Abstract
The effect of methamphetamine on the host response to an opportunistic pathogen has not been extensively described. Methamphetamine is a major public health and safety problem in the United States. Chronic methamphetamine abuse is associated with a 2-fold higher risk of human immunodeficiency virus infection and, possibly, additional infections. Histoplasma capsulatum is a dimorphic fungus that is endemic in the Midwest of the United States and that causes respiratory and systemic disease, particularly in individuals with impaired immunity. We showed that methamphetamine abrogates normal macrophage function, resulting in an inability to control histoplasmosis. Methamphetamine decreased phagocytosis and killing of yeast by primary macrophages by alkalization of the phagosome. Furthermore, mice that received methamphetamine prior to H. capsulatum infection were immunologically impaired, with increased fungal burden, increased pulmonary inflammation, and decreased survival. Immunosuppression by methamphetamine may be associated with deregulation of cytokines in the lungs of infected mice, aberrant processing of H. capsulatum within macrophages, and immobilization of MAC-1 receptors on the surface of macrophages that are involved in phagocytosis. Additionally, methamphetamine inhibits T cell proliferation and alters antibody production, which are important components of adaptive immunity. With use of a murine model of histoplasmosis, this study establishes that methamphetamine may alter the immune system of the host and enhance fungal pathogenesis.
Histoplasmosis due to Histoplasma capsulatum is the most prevalent fungal respiratory disease in the United States, affecting ~500,000 individuals each year [1]. Histoplasmosis usually manifests as a self-limited, mild respiratory illness, but infection may progress to life-threatening systemic disease in immunocompromised individuals. After inhalation, H. capsulatum conidia or hyphal fragments are ingested by resident pulmonary macrophages, where the fungus transforms to its yeast phase, replicates, and is subsequently disseminated. Macrophages are considered to be the most important effector cells in host resistance against histoplasmosis, functioning in both innate and cell-mediated immunity [2]. However, resolution depends on the activation of cell-mediated immunity, particularly, effective T cell responses [1]. Both CD4+ and CD8+ T cells contribute to host resistance in primary histoplasmosis. Reduction of CD4+ T cells results in fatal histoplasmosis in naive mice, and adoptive transfer of H. capsulatum–reactive CD4+ T cells confers protection [3, 4]. In mice lacking CD8+ T cells, clearance of H. capsulatum is impaired [3, 4]. Sublethal infection evokes a T helper (Th) 1–like response in mice, characterized by the dominance of interleukin (IL)–12, tumor necrosis factor (TNF)–α, and interferon (IFN)–γ during the acute phase of infection [5]. On induction of cell-mediated immunity and the production of cytokines, macrophages are activated, and the fungus is eliminated. Although B cell–deficient animals experienced an accelerated progression to death in the case of reactivation disease [6], B cells are less critical in primary histoplasmosis [3].
Methamphetamine is an extremely addictive central nervous system stimulant. Chronic abuse of methamphetamine has increased throughout the United States. A 2003 survey indicated that ~5% of the population aged >12 years has tried methamphetamine, and the rate of hospital admissions for treatment of primary methamphetamine abuse increased more than 3-fold from 1993 to 2003 [7, 8]. Also, the transmission of human immunodeficiency virus (HIV) [9, 10] and hepatitis B and C [11] are possible consequences of methamphetamine abuse. These diseases are often spread via contaminated needles, syringes, and other equipment that is used by multiple people who are injecting methamphetamine. The intoxicating effects of methamphetamine, regardless of administration route, can also alter judgement and reduce inhibitions, leading people to engage in unsafe activities [10]. Among homosexual and bisexual men [9], methamphetamine use is associated with high-risk sexual behavior and high rates of HIV acquisition [10]. Abuse may also facilitate the progression of HIV infection [12]. Methamphetamine addicts who are infected with HIV have more pronounced neuronal injury and cognitive impairment, compared with HIV-infected individuals who do not use methamphetamine [13, 14]. Furthermore, animal studies have demonstrated that methamphetamine suppresses both innate and adaptive immunity [15, 16] and alters gene expression of immune cells [17].
Recently, we began to elucidate the molecular basis for immune suppression by methamphetamine [18]. We demonstrated that methamphetamine is an immunosuppressive agent, because it alkalizes normally acidic organelles within immune cells, inhibits antigen presentation, and impairs phagocytosis. Maintenance of low endosomal and lysosomal pH serves many regulatory functions, including protein degradation, pathogen inactivation, and surface receptor expression. These functions require active transport via the endocytic pathway and fusion with lysosomal compartments. The interference of methamphetamine on pH maintenance likely compromises the immune response to opportunistic microbes.
The effects of methamphetamine on host responses to an opportunistic pathogen have not been extensively described. H. capsulatum is an ideal model organism to study, because this fungus is endemic to the midwest region of the United States, where methamphetamine is a major public health problem. Furthermore, H. capsulatum is unique among fungal pathogens in its ability to regulate phagosomal pH in macrophages [19–21], thereby avoiding damage from cellular products, such as hydrolytic enzymes, that are most active at low pH. We hypothesized that the capacity of methamphetamine to prevent acidification of phagosomes and interfere with antigen presentation would exacerbate histoplasmosis. In the present work, we investigate the effect of methamphetamine on the host response during histoplasmosis.
MATERIALS AND METHODS
Fungus.
H. capsulatum ATCC 26032 (G217B) is available from the American Type Culture Collection. A green fluorescent protein–expressing strain of G217B was a gift from J. P. Woods (University of Wisconsin, Madison). Yeast cells were grown for 48 h at 37°C in Ham’s F-12 medium with rotary shaking at 150 rpm.
Methamphetamine administration and infection.
Most high-dose methamphetamine abusers initially use small amounts intermittently before progressively increasing the dose [22]. To simulate this pattern, increasing doses (2, 4, 6, and 8 mg/kg/day on days 1, 2, 3, and 4, respectively, and 10 mg/kg/day from days 5–10) of methamphetamine (Sigma) were intraperitoneally administered to female C57BL/6 mice (age, 6–8 weeks; National Cancer Institute) over 10 days. At day 11, mice were infected with H. capsulatum. Sublethal infection was induced by anesthetization and intranasal inoculation of 5 × 106 H. capsulatum cells. Determinations of colony-forming units, cytokine levels, and antibody production and histological analyses were performed as described elsewhere [23]. Lethal infection was performed by intranasal inoculation with 1.25 × 107 H. capsulatum cells. Phosphate-buffered saline (PBS) was injected intraperitoneally into control mice before infection.
H. capsulatum antigens.
M antigen was purified from histoplasmin by cation-exchange chromatography in columns of carboxymethyl-Sepharose CL-6B, as described elsewhere [24]. Recombinant HSP60 (rHSP60) was prepared as described elsewhere [25].
Splenocyte preparation and proliferation assay.
At day 8 after infection, spleens were removed and homogenized. Red blood cells were lysed with RBC lysis solution (Gentra Systems). The cells were suspended in RPMI 1640 (Life Technologies Institute) with 10% fetal calf serum (HyClone Laboratories) and were centrifuged for 10 min at 500 g. Cells were cultured at a concentration of 2 × 105 cells/well in 96-well flat-bottom plates in a 200-μL volume, with 5, 25, or 50 μg/mL of M antigen or rHSP60. After 6 days at 37°C in 5% CO2, cells were pulsed with 0.5 μCi/well of [3H]-thymidine for 8 h and were harvested onto glass fiber filters. The incorporated radioactivity was counted using a β scintillation counter (PerkinElmer Life and Analytical Sciences).
Phagocytosis assay.
J774.16 cells were incubated in the absence or presence of methamphetamine (10 or 50 μmol/L) for 2 h. Chloroquine (20 μmol/L) was used as a positive control. For primary macrophages, mice were injected with methamphetamine or PBS as described above. After euthanasia, peritoneal lavage was performed [26] to isolate peritoneal macrophages. Monolayers of macrophage-like or primary cells were washed thrice with PBS or Dulbecco’s Modified Eagle’s Medium (Mediatech), respectively, followed by the addition of green fluorescent protein–expressing H. capsulatum G217B cells in a macrophage:yeast ratio of 1:10. The plates were incubated for 2 h at 37°C. The monolayer coculture was washed 3 times with PBS to remove nonadherent cells, fixed with cold methanol, and viewed with light and fluorescence microscopy with use of an Axiovert 200 M inverted microscope (Carl Zeiss); images were collected using a SPOT-RT slider CCD camera and image acquisition software (Diagnostic Instruments). The phagocytic index was determined to be the number of internalized yeast cells per number of macrophages per field. Internalized cells were differentiated from attached cells by the presence in a well-defined phagocytic vacuole.
Killing assay.
J774.16 cells and primary macrophages were incubated with H. capsulatum as described above. Quantification of viable yeast cells was determined by measuring colony-forming units after lysis of macrophages [27].
Flow cytometry.
For staining for fluorescence-activated cell sorting (FACS) analysis, primary macrophages were incubated with methamphetamine, chloroquine, or PBS as described above; were washed; and were then stained with the fluorescence-labeled antibodies. Anti-CD11b-APC, anti-CD11c-Cy7, anti-CD14-FITC, and anti-CD-18-PE were purchased from eBiosciences. Samples were processed on a FACSCalibur flow cytometer (BD) and were analyzed using the Cell Quest Pro software (BD).
Measurement of phagosomal pH.
The pH of H. capsulatum–containing phagosomes in primary macrophages was determined as described elsewhere [21], with minor modifications.
Measurement of nitric oxide (NO) and superoxide (O2−) production.
Primary macrophages were incubated in the absence or presence of methamphetamine (10, 25, 50, or 100 μmol/L) for 2 h. Chloroquine (20 μmol/L) was used as a positive control. To stimulate NO and O2− production, macrophages were coincubated with H. capsulatum in the presence of 350 U/mL of IFN-γ for 24 h at 37°C. NO production was quantified using a Griess method kit (Promega), according to the manufacturer’s protocol. SOD1 activity was measured using the superoxide dismutase assay kit (Calbiochem).
Statistical analysis.
All data were subjected to statistical analysis with use of Prism 5.0 (GraphPad Software). P values were calculated by analysis of variance and were adjusted by use of the Bonferroni correction or t-test analysis. P values of <.05 were considered to be statistically significant.
RESULTS
Physical and behavioral effects of methamphetamine.
C57BL/6 mice were given either methamphetamine (2–10 mg/kg; n = 10) or PBS (n = 10) daily for 10 days. Mice that received the daily injection of methamphetamine lost ~2 g of body weight, compared with control mice (P < .05) (figure 1, which appears only in the electronic version of the Journal).
Figure 1.
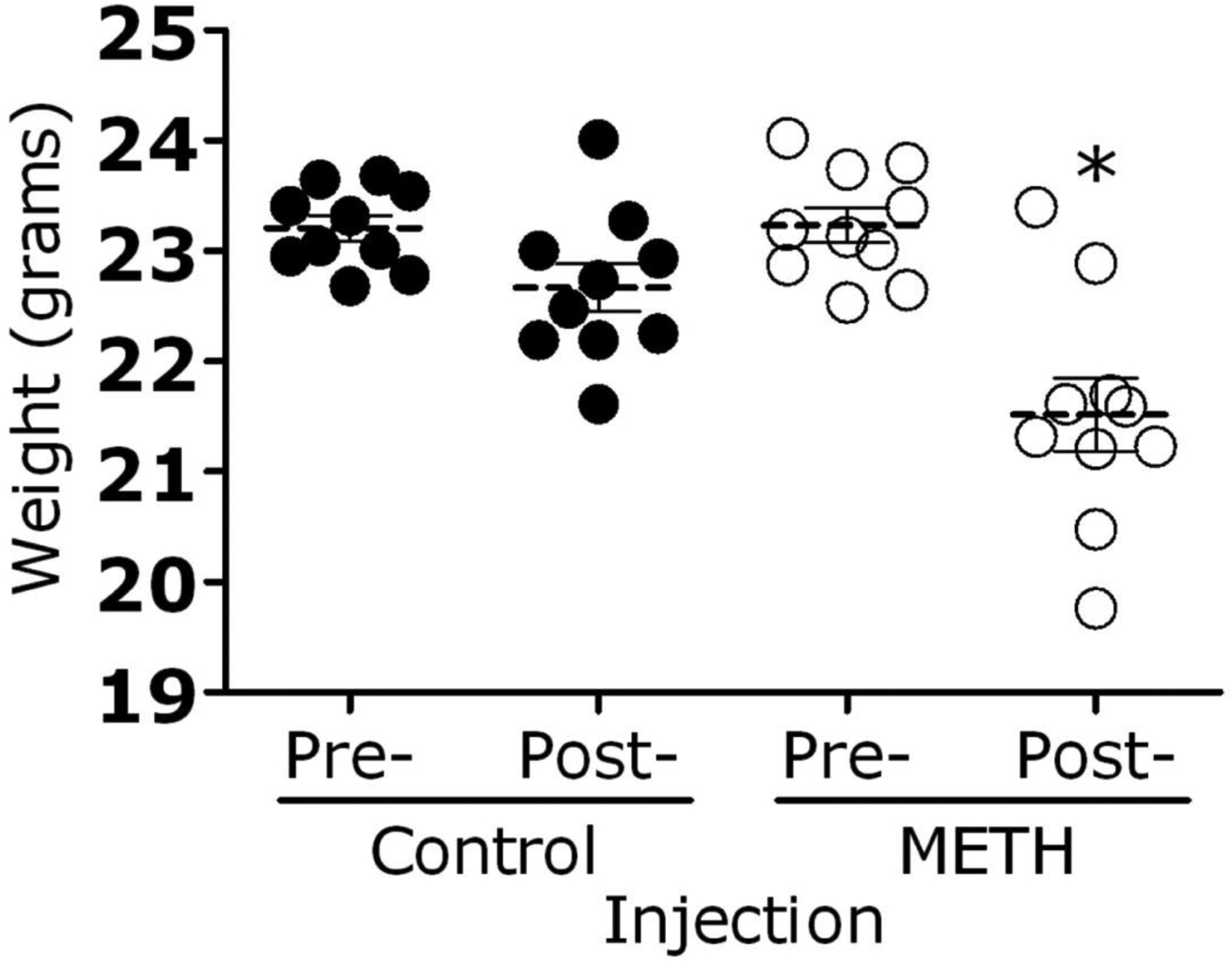
Methamphetamine (meth)–injected mice exhibited weight loss after 10 days of treatment. Mice (10 per group) were injected intraperitoneally with increasing doses (2–10 mg/kg/day) of methamphetamine or phosphate-buffered saline (control). Weight differences were compared by t test. Bars represent the means of 10 measurements, and error bars denote standard error. *P < .05.
Videotape analysis showed that mice injected with methamphetamine were significantly more active than those in the control group. Mice injected with methamphetamine exhibited marked stereotypic behaviors, including running in circles, constant grooming of their heads, standing up on their hind legs, and repetitive head movements. These behaviors were most apparent 5–10 min after methamphetamine injection and continued for several hours (figure 2, which appears only in the electronic version of the Journal).
Figure 2.
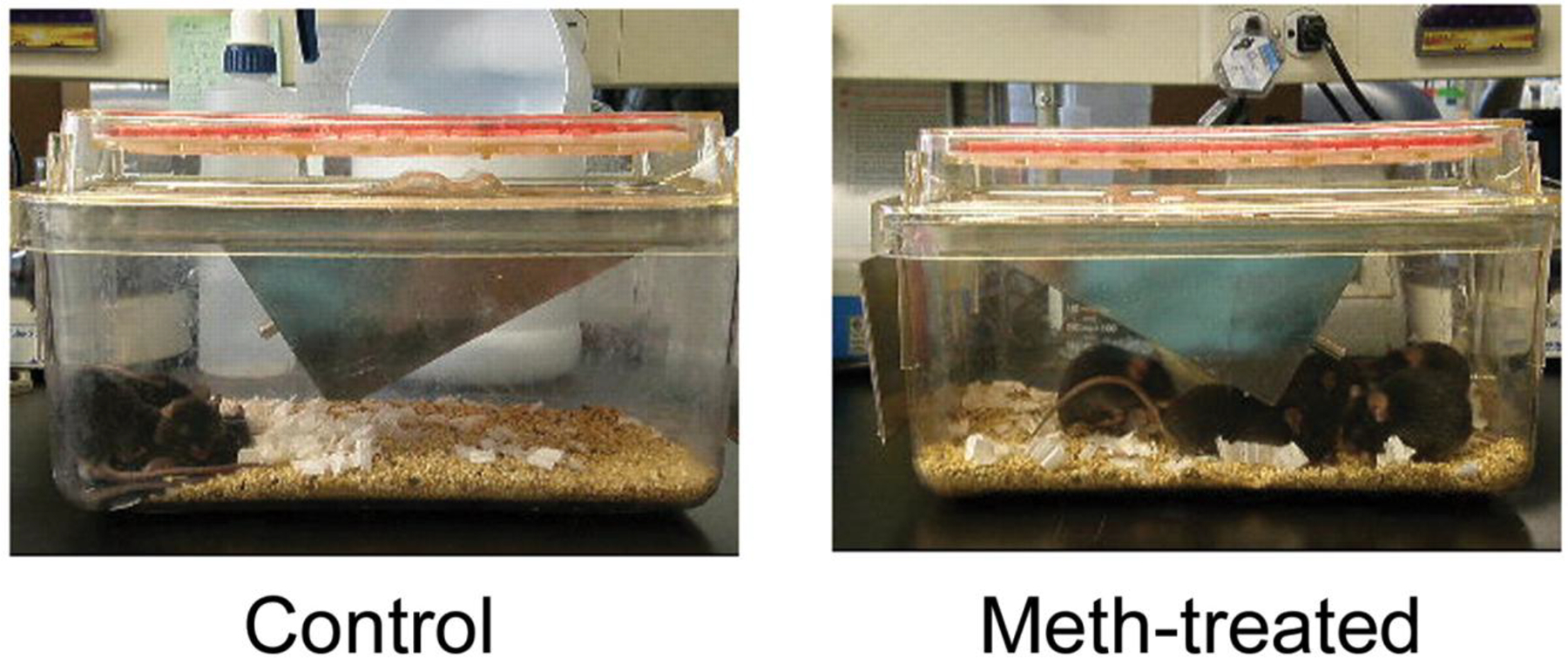
The behavior of methamphetamine (meth)–injected mice was videotaped 20 min after drug administration. Control and meth-injected mice (5 per group) were injected intraperitoneally with phosphate-buffered saline and 10 mg/kg of meth, respectively.
Enhancement of H. capsulatum infection by methamphetamine.
To study the effect of methamphetamine in histoplasmosis, groups of methamphetamine-treated and control C57BL/6 mice were lethally infected with 1.25 × 107 H. capsulatum cells. In this model of histoplasmosis, administration of methamphetamine significantly accelerated the progression to death of H. capsulatum–infected mice relative to control mice (P < .01). On day 14 after infection, 100% of methamphetamine-treated mice had died, compared with 38% of control mice. On average, methamphetamine-treated mice died 13 days after infection, whereas control mice died 16 days after infection (figure 3A).
Figure 3.
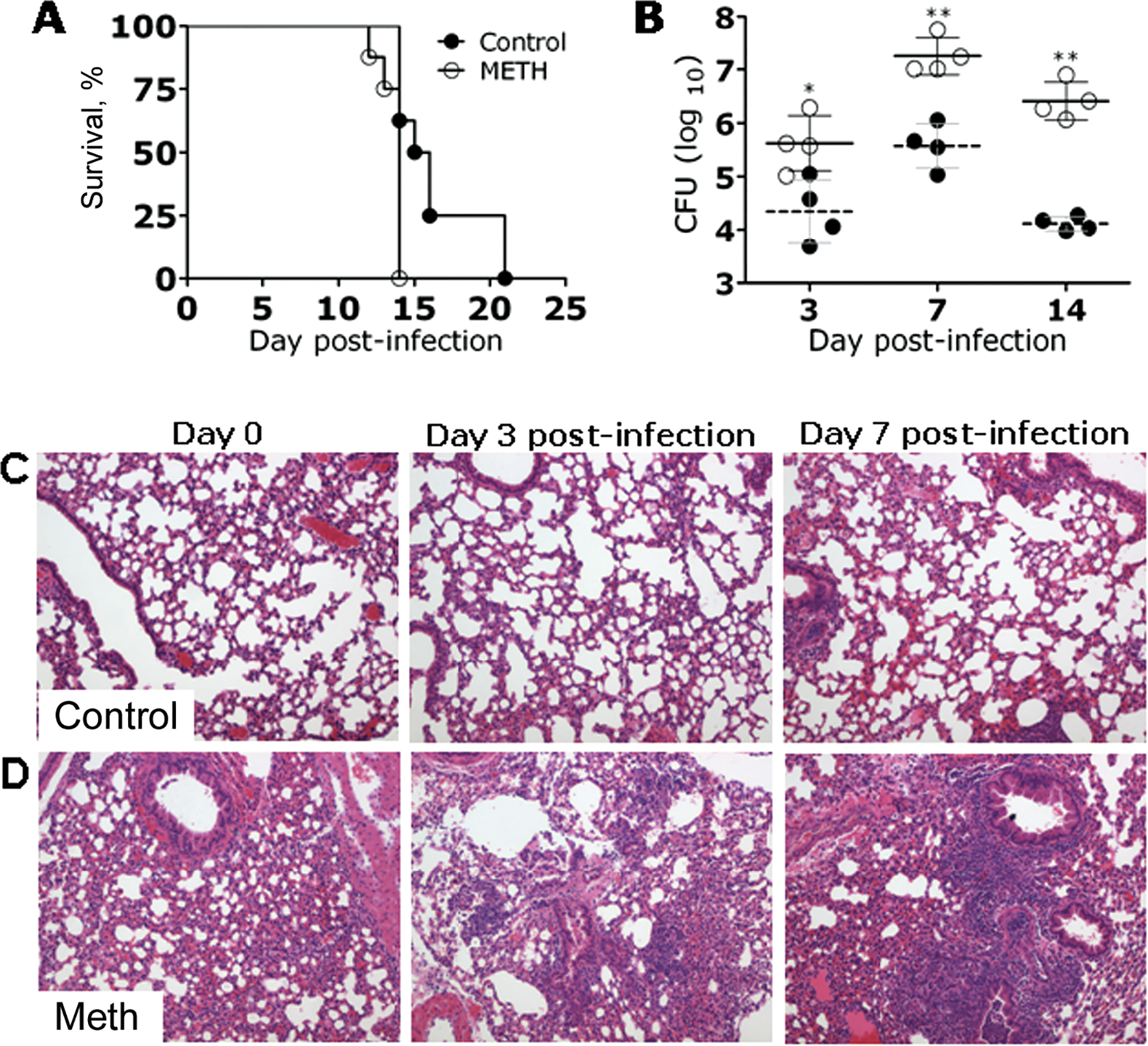
Methamphetamine (meth)–exacerbated histoplasmosis. A, Survival differences between meth-injected and phosphate-buffered saline(PBS)–injected (control) C57BL/6 mice after infection with Histoplasma capsulatum. Mice (8 per group) were infected intranasally with 1.25 × 107 yeast cells. Survival was compared by log-rank test. This experiment was performed twice, with similar results. B, Lung fungal burden in meth-treated mice infected intranasally with 5 × 106 H. capsulatum cells was significantly greater than that in PBS-injected mice (4 per group). Solid and dashed lines represent the mean values for the meth and PBS control groups, respectively; brackets denote standard deviations. Each circle represents 1 mouse. P values were calculated by analysis of variance and adjusted by use of the Bonferroni correction. This experiment was done twice, with similar results. C and D, Histological analysis of the lungs of H. capsulatum–infected PBS- (control) and meth-injected C57BL/6 mice at days 0, 3, and 7 after infection with 5 × 106 yeast cells. Representative hematoxylin and eosin–stained sections of the lungs are shown (original magnification, ×400). *P < .01; **P < .001. CFU, colony-forming units.
We examined the pulmonary fungal burden of mice infected with 5 × 106 H. capsulatum cells. The pulmonary fungal burden in methamphetamine-treated mice was significantly higher than that in control mice (P < .001) (figure 3B). Histological examination revealed that mice treated with methamphetamine prior to sublethal H. capsulatum infection were abnormal, with peribronchial inflammation and inflammatory cells present within alveoli (figure 3D). After infection, methamphetamine-treated mice developed progressive pneumonia, whereas the alveolar spaces of control mice were largely intact during the observed time intervals (figure 3C). At day 3 after infection, methamphetamine-treated mice had bronchointerstitial pneumonia, manifested by edema and perivascular inflammation with thickened alveolar walls, as well as some vascular thrombosis. By day 7 after infection, the disease in methamphetamine-treated mice had progressed to necrotizing inflammation with thickened alveolar walls and substantial neutrophil infiltration. In contrast, the inflammation in control mice was generally localized adjacent to bronchioles, with the alveolar airspaces appearing intact. In summary, these studies demonstrated that methamphetamine administration enhances disease by increasing pulmonary fungal burden and host inflammation.
Alteration of host cytokine expression by methamphetamine.
We measured the cytokine response in the lungs of mice that were sublethally infected with H. capsulatum. At day 7 after infection, lung tissue of infected mice treated with methamphetamine contained significantly higher quantities of TNF-α, IFN-γ, IL-4, IL-10, and transforming growth factor–β than that of control mice (figure 4A, B, D, E, and F). There was a trend toward an increase in IL-12 production in methamphetamine-treated animals, but it was not statistically significant (figure 4C).
Figure 4.
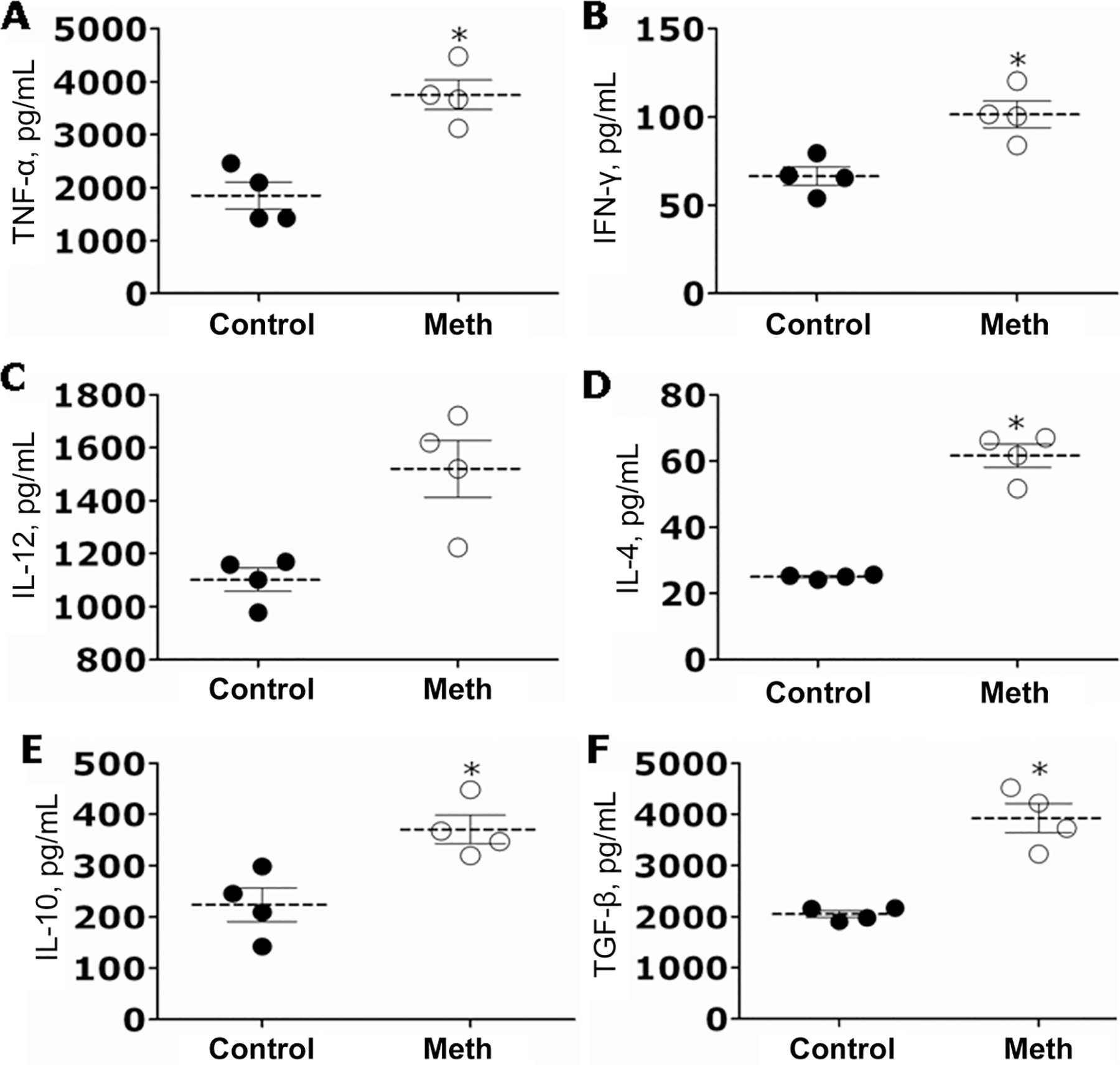
Cytokine profiles in the lung homogenates of control and methamphetamine (meth)–injected mice 7 days after infection with 5 × 106 yeast cells. Tumor necrosis factor (TNF)–α (A), interferon (IFN)–γ (B), interleukin (IL)-12 (C), IL-4 (D), IL-10 (E), and transforming growth factor (TGF)–β (F) levels were measured in lung homogenates derived from meth-injected or phosphate-buffered saline–injected (control) Histoplasma capsulatum–infected mice. Dashed lines represent the mean values for the meth and control groups. Brackets denote standard deviations. Each circle represents 1 mouse. P values were calculated by analysis of variance and adjusted by use of the Bonferroni correction. *P < .01.
Immunosuppression of mice by methamphetamine.
The effects of methamphetamine on T cell activation and the generation of antibodies was investigated using in vitro T cell proliferation assays and measurements of antibodies. For T cell proliferation assays, splenocytes of methamphetamine-treated or control C57BL/6 mice were isolated and exposed to H. capsulatum antigens, M antigen, or rHSP60. Splenocytes from methamphetamine-treated mice were severely limited in their capacity to proliferate in response to activation with 25 μg/mL M antigen (P < .05), 50 μg/mL M antigen (P < .001), or 50 μg/mL rHSP60 (P < .001) (figure 5A). In contrast, splenocytes from control mice demonstrated significantly increased proliferation in the presence of either H. capsulatum antigen. T cell proliferation in methamphetamine-treated mice was inhibited by 1 and 3 fold, compared with control mice, after exposure to 25 and 50 μg/mL of either protein, respectively.
Figure 5.
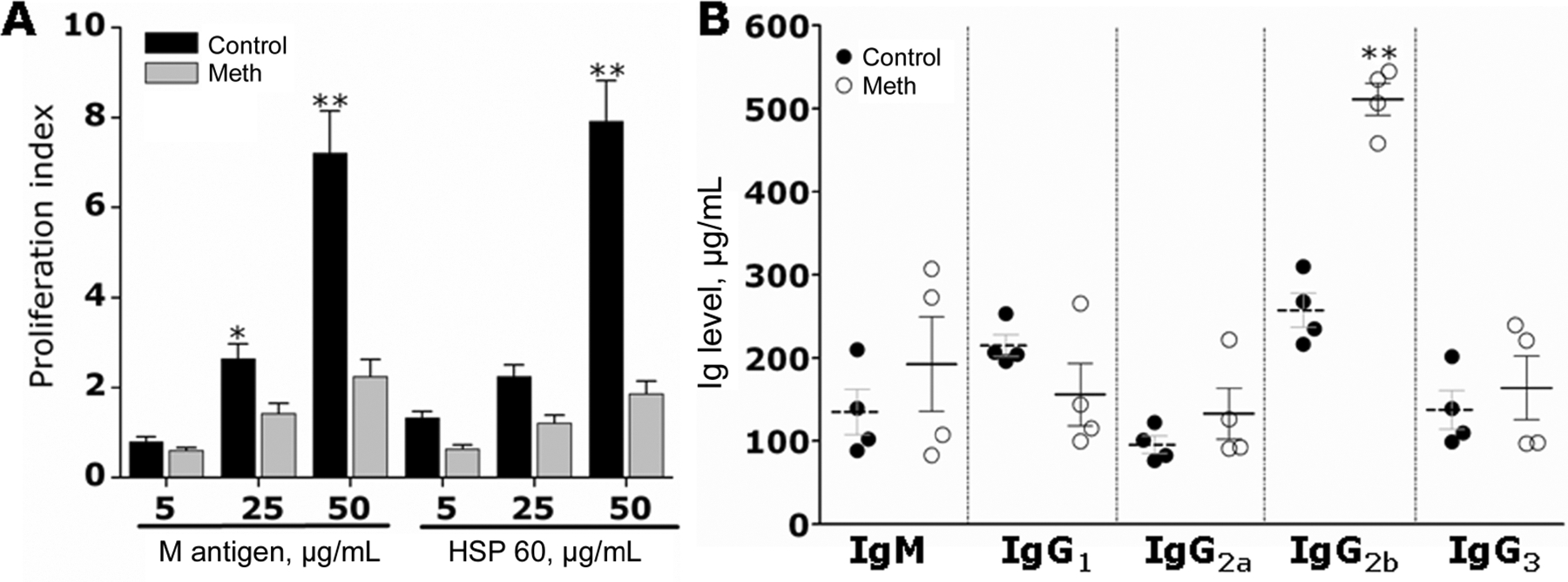
Methamphetamine (meth) inhibits lymphocyte proliferation and alters antibody production in mice infected with 5 × 106 Histoplasma capsulatum cells. A, Meth interferes with lymphocyte proliferation after exposure to M antigen and HSP60 from H. capsulatum. The concentrations used for each protein were 5, 25, and 50 μg/mL. Bars represent the means of 5 measurements, and error bars denote standard deviations. B, Immunoglobulin (Ig) M, IgG1, IgG2a, IgG2b, and IgG3 levels were measured in serum of infected mice. Solid and dashed lines represent the means of the meth and control groups, respectively; brackets denote standard deviations. Each circle represents 1 mouse. P values were calculated by analysis of variance and adjusted by use of the Bonferroni correction. *P < .05; **P < .001.
We investigated the total levels of antibodies after methamphetamine treatment and H. capsulatum infection. The levels of IgM, IgG1, IgG2a, and IgG3 isotypes were not significantly different. However, methamphetamine-treated mice showed higher levels of IgG2b production than did control mice (figure 5B). High levels of IgG2b production have been associated with a nonprotective immune response to histoplasmosis [28]. This finding suggests that methamphetamine induces a suppressive adaptive immune response, possibly through the inhibition of T cell proliferation and dysregulation of antibody production.
Inhibition of macrophage phagocytosis and facilitation of intracellular replication of H. capsulatum by methamphetamine.
We analyzed the effects of methamphetamine on H. capsulatum phagocytosis and killing by J774.16 macrophage-like cells. Methamphetamine (10 and 50 μmol/L) significantly inhibited phagocytosis of H. capsulatum by macrophages, compared with the control (P < .01 for 10 μmol/L methamphetamine and P < .001 for 50 μmol/L methamphetamine) or chloroquine-treated (20 μmol/L) cells (P < .05 for 50 μmol/L methamphetamine), although chloroquine also suppressed the uptake of yeast, compared with the control (figure 6A). Moreover, methamphetamine enhanced the proliferation of fungi within macrophages (figure 6B). Although chloroquine significantly reduced phagocytosis, treated macrophages were able to control the proliferation of H. capsulatum to the same extent as control macrophages. In figure 6C, light microscopy images show the effect of methamphetamine or chloroquine on H. capsulatum phagocytosis by J774.16 cells. The reduction in phagocytosis is most apparent in methamphetamine-treated (50 μmol/L) macrophages, compared with control macrophages.
Figure 6.
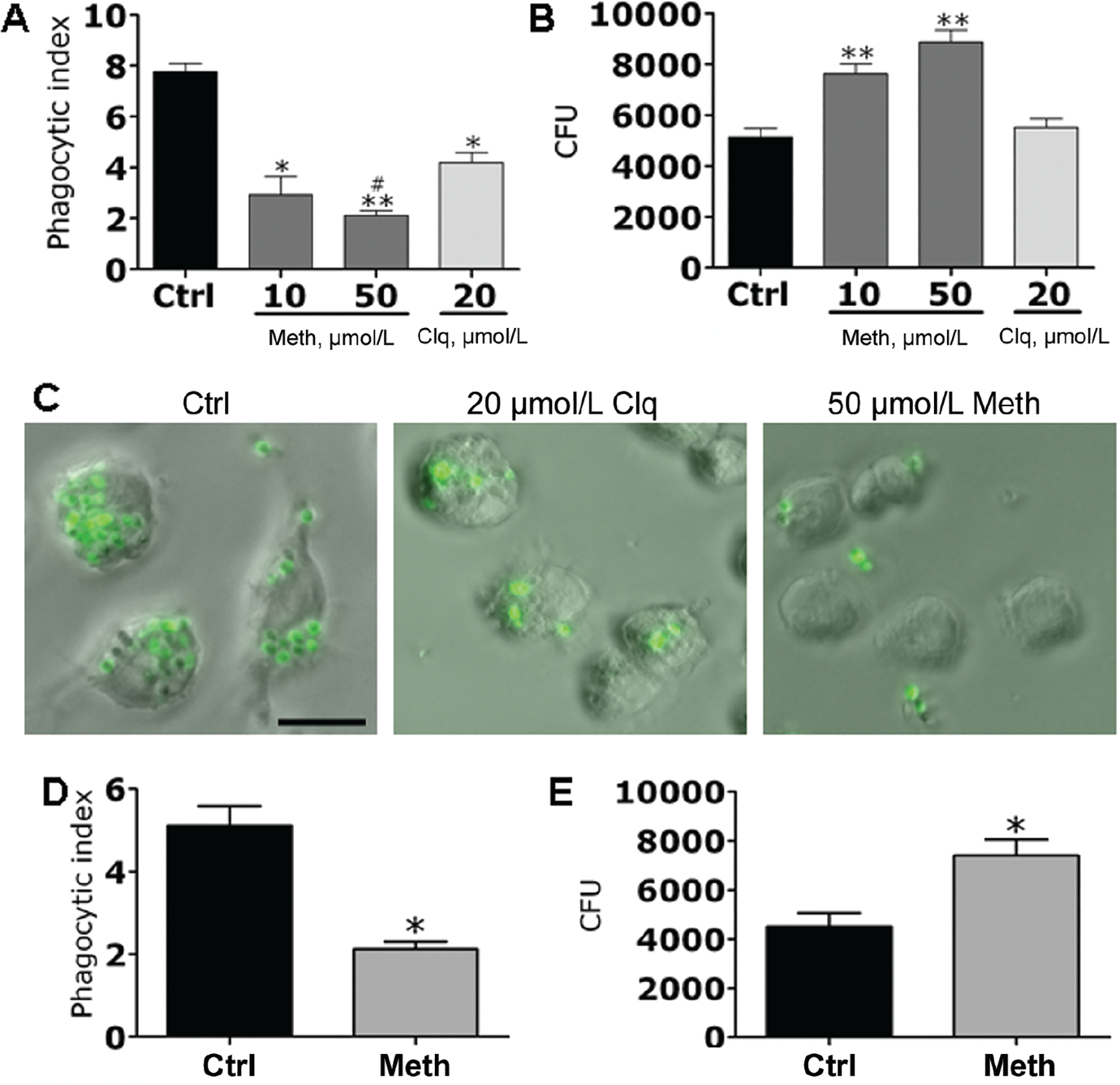
Methamphetamine (meth) inhibits macrophage phagocytosis and augments intracellular replication of Histoplasma capsulatum. A and B, J774.16 cells were exposed to phosphate-buffered saline (PBS; control [ctrl]), chloroquine (clq), or meth for 2 h followed by incubation with H. capsulatum. A, The phagocytic indices (ratio of number of intracellular yeast cells to the number of macrophages counted) were determined after 2 h. B, Colony-forming unit (CFU) determinations were performed after 24 h of incubation. C, Light microscopy images of J774.16 cells impaired in the phagocytosis of green fluorescent protein–labeled H. capsulatum at 2 h after PBS, clq, or meth exposure. The scale bar represents 10 μm. D and E, C57BL/6 mice were injected with PBS or meth for 10 days, followed by isolation of peritoneal macrophages and coculture with H. capsulatum for 2 h. D, The phagocytic indices (ratio of number of intracellular yeast to the number of macrophages counted) were determined after 2 h. E, CFU determinations were performed after 24 h of incubation. For panels A, B, D, and E, the values presented are the means and standard deviations from determinations made in quadruplicate wells (400 cells in each well). Experiments were repeated 3 times with similar results each time. *P < .01 and **P < .001, for comparison of meth and clq groups with untreated controls; #P < .05, for comparison of meth and clq groups by 2-tailed analysis of variance.
To confirm these data in primary macrophages, C57BL/6 mice were treated with methamphetamine for 10 days, and their peritoneal macrophages were isolated. Methamphetamine significantly inhibited phagocytic activity of primary macrophages, compared with their untreated counterparts (figure 6D). In addition, the fungus significantly proliferated in the methamphetamine-treated macrophages (figure 6E). Control experiments showed that, in the absence of macrophages, H. capsulatum proliferation was unaffected by the addition of chloroquine or methamphetamine to brain-heart infusion medium (figure 7, which appears only in the electronic version of the Journal). Moreover, dehydrogenase–release activity assays showed that macrophages were morphologically unaltered after treatment with these drugs (figure 8, which appears only in the electronic version of the Journal).
Figure 7.
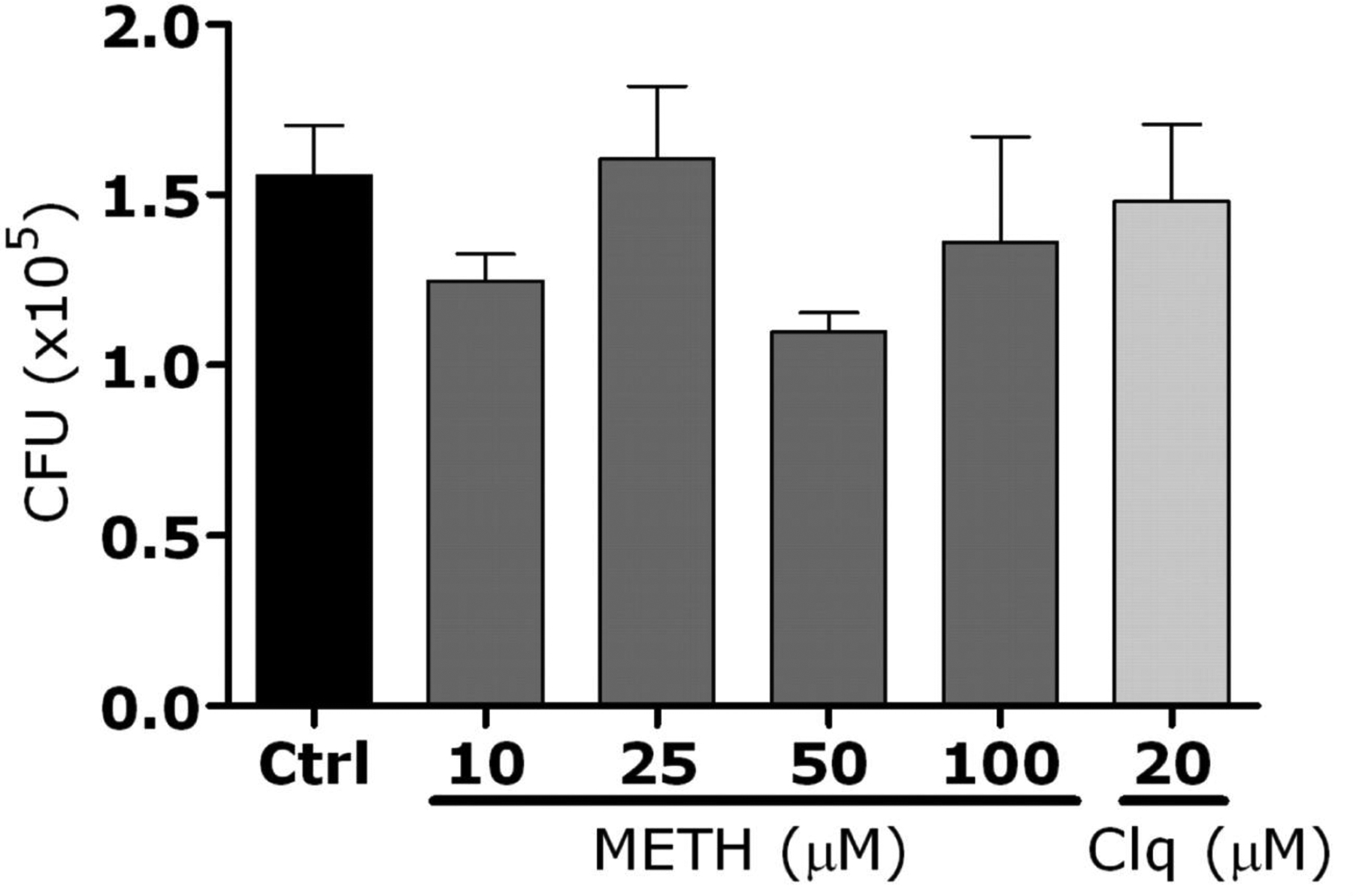
Addition of methamphetamine (meth) or chloroquine (clq) to the medium did not affect Histoplasma capsulatum growth. Yeasts were exposed to phosphate-buffered saline (control [ctrl]), meth (10, 25, 50, or 100 μmol/L), or clq (20 μmol/L) for 24 h. Bars represent the means of 3 measurements, and error bars denote standard errors. Analysis of variance was used to calculate significance and adjusted by use of the Bonferroni correction.
Figure 8.
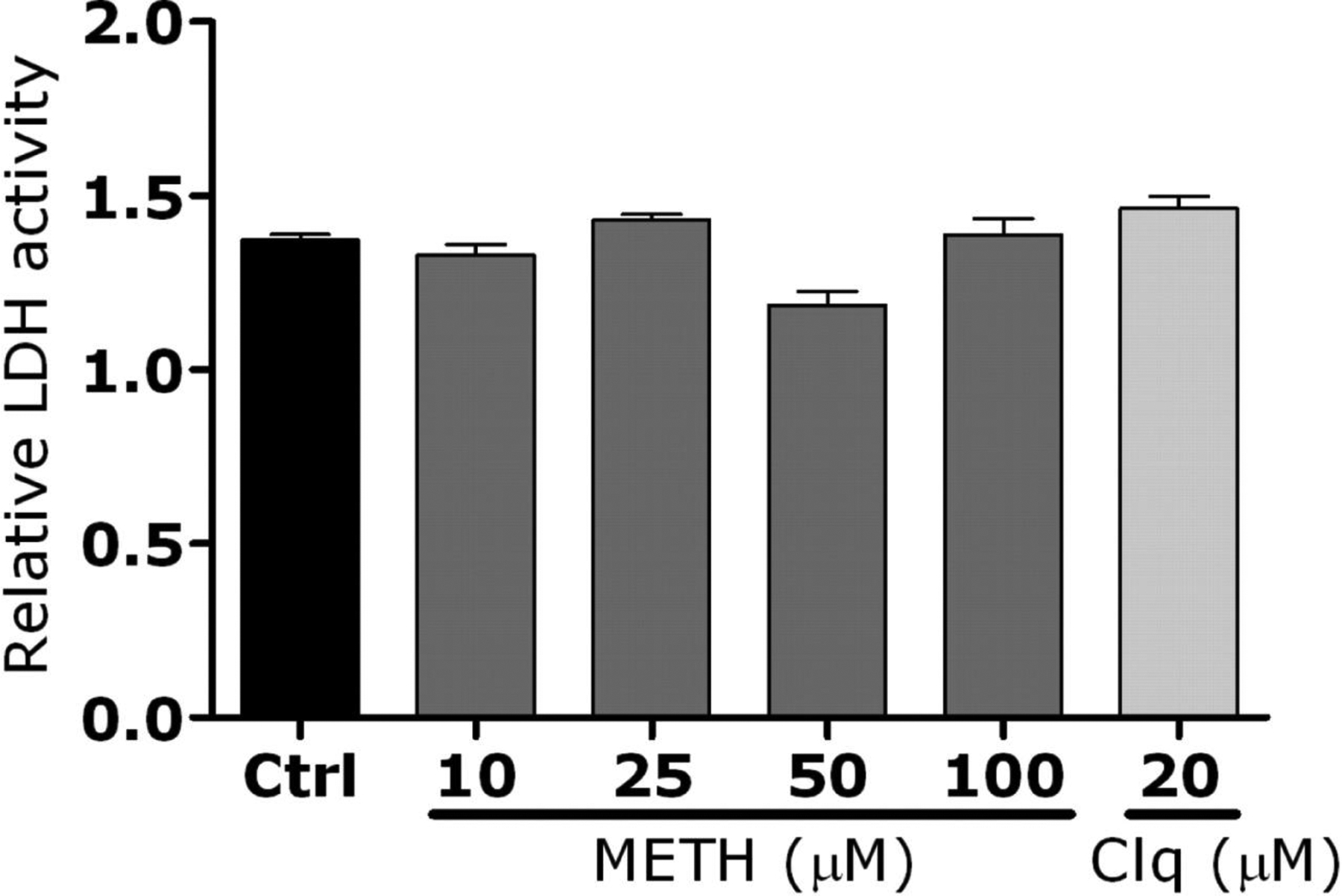
Relative lactate dehydrogenase (LDH) activity measured in primary macrophages culture supernatant after incubation with methamphetamine (meth). Macrophages were exposed to phosphate-buffered saline (control [ctrl]), meth (10, 25, 50, or 100 μmol/L), or chloroquine (clq) (20 μmol/L) for 24 h. Bars represent the means of the results for 3 measurements, and error bars denote standard errors. Analysis of variance was used to calculate significance and adjusted by use of the Bonferroni correction.
Up-regulation of CD14 and CD18 expression on macrophages by methamphetamine.
We investigated whether methamphetamine’s inhibition of macrophage phagocytic activity was in response to alterations in the complement receptor 3 (CD11b/CD18) or CD14 expression levels. Macrophages recognize H. capsulatum via complement receptor 3 for phagocytosis [29], whereas CD14 is involved in host immune responses against fungi [30]. FACS analysis showed that CD14 and CD18 were markedly up-regulated on macrophages treated with methamphetamine or chloroquine, compared with control macrophages. Interestingly, there were no differences in CD11b expression between drug-treated macrophages and control macrophages (figure 9). Furthermore, to test whether methamphetamine affected H. capsulatum–complement receptor 4 interactions, we measured the expression of macrophage CD11c and found that methamphetamine did not affect the expression of CD11c when comparing untreated or chloroquine-treated macrophages (data not shown).
Figure 9.
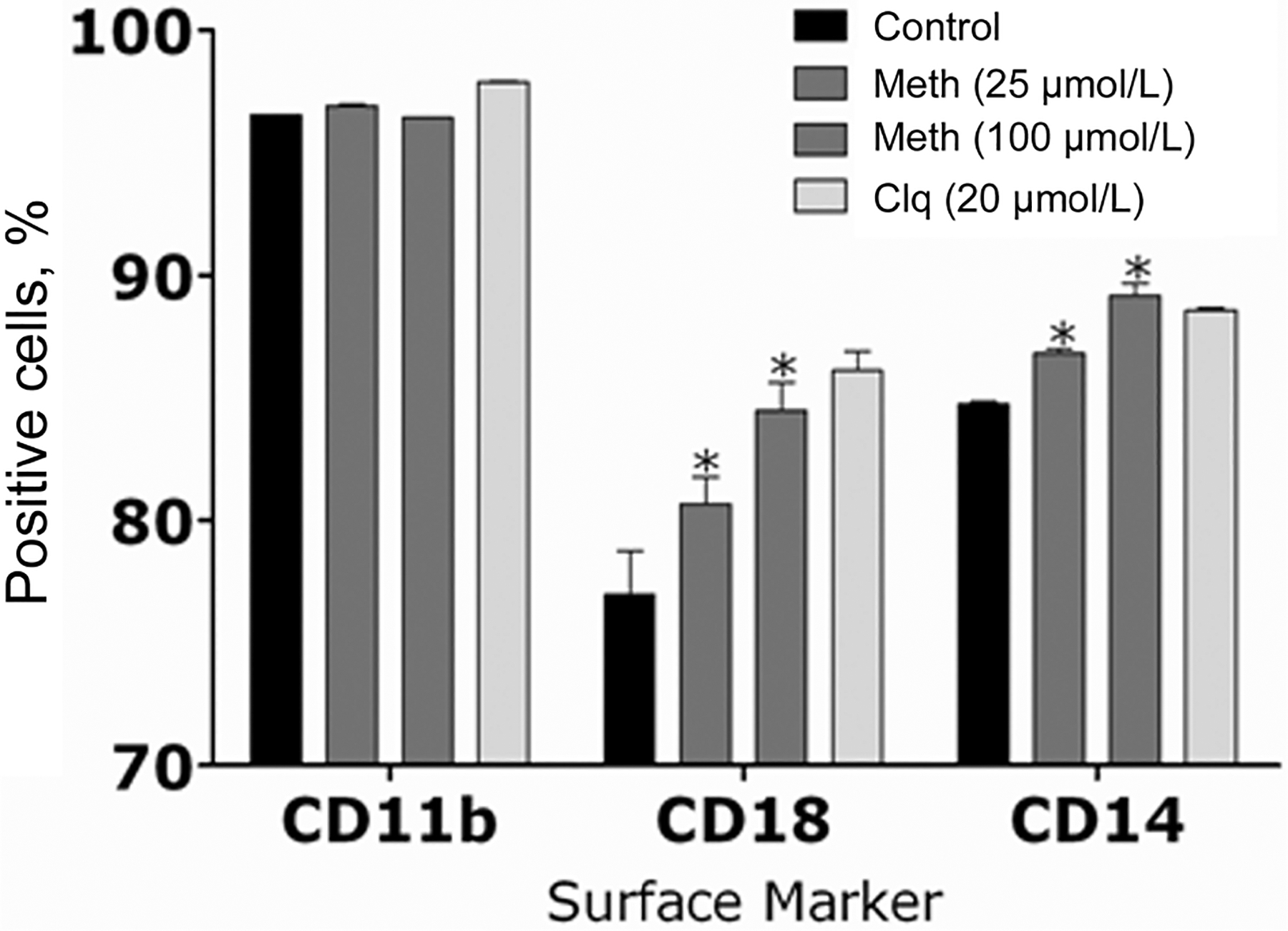
The expression levels of the complement receptor 3 (CR3; CD-11b/CD-18) and CD-14 were analyzed by fluorescence-activated cell sorting analysis. Primary macrophages were isolated and exposed to phosphate-buffered saline (control), methamphetamine (meth) (25 or 100 μmol/L), or chloroquine (clq) (20 μmol/L) for 2 h. Bars represent the means of 3 measurements, and error bars denote standard deviations. P values were calculated by analysis of variance and adjusted by use of the Bonferroni correction. *P < .001.
Induction of the alkalization of macrophage phagosomes that contain H. capsulatum cells by methamphetamine.
Phagosome acidification was studied to elucidate the mechanism by which methamphetamine interferes with H. capsulatum killing after phagocytosis. The acidification of phagosomes of primary macrophages containing H. capsulatum yeast labeled with pH-sensitive and pH-insensitive probes was measured using a spectrofluorometer. Standard curves were generated with the fluorophores (figure 10, which appears only in the electronic version of the Journal). Methamphetamine induces the alkalization of macrophage phagosomes that contain H. capsulatum cells. The pH of phagosomes in macrophages infected with the fungus for 2 h was analyzed. In macrophages treated with methamphetamine (25 or 50 μmol/L) or chloroquine (20 μmol/L), phagosomal pH was elevated (pH, 7.3, 7.7, and 7.5 respectively) significantly, compared with the pH in untreated control macrophages (pH, 5.9; P < .001) (figure 11).
Figure 10.
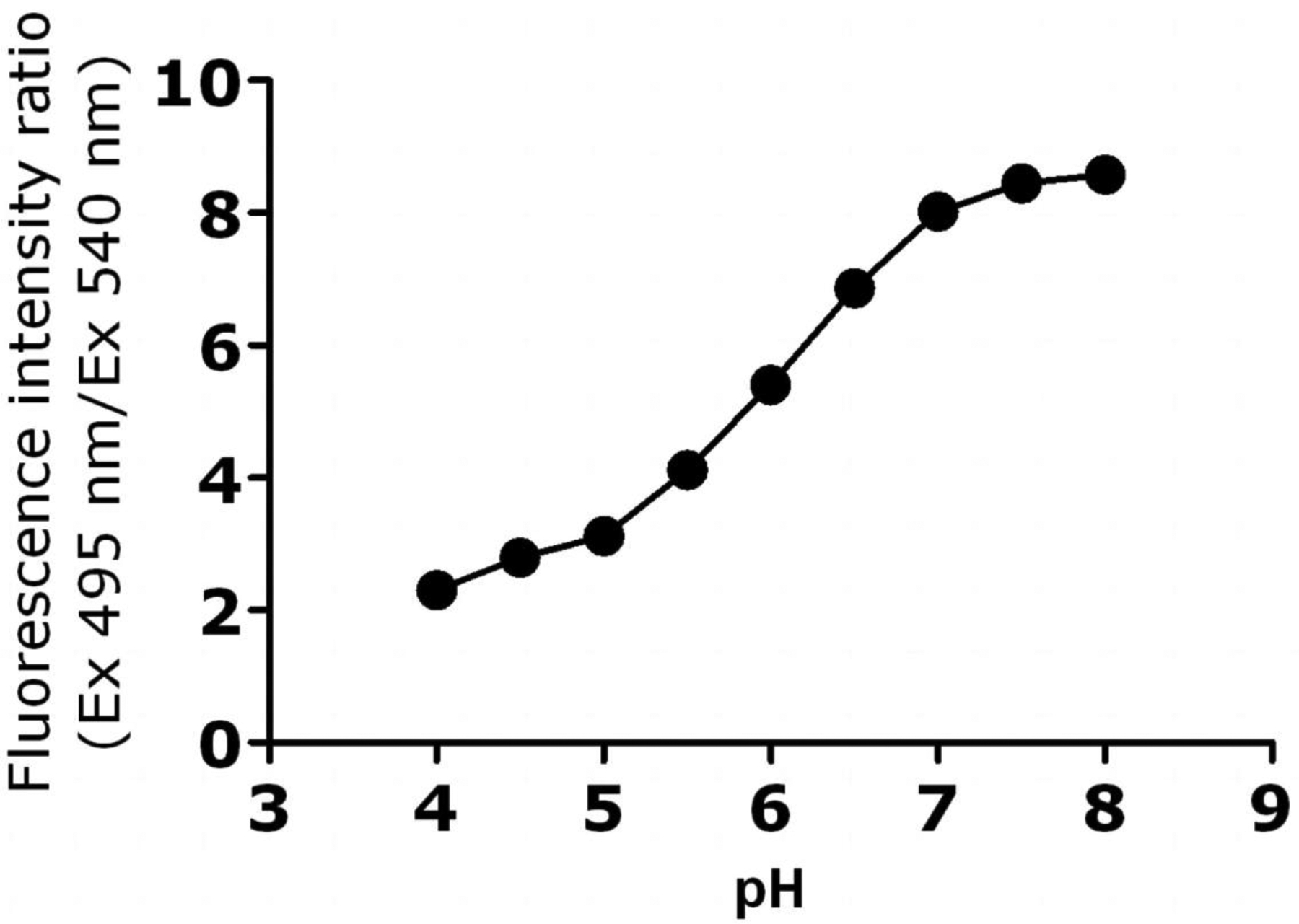
Calibration curve obtained by plotting CF/Rho fluorescent intensity ratios versus pH for primary macrophages. The excitation and emission wavelengths for CF were 495 and 520 nm, whereas for Rho, they were 540 and 580 nm.
Figure 11.
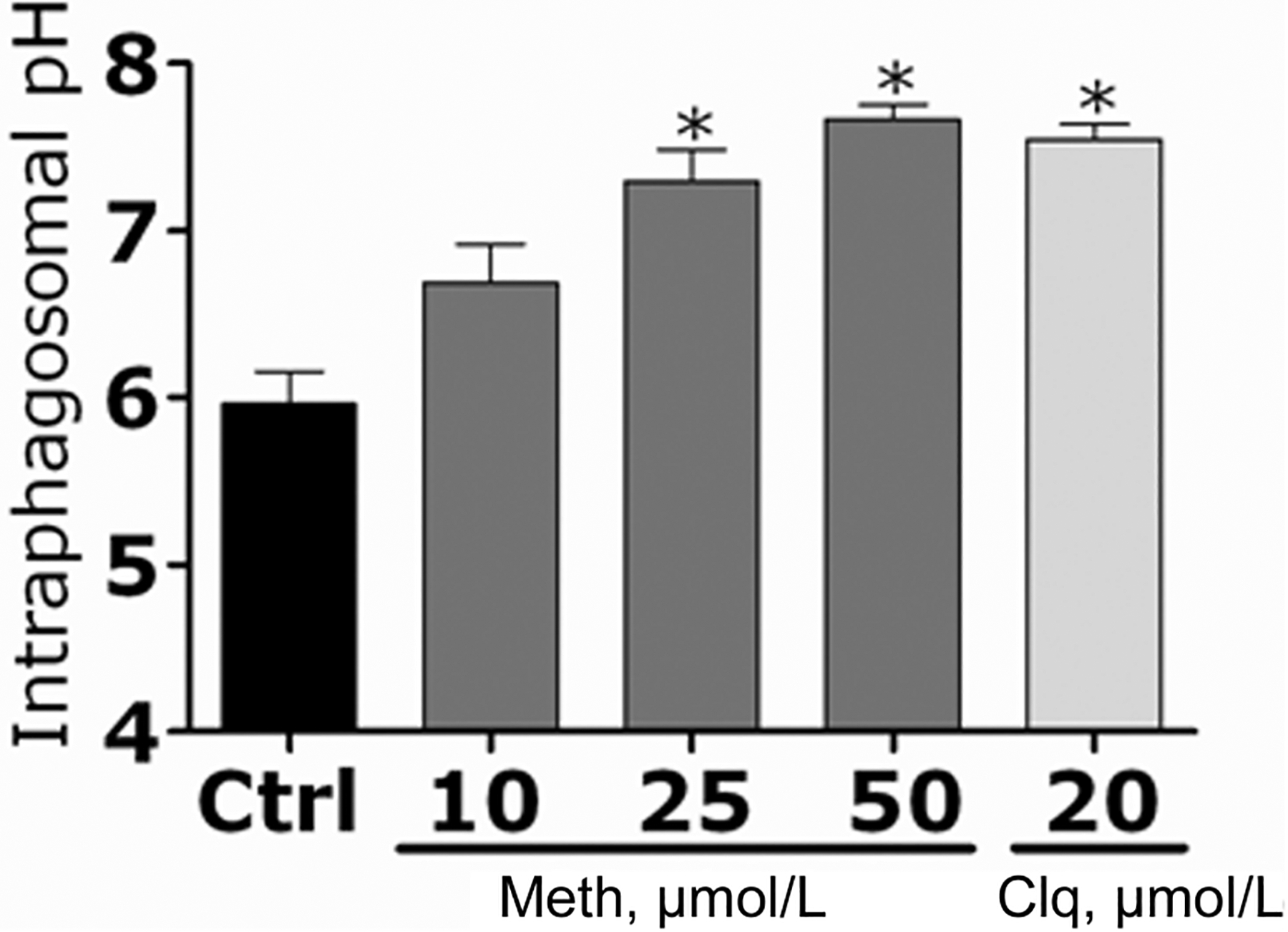
Methamphetamine (meth) causes alkalization of phagosomes in primary macrophages. The pH of phagosomes of primary macrophages that contain Histoplasma capsulatum yeast labeled with pH-sensitive and pH-insensitive probes was measured using a spectrofluorometer after treatment with phosphate-buffered saline (control [ctrl]), meth (10, 25, or 50 μmol/L), or chloroquine (clq) (20 μmol/L). Bars represent the means of 4 measurements, and error bars denote standard deviations. P values were calculated by analysis of variance and adjusted by use of the Bonferroni correction. *P < .001.
Inhibition of NO and O2− production by methamphetamine.
After phagocytosis, macrophages produce NO and O2− in response to pathogens within the phagosome. Therefore, we assessed whether methamphetamine inhibited NO and O2− production in primary macrophages coincubated with H. capsulatum. Macrophages treated with methamphetamine (25 or 50 μmol/L) or chloroquine (20 μmol/L) showed significantly lower levels of NO production after H. capsulatum infection than did control macrophages. Similarly, the release of O2− by primary macrophages was greatly decreased by these drugs (figure 12).
Figure 12.
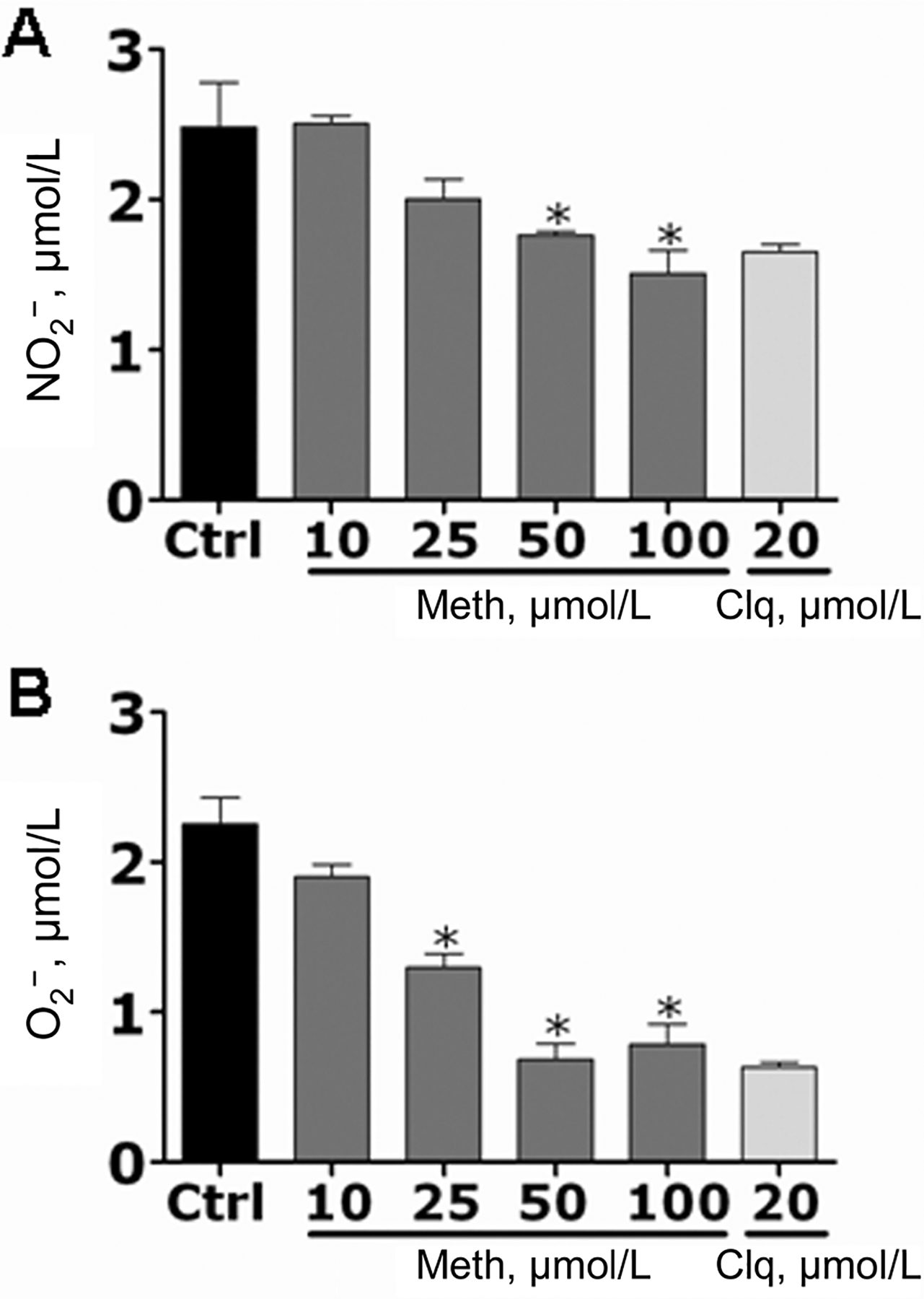
Methamphetamine (meth) inhibits nitric oxide (NO) and superoxide (O2−) production by primary macrophages. NO (A) and O2− (B) production was quantified using the Griess method and by measurement of SOD1 activity, respectively, after primary macrophages were treated with phosphate-buffered saline (control [ctrl]), meth (10, 25, 50, or 100 μmol/L), or chloroquine (clq) (20 μmol/L) and coincubated with Histoplasma capsulatum. Bars represent the means of 4 measurements, and error bars denote standard deviations. P values were calculated by analysis of variance and adjusted by use of the Bonferroni correction. *P < .05.
DISCUSSION
Methamphetamine is a potent central nervous system stimulant, and its abuse causes dramatic detrimental physiological and psychological effects. We developed a murine model to investigate the effects of methamphetamine on the immune system during H. capsulatum infection. Similar to humans who abuse methamphetamine, mice injected with the compound were agitated and displayed marked stereotypic behaviors. The aberrant behavior began shortly after the administration of methamphetamine and continued for several hours. Additionally, methamphetamine-treated mice lost weight with treatment at physiological doses.
During the early phase of histoplasmosis, macrophages recognize and phagocytose H. capsulatum [31], thereby providing the fungus access to a intracellular environment that is permissive for replication. This initial interaction is crucial for the pathogenesis of histoplasmosis. Our results showed that methamphetamine exerts a direct immunosuppressive effect on macrophages that is initially apparent by the reduction in H. capsulatum phagocytosis. In this regard, FACS analysis showed that methamphetamine induces a higher expression of CD18 and CD14 receptors that are involved in the recognition and uptake of the fungus. However, it is possible that methamphetamine has a negative effect on actin polymerization, because actively expressed receptors in the plasma membrane are unable to attach to fungal cells. For efficient attachment of H. capsulatum, receptors must be mobile within the macrophage membrane, which requires intact actin microfilaments of the cellular cytoskeleton [29]. Furthermore, because complement receptor 3 is a heterodimer, there is a possibility that the up-regulation of CD18 without up-regulation of CD11b might affect the interaction of these subunits during H. capsulatum recognition, impairing phagocytosis.
Once inside the phagosome, H. capsulatum is rapidly exposed to the microbicidal armamentarium of the macrophages, which consists of toxic reactive species and lysosomal hydrolases. Macrophage-derived NO and O2− species can inhibit H. capsulatum growth [32]. However, in the absence of antibody, H. capsulatum does not induce NO or O2− release from murine macrophages [33], although increased production of reactive species occurs if macrophages are exposed to IFN-γ [34]. Our data show that free-radical generation is significantly altered in murine macrophages that are exposed to methamphetamine. Impaired production of toxic reactive species might create an ideal environment for fungal survival, facilitating intracellular replication and regulation of the phagolysosomal milieu.
We recently showed that methamphetamine acts as an immunosuppressive agent, because of its inhibition of endosomal acidification in macrophages [18]. These actions reduce antigen presentation and phagocytosis. Normally, maintenance of low phagolysosomal pH upon ingestion of a microbe serves many functions, including pathogen inactivation, regulation of protein degradation, and modulation of surface receptor expression. Our results demonstrate that methamphetamine inhibits phagosomal acidification, preventing intracellular killing of H. capsulatum. This fungus normally survives within phagosomes by regulating the intracellular milieu of macrophages [19–21]. By maintaining a neutral pH in macrophages, H. capsulatum yeast avoids damage by host defenses, such as lysosomal hydrolases. A neutral pH has other beneficial effects for the fungus during infection of macrophages, such as inhibition of intracellular trafficking and antigen presentation [35]. Methamphetamine further destabilizes the capacity of infected macrophages to regulate their intracellular milieu. These effects are expected to be particularly devastating in HIV-infected methamphetamine abusers in regions where this fungus is endemic. Hence, methamphetamine apparently has complex effects on macrophages that result in an intracellular milieu that enables the replication of this pathogenic fungus.
The induction of cell-mediated immunity is required for host defense against H. capsulatum [1]. Our results showed that methamphetamine induces T cells to produce large amounts of IL-4 and IL-10, and the secretion of these cytokines contributes to the inhibition of T cell proliferation [36, 37] after exposure to M antigen and rHSP60 from H. capsulatum. The methamphetamine-induced production of IL-4 and IL-10 likely contributes to enhanced H. capsulatum survival in the host by inhibiting T cell activation and proliferation. Because methamphetamine also impairs humoral immune responses and phagocytic defenses, this could lead to a situation in which the host is left without effective innate or acquired immunologic defenses.
One of the mechanisms by which T cells can cause exacerbation of H. capsulatum infection is, in part, linked to a shift in the immune response from a dominant Th1 to a mixed Th1-Th2 phenotype. Elevated levels of IL-4 and IL-10 have been associated with inhibition of apoptosis [38]. In histoplasmosis, apoptosis of T cells is a protective response, and inhibition of this process greatly intensifies the severity of disease. Furthermore, IL-4 and IL-10 impair the protective immune response to this fungus, even when Th1 cytokines are present [36, 39–42]. In our infection model, we found high levels of endogenous TNF-α and IFN-γ in methamphetamine-treated mice. However, the increased levels of both Th1 cytokines were not sufficient to rescue mice from the lethal effects of H. capsulatum. Thus, methamphetamine might play a role in inducing a dominant nonprotective Th2 response to H. capsulatum.
We study the complex impact of antibodies to H. capsulatum on disease pathogenesis. Recently, we demonstrated that administration of monoclonal antibodies to H. capsulatum before infection alters the intracellular fate of H. capsulatum [43], by affecting its ability to regulate the milieu of the phagosome, conferring protection against the fungus [44]. However, we are aware that antibodies can also be nonprotective or enhance disease, as shown in studies of the encapsulated fungus, Cryptococcus neoformans [45]. Results of the present study demonstrated that methamphetamine induces production of high levels of IgG2b antibodies, an isotype that data from our laboratory suggests can be detrimental to the host in histoplasmosis.
In conclusion, the immunosuppressive effects of methamphetamine are consistent with reports that methamphetamine-treated mice demonstrate decreased immunity [15, 16]. The collapse of the phagosomal pH gradient that is caused by methamphetamine and the resulting decrease of normal immune response provide an explanation for the compromised immunity and exacerbate infections, such as histoplasmosis, that occur in methamphetamine abusers. In fact, methamphetamine is strongly suspected to more dramatically inhibit normal immune responses than other drugs, because methamphetamine users often present with cutaneous infections and “methamphetamine mouth,” a devastating periodontal disease [7, 8, 46].
Acknowledgments
We are grateful to Allan J. Guimaraes for assistance with the FACS and for valuable and constructive criticism.
Financial support:
Molecular pathogenesis training grant (to L.R.M.); National Institutes of Health (grant AI056070-01A2 to J.D.N.).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Deepe GS Jr. Immune response to early and late Histoplasma capsulatum infections. Curr Opin Microbiol 2000;3:359–62. [DOI] [PubMed] [Google Scholar]
- 2.Newman SL. Macrophages in host defense against Histoplasma capsulatum. Trends Microbiol 1999;7:67–71. [DOI] [PubMed] [Google Scholar]
- 3.Allendorfer R, Brunner GD, Deepe GS Jr. Complex requirements for nascent and memory immunity in pulmonary histoplasmosis. J Immunol 1999;162:7389–96. [PubMed] [Google Scholar]
- 4.Zhou P, Seder RA. CD40 ligand is not essential for induction of type 1 cytokine responses or protective immunity after primary or secondary infection with Histoplasma capsulatum. J Exp Med 1998;187:1315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cain JA, Deepe GS Jr. Evolution of the primary immune response to Histoplasma capsulatum in murine lung. Infect Immun 1998;66: 1473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen HL, Deepe GS Jr. B cells and CD4−CD8− T cells are key regulators of the severity of reactivation histoplasmosis. J Immunol 2006;177: 1763–71. [DOI] [PubMed] [Google Scholar]
- 7.Drug and Alcohol Services Information System. Trends in methamphetamine/amphetamine admissions to treatment: 1993–2003. 2006. Available at: http://www.oas.samhsa.gov/2k6/methTX/methTX.htm. Accessed 12 June 2007. [Google Scholar]
- 8.Colfax G, Shoptaw S. The methamphetamine epidemic: implications for HIV prevention and treatment. Curr HIV/AIDS Rep 2005;2:194–9. [DOI] [PubMed] [Google Scholar]
- 9.Urbina A, Jones K. Crystal methamphetamine, its analogues, and HIV infection: medical and psychiatric aspects of a new epidemic. Clin Infect Dis 2004;38:890–4. [DOI] [PubMed] [Google Scholar]
- 10.Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I;HIV Neurobehavioral Research Center Group. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis 2003;188:1820–6. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales R, Marinelli-Casey P, Shoptaw S, Ang A, Rawson RA. Hepatitis C virus infection among methamphetamine-dependent individuals in outpatient treatment. J Subst Abuse Treat 2006;31:195–202. [DOI] [PubMed] [Google Scholar]
- 12.Meth’s impact on HIV epidemic being studied: drug’s use is growing problem among MSMs. AIDS Alert 2005;20:79–80. [PubMed] [Google Scholar]
- 13.Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I. Additive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS Behav 2006;10:185–90. [DOI] [PubMed] [Google Scholar]
- 14.Cherner M, Letendre S, Heaton RK, et al. ; HIV Neurobehavioral Research Center Group. Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology 2005;64: 1343–7. [DOI] [PubMed] [Google Scholar]
- 15.Yu Q, Zhang D, Walston M, et al. Chronic methamphetamine exposure alters immune function in normal and retrovirus-infected mice. Int Immunopharmacol 2002;2:951–62. [DOI] [PubMed] [Google Scholar]
- 16.In SW, Son EW, Rhee DK, Pyo S. Methamphetamine administration produces immunomodulation in mice. J Toxicol Environ Health A 2005;68:2133–45. [DOI] [PubMed] [Google Scholar]
- 17.Mahajan SD, Hu Z, Reynolds JL, Aalinkeel R, Schwartz SA, Nair MP. Methamphetamine modulates gene expression patterns in monocyte derived mature dendritic cells: implications for HIV-1 pathogenesis. Mol Diagn Ther 2006;10:257–69. [DOI] [PubMed] [Google Scholar]
- 18.Tallóczy Z, Martinez J, Joset D, et al. Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathog 2008; 4:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman SL. Macrophages in host defense against Histoplasma capsulatum. Trends Microbiol 1999;7:67–71. [DOI] [PubMed] [Google Scholar]
- 20.Sebghati TS, Engle JT, Goldman WE. Intracellular parasitism by Histoplasma capsulatum: fungal virulence and calcium dependence. Science 2000;290:1368–72. [DOI] [PubMed] [Google Scholar]
- 21.Strasser JE, Newman SL, Ciraolo GM, Morris RE, Howell ML, Dean GE. Regulation of the macrophage vacuolar ATPase and phagosomelysosome fusion by Histoplasma capsulatum. J Immunol 1999;162: 6148–54. [PubMed] [Google Scholar]
- 22.Simon SL, Richardson K, Dacey J, et al. A comparison of patterns of methamphetamine and cocaine use. J Addict Dis 2002;21:35–44. [DOI] [PubMed] [Google Scholar]
- 23.Nosanchuk JD, Steenbergen JN, Shi L, Deepe GS Jr, Casadevall A. Antibodies to a cell surface histone-like protein protect against Histoplasma capsulatum. J Clin Investig 2003;112:1164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zancopé-Oliveira RM, Bragg SL, Hurst SF, Peralta JM, Reiss E. Evaluation of cation exchange chromatography for the isolation of M glycoprotein from histoplasmin. J Med Vet Mycol 1993;31:29–41. [PubMed] [Google Scholar]
- 25.Gomez FJ, Allendoerfer R, Deepe GS Jr. Vaccination with recombinant heat shock protein 60 from Histoplasma capsulatum protects mice against pulmonary histoplasmosis. Infect Immun 1995;63:2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi L, Albuquerque PC, Lazar-Molnar E, et al. A monoclonal antibody to Histoplasma capsulatum alters the intracellular fate of the fungus in murine macrophages. Eukaryot Cell 2008;7:1109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steenbergen JN, Nosanchuk JD, Malliaris SD, Casadevall A. Interaction of Blastomyces dermatitidis, Sporothrix schenckii, and Histoplasma capsulatum with Acanthamoeba castellanii. Infect Immun 2004;72: 3478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guimarães AJ, Williams DA Sr, Zancope-Oliveira RM, Nosanchuk JD. Protective antibodies to histoplasma capsulatum cell surface antigens: HSP60 and recombinant M antigen [abstract B35]. In: Program and abstracts of the American Society of Microbiology Conference on Dimorphic Fungal Pathogens. Washington DC: American Society for Microbiology, 2006:42. [Google Scholar]
- 29.Newman SL, Bucher C, Rhodes J, Bullock WE. Phagocytosis of Histoplasma capsulatum yeasts and microconidia by human cultured macrophages and alveolar macrophages: cellular cytoskeleton requirement for attachment and ingestion. J Clin Invest 1990;85:223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yauch LE, Mansour MK, Shoham S, Rottman JB, Levitz SM. Involvement of CD14, toll-like receptors 2 and 4, and MyD88 in the host response to the fungal pathogen Cryptococcus neoformans in vivo. Infect Immun 2004;72:5373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deepe GS Jr, Gibbons RS, Smulian AG. Histoplasma capsulatum manifests preferential invasion of phagocytic subpopulations in murine lungs. J Leukoc Biol 2008;84:669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaffner A, Davis CE, Schaffner T, Markert M, Douglas H, Braude AI. In vitro susceptibility of fungi to killing by neutrophils granulocytes discriminates between primary pathogenecity and opportunism. J Clin Invest 1986;78:511–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eissenberg LG, Goldman WE. Histoplasma capsulatum fails to trigger release of superoxide from macrophages. Infect Immun 1987;55:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf JE, Kerchberger V, Kobayashi GS, Little JR. Modulation of the macrophage oxidative burst by Histoplasma capsulatum. J Immunol 1987;138:582–6. [PubMed] [Google Scholar]
- 35.Eissenberg LG, Goldman WE, Schlesinger PH. Histoplasma capsulatum modulates the acidification of phagolysosomes. J Exp Med 1993;177: 1605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding L, Shevach EM. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol 1992;148:3133–9. [PubMed] [Google Scholar]
- 37.Gildea LA, Gibbons R, Finkelman FD, Deepe GS Jr. Overexpression of interleukin-4 in lungs of mice impairs elimination of Histoplasma capsulatum. Infect Immun 2003;71:3787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen HL, Deepe GS Jr. Apoptosis modulates protective immunity to the pathogenic fungus Histoplasma capsulatum. J Clin Invest 2005;115: 2875–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allendoerfer R, Deepe GS Jr. Blockade of endogenous TNF-α exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J Immunol 1998;160:6072–82. [PubMed] [Google Scholar]
- 40.Allendoerfer R, Biovin GP, Deepe GS Jr. Modulation of immune responses in murine pulmonary histoplasmosis. J Infect Dis 1997;175: 905–14. [DOI] [PubMed] [Google Scholar]
- 41.Zhou P, Sieve MC, Bennett J, et al. IL-12 prevents mortality in mice infected with Histoplasma capsulatum through induction of IFN-γ. J Immunol 1995;155:785–95. [PubMed] [Google Scholar]
- 42.Deepe GS Jr, Gibbons RS. Protective and memory immunity to Histoplasma capsulatum in the absence of IL-10. J Immunol 2003;171: 5353–62. [DOI] [PubMed] [Google Scholar]
- 43.Nosanchuk JD, Steenbergen JN, Shi L, Deepe GS Jr, Casadevall A. Antibodies to a cell surface histone-like protein protect against Histoplasma capsulatum. J Clin Investig 2003;112:1164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi L, Albuquerque PC, Lazar-Molnar E, et al. A monoclonal antibody to Histoplasma capsulatum alters the intracellular fate of the fungus in murine macrophages. Eukaryot Cell 2008;7:1109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan RR, Spira G, Oh J, Paizi M, Casadevall A, Scharff MD. Isotype switching increases efficacy of antibody protection against Cryptococcus neoformans infection in mice. Infect Immun 1998;66:1057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.The Partnership for a Drug-Free America Partnership for a Drug-Free America. 2007. Available at: http://www.drugfree.org/. Accessed 4 August 2008. [Google Scholar]


