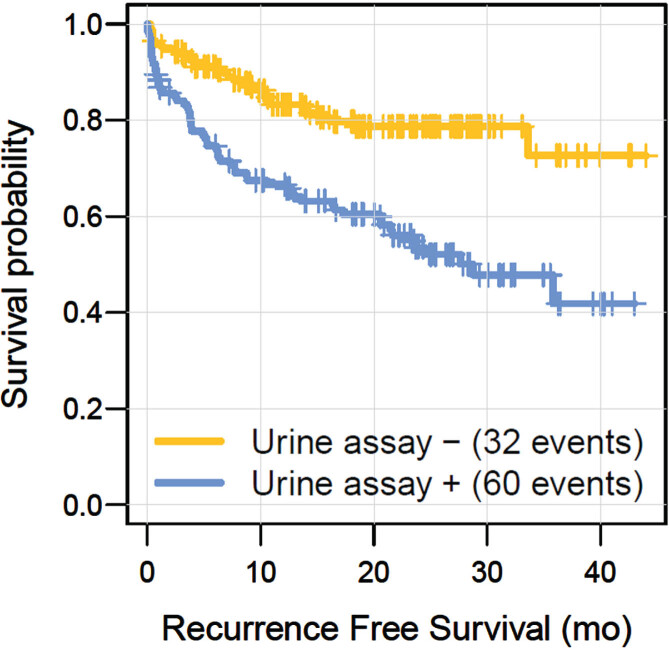
Twenty-nine additional tumors were diagnosed without available concomitant urine samples, including recurrences detected during follow-up after the urine collection period. Table 4B lists clinicopathological characteristics of these 29 tumors, of which 25 were confirmed histologically (Table 4B). Six (21%) out of these 29 tumors showed disease progression, of which one was confirmed radiologically (Table 4). The total number of recurrences during the study period eventually comprised 92. In previous publications, we and others recognized an ‘anticipatory effect’ of a positive urine assay, i.e., the urine assay detects recurrent tumors earlier than cystoscopy does [5, 12, 13]. To model this anticipatory effect over time, we conducted a cox proportional hazard analysis for which consecutive urine assay results were taken as a time-dependent covariate. As listed in Table 6, this analysis revealed that a positive urine assay showed a significant (p < 0.001) HR of 3.5 for predicting a recurrence over time. Furthermore, having had a previous recurrence before study inclusion also appeared a significant predictor for a tumor recurrence. To illustrate the urine assay’s anticipatory effect, we also generated Kaplan Meier graphs, showing that a positive urine assay was more frequently followed by a recurrence over time (Fig. 1, p < 0.001).
Table 4B.
Characteristics of N = 29 diagnosed recurrences without concomitant urine samples, including recurrences detected after urine sample collection ended
| Variables | N (%) | |
| Total | 29 (100%) | |
| Histologically confirmed | Solitary CIS / Tis | 6 |
| TaG1 | 8 | |
| TaG2 LG | 5 | |
| TaG3 | 2 | |
| T2G3 | 4 | |
| Radiologically confirmed | cM+ | 1 |
| Clinical presence | Watchful Waiting | 2 |
| No show at TURBT | 1 |
•Disease progression.
Table 6.
Multivariable time-dependent cox proportional hazard model analysis for the association between assay test results and tumor recurrence detection over time
| Variable | Coefficient | P Value | HR (95% CI) |
| Positive Urine Assay | 1.245 | *8.21×10–8 | 3.47 (2.20–5.48) |
| Age | 0.023 | 0.052 | 1.02 (0.97–1.05) |
| Recurrence during FU | 0.743 | *1.23×10–15 | 2.10 (1.75–2.52) |
| Male sex | 0.22 | 0.488 | 1.25 (0.67–2.32) |
Fig. 1.
Kaplan Meier curves illustrating the anticipatory effect of a positive urine assay result (P < 0.001, Log-Rank).
DISCUSSION
NMIBC is associated with a considerable burden regarding both health economics and health-related quality of life [3]. As such, reducing the number of follow-up cystoscopies may improve patient quality of life and value-based care for NMIBC. A recent meta-analysis suggested several molecular urine assays might be efficient in reducing the number of avoidable cystoscopies, with pooled sensitivities varying between 57–93% and pooled specificities between 61–84% [14]. In this context, the present study has validated our 3-gene assay performance within an unselected prospective cohort of high-risk NMIBC patients, revealing 75% sensitivity and 70% specificity for detecting recurrences in cross-sectional analysis, which is even better than in the previous study. Furthermore, we have modeled the anticipatory effect in a time-dependent fashion, confirming a strong anticipatory effect for positive assay results, which is a 3.5 fold increased risk of recurrences when the test is positive, independent of a specific time point.
Importantly, results from this multi-center validation study confirm assay robustness with an 98% success rate (94% when a single urine was analyzed), encouraging clinical application of our molecular assay. Ideally, a molecular urine assay facilitates clinical decision making during NMIBC follow-up. With the strong anticipatory effect, as confirmed in the present study, a positive assay result may necessitate execution of enhanced diagnostics, such as blue light cystoscopy and/or biopsy in case no tumor is seen at cystoscopy, or at least increased vigilance during follow-up visits [5, 15]. In the BLFC trial, white light cystoscopy detected cancer in 50/63 (79%) of patients who were ultimately found to have cancer, suggesting a biopsy based on a positive molecular assay could assist in earlier detection of tumor recurrences [16]. We previously found that awareness of a positive test increased recurrence detection by the physician, providing an additional argument for clinical implementation of the assay [4]. Furthermore, turnaround for molecular urine testing is less than four weeks, even in a trial setting, it is easy and cheap to execute and therefore could be of real added value for bladder cancer treating physicians.
Uromonitor® is similar to our 3-plex assay and includes mutations in FGFR3 and TERTp. The latest version of Uromonitor® also includes KRAS mutations [18]. Our group previously reported that the frequency of RAS (HRAS, KRAS, and NRAS) alterations in a general NMIBC population under surveillance is low (<1%) and thus we stopped analyzing KRAS [19]. A meta-analysis of Uromonitor® showed a pooled sensitivity of 93% and specificity of 79% [14]. The results are excellent, but the included studies had a different design. Batista et al. showed outcomes for a urine test with cystoscopy, as compared to cystoscopy with cytology [18]. Uromonitor® without cystoscopy had a sensitivity of 74%, which is comparable to our results. Sieverink et al. did not make use of an unselected cohort, but a case-enriched cohort of high-risk NMIBC patients [18].
In case of a negative assay result, office visits for cystoscopies may be replaced for molecular testing of a voided urine sample. Of note, this approach currently is investigated for low-risk NMIBC within the context of the UroFollow Trial [20]. Importantly, this approach may be more suited for low and/or intermediate NMIBC risk groups, since probabilities of disease progression for these groups are lower than for high-risk NMIBC [20]. However, previous work revealed lower performance of our assay in a low-risk NMIBC subgroup (∼60% sensitive) [5, 12]. Nevertheless, time dependent survival analyses from the present study showed a lower incidence of recurrences following negative assay results. Therefore, rather than avoiding follow-up cystoscopies based on a single negative assay result, the frequency of cystoscopic follow-up for high-risk NMIBC might be decreased in combination with intermittent molecular assays. In addition, future management may involve tissue-based biomarkers to aid in estimating the risk of progression prior to follow-up in high-risk NMIBC patients [21, 22].
Though less evident due to the high test performance, we anticipated that using two urine samples instead of one increased sensitivity for recurrence detection [7]. Furthermore, using two urine samples resulted in a reduced number of inconclusive assay results, since we had two opportunities to define the biomarker status for all 3 genes. Indeed, in our recent hematuria trial, we found that urines without a complete biomarker result, had a significantly lower DNA yield. The low number of inconclusive assay results in the present study are therefore most likely due to the use of two urine samples instead of one.
Limitations of this study include missing information about follow-up time before inclusion, together with a lack of detailed information on previous intravesical therapies. Therefore, we were not able to determine which patients would for instance be considered BCG unresponsive. In addition, surveillance bias is increased with a high number of participating urological centers, while a lack of centralized pathology review increases interobserver variability. On the other hand, a multicenter setting better reflects current clinical practice. To control for random effects caused by a different number of follow-up moments per patient, we conducted multivariable generalized mixed effects model analyses. Of interest, these analyses revealed that cross-sectionally FGFR3 mutation status showed the least predictive performance in the present study. This observation may be attributed to the fact that we assembled a high-risk NMIBC cohort, whereas FGFR3 mutations are generally present in the majority of low- and intermediate risk NMIBC, representing a subgroup of bladder cancer that harbors less aggressive cancer biology [12, 23, 24]. Importantly, the odds ratio for FGFR3 mutation status was substantially high, leading us to anticipate statistical significance could have been achieved in a larger patient cohort or when more intermediate risk recurrences would have occurred. Furthermore, the present study was designed to validate our 3-gene assay, and previous work has yet convincingly suggested efficacy for FGFR3 mutation analysis on voided urine samples in patients with a FGFR3-mutated tumors, together with advocated cost-effectiveness [12, 23]. To conclude; TERT and OTX1 were significant predictors of recurrences. Previous research showed that TERT mutations were common and unrelated to clinical outcomes in NMIBC. Yet, recently it was shown that TERT mutations are detectable up to ten years prior to the diagnosis of bladder cancer, further emphasizing that mutations in TERT have an important anticipatory value [25, 26]. As for OTX1, genome-wide analysis of CpG islands showed it is often hypermethylated in bladder cancer as compared to normal urothelium [27]. To our knowledge, no information exists on the predictive value of hypermethylation of OTX1 in HR-NMIBC. However, a methylation signature including hypermethylated OTX1 was associated with a poor overall survival in muscle-invasive bladder cancer, but also with increased gene expression. Silencing OTX1 significantly reduced cell viability and inhibited tumor growth and motility in vivo, with overexpression leading to opposite effects [28]. This is seemingly contradictory as hypermethylation usually leads to reduced expression, hence, future research is needed to help us to elucidate OTX1’s role as biomarker [29].
CONCLUSIONS
This multi-institutional, prospective cohort study validates the performance of a molecular urine assay in an unselected cohort of high-risk NMIBC patients. This DNA-based assay includes mutation and methylation markers and represents a valuable adjunct for the follow-up of high-risk NMIBC. Analyzing two samples increased sensitivity for detecting NMIBC recurrence and led to an increased number of successive urines. With its strong anticipatory effect, we anticipate implementation of the assay enables earlier diagnosis of recurrences.
Supplementary Material
ACKNOWLEDGMENTS
Prof. Dr. E.C. Zwarthoff had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/BLC-240017.
FUNDING
The study was funded by a Dutch ZonMW grant (80-83700-98-51018, Doelmatigheidsonderzoek).
AUTHOR CONTRIBUTIONS
Conceptualization, JJ, FJ, JB, TZ and EZ; methodology, JJ, FJ and EZ; validation, JJ, FJ and EZ; formal analysis, JJ and EW; investigation, JJ, FJ and AM; resources, NC, HR, EO, RP and CB; data curation, JJ and FJ; writing—original draft preparation, JJ; writing—review and editing, FJ, JB and EZ; visualization, JJ; supervision, JB, TZ and EZ; project administration, EZ; funding acquisition, EZ.
CONFLICTS OF INTEREST
TZ and EZ are Editorial Board members of this journal, but were not involved in the peer-review process nor had access to any information regarding its peer-review.
TZ declares Grant/Research support from: Genecentric/Janssen, InnoSign and Merk AG (all paid to Erasmus MC). EZ declares a License agreement with MDxHealth / Vesica and a patent for OTX1 and ONECUT2 methylation issued in EU and Canada, and a pending patent in the USA. JB declares consultantcy / speaker’s fee (all paid to Erasmus MC) from: Janssen, BMS, AstraZeneca, Merck AG/Pfizer, MSD, Bayer, Ismar Healtcare. Research collaborations with: Merck AG, MSD, Janssen, VitroScan and Merk Serono. Member of the Scientific Congress Committee European Association of Urology.
JJ, FJ, AM, NC, HR, EO, RP, EW and CB declare no conflicts of interest related to this topic nor to the contents of this manuscript.
DATA AVAILBILITY
Only trivial code has been used for the analyses.
REFERENCES
- [1]. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021. [DOI] [PubMed] [Google Scholar]
- [2]. Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol. 2022;81(1):75–94. [DOI] [PubMed] [Google Scholar]
- [3]. Lee LJ, Kwon CS, Forsythe A, Mamolo CM, Masters ET, Jacobs IA. Humanistic and Economic Burden of Non-Muscle Invasive Bladder Cancer: Results of Two Systematic Literature Reviews. Clinicoecon Outcomes Res. 2020;12:693–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. van der Aa MN, Steyerberg EW, Bangma C, van Rhijn BW, Zwarthoff EC, van der Kwast TH. Cystoscopy revisited as the gold standard for detecting bladder cancer recurrence: diagnostic review bias in the randomized, prospective CEFUB trial. The Journal of Urology. 2010;183(1):76–80. [DOI] [PubMed] [Google Scholar]
- [5]. Beukers W, van der Keur KA, Kandimalla R, Vergouwe Y, Steyerberg EW, Boormans JL, et al. FGFR3, TERT and OTX1 as a Urinary Biomarker Combination for Surveillance of Patients with Bladder Cancer in a Large Prospective Multicenter Study. J Urol. 2017;197(6):1410–8. [DOI] [PubMed] [Google Scholar]
- [6]. Hruban RH, van der Riet P, Erozan YS, Sidransky D. Brief report: molecular biology and the early detection of carcinoma of the bladder–the case of Hubert H. Humphrey. N Engl J Med. 1994;330(18):1276–8. [DOI] [PubMed] [Google Scholar]
- [7]. Zuiverloon TC, Tjin SS, Busstra M, Bangma CH, Boevé ER, Zwarthoff EC. Optimization of nonmuscle invasive bladder cancer recurrence detection using a urine based FGFR3 mutation assay. J Urol. 2011;186(2):707–12. [DOI] [PubMed] [Google Scholar]
- [8]. Kompier LC, Lurkin I, van der Aa MNM, van Rhijn BWG, van der Kwast TH, Zwarthoff EC. FGFR3, HRAS, KRAS, NRAS and PIK3CA Mutations in Bladder Cancer and Their Potential as Biomarkers for Surveillance and Therapy. PLOS ONE. 2010;5(11):e13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Allory Y, Beukers W, Sagrera A, Flández M, Marqués M, Márquez M, et al. Telomerase Reverse Transcriptase Promoter Mutations in Bladder Cancer: High Frequency Across Stages, Detection in Urine, and Lack of Association with Outcome. European Urology. 2014;65(2):360–6. [DOI] [PubMed] [Google Scholar]
- [10]. Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67(1):1–48. [Google Scholar]
- [11]. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model: Springer; 2014. [Google Scholar]
- [12]. Zuiverloon TC, van der Aa MN, van der Kwast TH, Steyerberg EW, Lingsma HF, Bangma CH, Zwarthoff EC. Fibroblast growth factor receptor 3 mutation analysis on voided urine for surveillance of patients with low-grade non-muscle-invasive bladder cancer. Clin Cancer Res. 2010;16(11):3011–8. [DOI] [PubMed] [Google Scholar]
- [13]. Sánchez-Carbayo M, Herrero E, Megias J, Mira A, Espasa A, Chinchilla V, Soria F. Initial evaluation of the diagnostic performance of the new urinary bladder cancer antigen test as a tumor marker for transitional cell carcinoma of the bladder. J Urol. 1999;161(4):1110–5. [PubMed] [Google Scholar]
- [14]. Laukhtina E, Shim SR, Mori K, D’Andrea D, Soria F, Rajwa P, et al. Diagnostic Accuracy of Novel Urinary Biomarker Tests in Non-muscle-invasive Bladder Cancer: A Systematic Review and Network Meta-analysis. Eur Urol Oncol. 2021;4(6):927–42. [DOI] [PubMed] [Google Scholar]
- [15]. Lotan Y, Black PC, Caba L, Chang SS, Cookson MS, Daneshmand S, et al. Optimal Trial Design for Studying Urinary Markers in Bladder Cancer: A Collaborative Review. Eur Urol Oncol. 2018;1(3):223–30. [DOI] [PubMed] [Google Scholar]
- [16]. Daneshmand S, Patel S, Lotan Y, Pohar K, Trabulsi E, Woods M, et al. Efficacy and Safety of Blue Light Flexible Cystoscopy with Hexaminolevulinate in the Surveillance of Bladder Cancer: A Phase III, Comparative, Multicenter Study. J Urol. 2018;199(5):1158–65. [DOI] [PubMed] [Google Scholar]
- [17]. Batista R, Vinagre J, Prazeres H, Sampaio C, Peralta P, Conceição P, et al. Validation of a Novel, Sensitive, and Specific Urine-Based Test for Recurrence Surveillance of Patients With Non-Muscle-Invasive Bladder Cancer in a Comprehensive Multicenter Study. Front Genet. 2019;10:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Sieverink CA, Batista RPM, Prazeres HJM, Vinagre J, Sampaio C, Leão RR, et al. Clinical Validation of a Urine Test (Uromonitor-V2®) for the Surveillance of Non-Muscle-Invasive Bladder Cancer Patients. Diagnostics. 2020;10(10):745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Zuiverloon TC, Beukers W, van der Keur KA, Nieuweboer AJ, Reinert T, Dyrskjot L, et al. Combinations of urinary biomarkers for surveillance of patients with incident nonmuscle invasive bladder cancer: the European FP7 UROMOL project. The Journal of Urology. 2013;189(5):1945–51. [DOI] [PubMed] [Google Scholar]
- [20]. Benderska-Söder N, Hovanec J, Pesch B, Goebell PJ, Roghmann F, Noldus J, et al. Toward noninvasive follow-up of low-risk bladder cancer – Rationale and concept of the UroFollow trial. Urol Oncol. 2020;38(12):886–95. [DOI] [PubMed] [Google Scholar]
- [21]. van Kessel KEM, van der Keur KA, Dyrskjøt L, Algaba F, Welvaart NYC, Beukers W, et al. Molecular Markers Increase Precision of the European Association of Urology Non-Muscle-Invasive Bladder Cancer Progression Risk Groups. Clin Cancer Res. 2018;24(7):1586–93. [DOI] [PubMed] [Google Scholar]
- [22]. de Jong FC, Laajala TD, Hoedemaeker RF, Jordan KR, van der Made ACJ, Boevé ER, et al. Non-muscle-invasive bladder cancer molecular subtypes predict differential response to intravesical Bacillus Calmette-Guérin. Sci Transl Med. 2023;15(697):eabn4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. van Kessel KE, Kompier LC, de Bekker-Grob EW, Zuiverloon TC, Vergouwe Y, Zwarthoff EC, Steyerberg EW. FGFR3 mutation analysis in voided urine samples to decrease cystoscopies and cost in nonmuscle invasive bladder cancer surveillance: a comparison of 3 strategies. J Urol. 2013;189(5):1676–81. [DOI] [PubMed] [Google Scholar]
- [24]. Tomlinson DC, Baldo O, Harnden P, Knowles MA. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J Pathol. 2007;213(1):91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Allory Y, Beukers W, Sagrera A, Flández M, Marqués M, Márquez M, et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur Urol. 2014;65(2):360–6. [DOI] [PubMed] [Google Scholar]
- [26]. Hosen MI, Sheikh M, Zvereva M, Scelo G, Forey N, Durand G, et al. Urinary TERT promoter mutations are detectable up to 10 years prior to clinical diagnosis of bladder cancer: Evidence from the Golestan Cohort Study. EBioMedicine. 2020;53:102643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Kandimalla R, van Tilborg AA, Kompier LC, Stumpel DJ, Stam RW, Bangma CH, Zwarthoff EC. Genome-wide analysis of CpG island methylation in bladder cancer identified TBX2, TBX3, GATA2, and ZIC4 as pTa-specific prognostic markers. Eur Urol. 2012;61(6):1245–56. [DOI] [PubMed] [Google Scholar]
- [28]. Jiang L, Zuo Z, Lin J, Yang C. Orthodenticle homeobox OTX1 is a potential prognostic biomarker for bladder cancer. Bioengineered. 2021;12(1):6559–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Xu Z, Gujar H, Fu G, Ahmadi H, Bhanvadia S, Weisenberger DJ, et al. A Novel DNA Methylation Signature as an Independent Prognostic Factor in Muscle-Invasive Bladder Cancer. Frontiers in Oncology. 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.