Highlights
-
•
Antibodies (Abs) were a more sensitive indicator of transmission than antigen (Ag).
-
•
Higher seroprevalence of Bm14 Ab, Bm33 Ab, and Wb123 Ab compared to Ag was found.
-
•
Ab seroprevalence was significantly higher among older males in suspected hotspots.
-
•
Significant clustering of Ab seropositivity was seen at the household-level.
-
•
Further research is needed to define Ab thresholds for active versus past infection.
Keywords: Lymphatic filariasis, Antifilarial antibodies, Seroprevalence, Age-specific associations, Samoa
Abstract
Objectives
Circulating filarial antigen (Ag) is used by elimination programs to monitor lymphatic filariasis (LF) transmission; however, antifilarial antibodies (Ab) may be more sensitive than Ag for detecting LF. Our objectives were to describe Ab seroprevalence, identify risk factors for Ab seropositivity, investigate age-specific associations between Ag and Ab, and evaluate geographic clustering of seropositivity.
Methods
Community-based serosurveys of participants aged ≥5 years were conducted in 35 primary sampling units (PSUs). Ag-positivity was detected using Alere™ Filariasis Test Strips and Ab-seropositivity using multiplex bead assays. Seroprevalence was adjusted for study design.
Results
Of 3795 participants (range:5-90 years), adjusted prevalence for Ag, Bm14 Ab, Wb123 Ab, and Bm33 Ab were 3.7% (n=117), 20.3% (n=583), 32.2% (n=987), and 51.0% (n=1659), respectively. Male sex, older age, and residents of suspected hotspots had higher odds of seropositivity to all seromarkers. Seroprevalence was lower in 5-9-year-olds vs ≥10-year-olds (P<0.001). Clustering was significantly higher in households (intra-cluster correlation for Ag:0.45; Bm14 Ab:0.32; Bm33 Ab:0.31; Wb123 Ab:0.29) compared to PSUs or region.
Conclusions
Abs enabled identification of risk factors for seropositivity and geographical clustering to inform targeted interventions for LF programmes. Further research is needed to define Ab thresholds for active versus past infection and elimination targets.
Introduction
Lymphatic filariasis (LF), a mosquito-borne helminth infection caused by the filarial nematodes Wuchereria bancrofti, Brugia malayi, and Brugia timori, is an important cause of chronic disability globally. Multiple demographic and environmental risk factors for LF have been identified, which vary geographically due to the behavioural versatility and diversity of both the mosquito vectors and filarial species. In the Western Pacific Region, where filarial worms are diurnal sub-periodic [1], known risk factors include male sex [2], increasing age [2,3], lower household socioeconomic status [4], and living in a known hotspot [5].
The Global Programme to Eliminate LF (GPELF) was launched by the World Health Organization (WHO) in 2000 with the aim to (i) interrupt transmission through mass drug administration (MDA) of anthelminthic medicines, and (ii) control morbidity of affected populations by 2020 [6]. Since its start, the number of LF infections has reduced by 74% globally and as of 2022, 19/72 endemic countries have achieved elimination of LF as a public health problem [7]. New milestones and targets beyond 2020 are being developed, outlined in the WHO Neglected Tropical Diseases Roadmap 2030, which aims for 58/72 (81%) of countries to have eliminated LF by 2030 [8]. It further proposes that all countries complete their MDA programmes and implement post-MDA or post-validation surveillance by 2030 [9].
Despite multiple MDA rounds, several countries including Samoa have reported persistent foci of LF transmission. Resurgence has also been reported by countries that have reached very low antigen (Ag) prevalence based on Transmission Assessment Surveys (TAS) of young children [10]. Measuring antibodies (Ab) to LF Ags (including Wb123 Ab, Bm33 Ab, and Bm14 Ab) may be more sensitive than Ag testing alone [11], particularly in areas with low population-level prevalence of microfilariae (Mf) and Ag, which make the identification of residual positive cases increasingly challenging [11]. Antifilarial Abs can be detected before Ag and Mf [12], providing earlier indication of infection and allowing for earlier intervention. Testing serum for LF Abs using serologic techniques such as multiplex bead assays (MBA), which measure Ab seroprevalence to multiple diseases simultaneously, also meets integrated approaches to epidemiologic surveillance of neglected tropical diseases as stressed in the WHO Neglected Tropical Diseases Roadmap 2030 [8].
Samoa, a tropical island in the Western Pacific Region, remains endemic for LF despite continued elimination efforts since 1965 [13]. Samoa underwent 8 national MDA rounds using diethylcarbamazine and albendazole between 1999 and 2011, and 2 targeted MDA rounds in 2015 and 2017 [14]. A nationwide TAS was conducted in 2013, in which 2 of 3 evaluation units (EU) passed target thresholds, and a second TAS took place in 2017 where all 3 EUs failed [14]. In 2017, Samoa prepared a National Action Plan to Eliminate LF, and in August 2018, Samoa was the first country in the world to distribute nationwide triple-drug MDA (ivermectin, diethylcarbamazine, albendazole [IDA]) [14]. A second round of IDA MDA was planned in 2019 but was delayed due to a measles outbreak in 2019 and the global COVID-19 pandemic. It was subsequently delivered in September 2023.
The Surveillance and Monitoring to Eliminate LF and Scabies from Samoa (SaMELFS) project is an ongoing operational research program to monitor and evaluate the effectiveness of triple-drug MDA on LF transmission [14]. The SaMELFS 2018 survey took place immediately following national distribution of IDA; this study found a 3-fold higher Ag prevalence among participants ≥10 years old compared to those 5-9 years old, significantly higher Mf prevalence among participants ≥10 years old compared to those 5-9 years old, and significant clustering of Ag and Mf at the household level relative to the primary sampling unit (PSU) and regional level [14]. This current study aims to evaluate the utility of Ab seroprevalence estimates as a surveillance tool for LF transmission. The objectives of the study were to (i) describe the seroprevalence of Bm14 Ab, Bm33 Ab, and Wb123 Ab from the SaMELFS 2018 human survey, (ii) identify risk factors for Ab seropositivity, (iii) investigate age-specific associations between Ag and Ab, and (iv) evaluate any geographic clustering of seropositive participants.
Methods
Data source
The SaMELFS study design has been described elsewhere [14]. Briefly, a community-based sero-survey of participants ≥5 years old was conducted in 2018 in 35 PSUs consisting of either 1 or 2 villages. Thirty PSUs were randomly selected and 5 PSUs were purposively selected by the Ministry of Health as ‘suspected LF hotspots’ based on local knowledge and high prevalence from previous surveys [15]. In addition, a convenience survey of 5-9-year-old children was conducted in each PSU. Each participant completed a questionnaire to collect demographic information and participation in previous MDA programs and had a finger prick sample of up to 400μL of blood collected into heparin microtainers. Ag-positivity was detected using Alere™ Filariasis Test Strips (FTS) (Abbott, Scarborough, ME), and thick blood smears were prepared from Ag-positive samples to detect Mf.
Multiplex bead assay
Dried blood spots (DBS) were eluted into 96-well plates and then diluted to a final concentration of 1:400 [16]. All samples were tested using MBA for antifilarial Ab to Bm14, Bm33, and Wb123 [17]. Sample plates were read on a Bio-Plex 200 instrument (Bio-Rad, Hercules, CA). For internal quality control purposes, 4 controls were used for the MBA analyses: a buffer blank containing the assay buffer only (in order to subtract any background noise), 2 pools of reference sera from known Ab-positives, and finally a negative control serum with known negative LF status. For each antigen, the mean plus 3 standard deviations of the median fluorescence intensity-background (MFI-bg) of a panel of 81 individuals from non-endemic regions were used to determine a threshold for seropositivity.
Definition of subgroups used in the analysis
Participants were considered (i) Ag-positive if they had a positive FTS result, (ii) Ab-positive if they were seropositive to at least one Ab, and (iii) LF-positive if they were seropositive to Ag and/or any Ab.
Statistical analysis
Data were analyzed using Stata (StataCorp, Version 17.0, College Station, TX). Summary statistics with 95% confidence intervals (95% CI) for seroprevalence was calculated for age groups (5-9 years old vs ≥10 years old), gender (male or female), PSU selection (randomly selected vs purposively selected), and region (Apia Urban area [AUA], Northwest Upolu [NWU], Rest of Upolu [ROU], and Savai'i [SAV]). Where overall Ab and Ag prevalence were reported in all 35 PSUs, summary statistics were reported without 95% CIs.
Adjustment for selection probability and clustering has been described previously [14]. Briefly, weighting for selection probability and clustering were based on the 2016 Samoa Census [18] and performed using the ‘svyset’ command in Stata with PSU as the unit of clustering. Age group and gender standardized weights were applied using information from the 2016 Samoa Census [18]. Prevalence estimates for the 2 main age groups were adjusted for selection probability and clustering and standardized for gender but not age. The prevalence estimates for all ages ≥5 years were adjusted for selection probability and clustering and standardized for gender (except when comparing between genders) and age using 5-year age bands.
Prevalence ratios were used to compare the seroprevalence of poly-seropositivity with Ag and Ab by PSU selection and age group with differences tested using Fisher's Exact test. P-values were adjusted for multiple comparisons using the ‘qqvalue’ package in Stata. Associations between Ag and Ab prevalence at the PSU level in participants 5-9 years old and participants ≥5 years old were investigated using Spearman's correlation coefficient (Stata command ‘pwcorr’). Logistic regression was used to assess associations between demographic and behavioural data and LF positivity. Variables with P<0.2 on univariable analyses were tested using multivariable logistic regression; variables were sequentially removed from the multivariable models to arrive at the most parsimonious models, in which variables with P<0.05 were retained. Geographic clustering at the household, PSU, and regional levels were assessed using intra-cluster correlation coefficient (ICC) estimated using a multi-level logistic regression model (Stata commands ‘melogit’ and ‘estat icc’) adjusting for age and sex. Intra-cluster correlation assumes that observations in a cluster are more similar (homogenous) than observations outside a cluster, therefore observations within a cluster are correlated and those outside a cluster are independent [19]. ICC values range from 0 to 1 and measure how similar observations are; a higher ICC indicates that the outcome measure is more homogeneous (there is higher degree of clustering at that level).
Results
Sample inclusion and exclusion
Of 4420 participants recruited in 2018; 480 aged <5 years were excluded. The remaining 3940 participants aged ≥5 years had DBS collected for MBA, of whom 89 had no results available (reasons included low bead count, no consent to use samples for additional testing, and duplicate samples). A further 56 participants without an FTS result were excluded (reasons included invalid FTS results, insufficient blood), giving a total sample of 3795 participants included in the current study (please refer to Supplementary Figure 1).
Study population demographics
The mean participant age was 20.7 years (range: 5-90 years); 51.2% were female and 40.9%, 19.2%, 22.9%, and 17.0% were from NWU, ROU, SAV, and AUA, respectively. Fourteen percent were recruited from purposively selected PSUs. Overall, 39.8% of participants aged 5-9 years were recruited via the convenience survey. There were no significant differences in demographic characteristics between purposively selected and randomly selected PSUs (Supplementary Table 1). However, significantly more participants from purposively selected PSUs had taken MDA in the past (80.7% vs 62.6%; P<0.001) and had spent their whole life in Samoa (95.0% vs 88.3%; P<0.001). No purposive PSUs were selected from AUA.
Unadjusted seroprevalence of Ag and Ab
Overall, 117 (3.1%) participants were Ag-positive, 1889 (49.8%) seropositive for at least one Ab, and 1892 (49.9%) were LF-positive. The most prevalent Ab was Bm33 Ab (43.7%), followed by Wb123 Ab (26.0%), and Bm14 Ab (15.4%). Of 3277 participants from randomly selected PSUs (1641 aged 5-9 years, 1636 aged ≥10 years), 1573 (48%) were LF-positive (37.1% [n=608] aged 5-9 years and 59.0% [n=965] aged ≥10 years). Of 518 participants from purposively selected PSUs (255 aged 5-9 years, 263 aged ≥10 years) 319 (61.6%) were LF-positive (47.1% [n=120] aged 5-9 years and 75.7% [n=199] aged ≥10 years).
Adjusted seroprevalence by PSU selection, age, and sex
Adjusted Ag, Bm14 Ab, Wb123 Ab, and Bm33 Ab prevalence in all 35 PSUs were 3.7%, 20.3%, 32.2%, and 51.0%, respectively. Significantly more participants from purposively selected PSUs were seropositive for Ag (P<0.001), Bm14 Ab (P<0.001), Bm33 Ab (P=0.02), and Wb123 Ab (P=0.01) compared to randomly selected PSUs. Significantly higher prevalence of Ag and individual Abs was seen among participants aged ≥10 years compared to those aged 5-9 years. There were no significant differences in prevalence of Ag, Ab, and LF-positivity when 5-9-year-olds in purposively and randomly selected PSUs were compared. However, participants aged ≥10 years from purposively selected PSUs had significantly higher prevalence of Ag (P=0.001), Bm14 Ab (P=0.003), Bm33 Ab (P=0.05), and Wb123 Ab (P=0.01) (Table 1 and Supplementary Figure 2).
Table 1.
Antibody and antigen prevalence by age (adjusted for sampling design and standardised by sex) and PSU (adjusted for survey design and standardised by age and sex), Samoa 2018.
| Total (N=3795) | Overall |
Purposively selected PSUs |
Randomly selected PSUs |
P-value 1 | P-value 2 | P-value 3 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total testing positive^ |
Total testing positive^ |
Age 5-9 years old testing positive* |
Age ≥10 years old testing positive* |
Total testing positive^ |
Age 5-9 years old testing positive* |
Age ≥10 years old testing positive* |
|||||||||||||||||
| N | % | N | % | 95% CI | N | % | 95% CI | N | % | 95% CI | N | % | 95% CI | N | % | 95% CI | N | % | 95% CI | ||||
| Ag | 117 | 3.7 | 31 | 10 | (7.4-13.4) | 4 | 2.1 | (1.0-4.3) | 27 | 11.4 | (7.9-16.1) | 86 | 3.5 | (2.4-5.0) | 24 | 1.3 | (0.8-2.1) | 62 | 4.1 | (2.7-6.3) | <0.001 | 0.28 | 0.001 |
| Bm14 Ab | 583 | 20.3 | 125 | 36.4 | (31.9-41.1) | 24 | 9.6 | (4.9-17.4) | 101 | 37.8 | (30.5-45.7) | 458 | 19.8 | (16.3-23.9) | 123 | 6.8 | (4.8-9.7) | 335 | 22.2 | (17.1-28.2) | <0.001 | 0.38 | 0.003 |
| Bm33 Ab | 1659 | 51.0 | 297 | 66.6 | (57.1-74.8) | 111 | 41.3 | (30.3-53.3) | 186 | 67.2 | (56.8-76.2) | 1362 | 50.5 | (44.8-56.2) | 509 | 30.3 | (24.8-36.5) | 853 | 54.3 | (47.2-61.2) | <0.001 | 0.08 | 0.048 |
| Wb123 Ab | 987 | 32.2 | 184 | 47.9 | (42.4-53.4) | 49 | 18.6 | (15.8-21.4) | 135 | 49.7 | (41.5-57.9) | 803 | 31.7 | (25.9-38.0) | 265 | 15.6 | (11.6-20.7) | 538 | 34.4 | (27.3-42.3) | <0.001 | 0.30 | 0.012 |
| Any Ab | 1889 | 57.8 | 317 | 70.7 | (61.1-78.7) | 119 | 44.4 | (34.3-55.0) | 198 | 72.0 | (61.4-80.6) | 1572 | 57.4 | (51.2-63.4) | 608 | 36.3 | (30.5-42.6) | 964 | 61.2 | (53.5-68.4) | <0.001 | 0.18 | 0.11 |
| Any Ag or Ab | 1892 | 57.9 | 319 | 71.7 | (61.3-80.2) | 120 | 45.1 | (34.9-55.8) | 199 | 72.9 | (61.8-81.6) | 1573 | 57.4 | (51.2-63.4) | 608 | 36.4 | (30.5-42.6) | 965 | 61.3 | (53.5-68.5) | <0.001 | 0.15 | 0.09 |
Adjusted for sampling design and standardised by sex.
Adjusted for survey design and standardised by age and sex. Ag: Antigen; Ab: Antibody; CI: Confidence Interval; PSU: Primary Sampling Unit.
P-value 1: Testing for significant differences in prevalence between all study participants 5-9 years old and ≥10 years old; P-value 2: Testing for significant differences in prevalence between participants 5-9 years old in randomly and purposively selected PSUs; P-value 3: Testing for significant differences in prevalence between participants ≥10 years old in randomly and purposively selected PSUs.
Significantly higher prevalence of Bm14 Ab (P<0.001) and Wb123 Ab (P<0.001) were seen in males compared to females, but not for Bm33 Ab, Ag or LF-positivity. Significantly more males from purposively selected PSUs were Ag (P<0.001), Bm14 Ab (P=0.001), Bm33 Ab (P=0.01), and Wb123 Ab-positive (P=0.02) compared to males from randomly selected PSUs. Similarly, more females from purposively selected PSUs were positive for Bm14 Ab (P=0.02), and Wb123 Ab (P=0.05) compared to females from randomly selected PSUs (Table 2).
Table 2.
Antibody, antigen, and microfilariae prevalence by sex (adjusted for sampling design and standardised by age), Samoa 2018.
| Total population |
Purposively selected PSUs |
Randomly selected PSUs |
P-value 1 | P-value 2 | P-value 3 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male |
Female |
Male |
Female |
Male |
Female |
||||||||||||||
| N | % | N | % | N | % | 95% CI | N | % | 95% CI | N | % | 95% CI | N | % | 95% CI | ||||
| Ag | 68 | 4.5 | 49 | 2.9 | 17 | 11.7 | (10.3-13.3) | 14 | 8.2 | (4.5-14.5) | 51 | 4.2 | (3.0-5.8) | 35 | 2.8 | (1.4-5.3) | 0.17 | <0.001 | 0.06 |
| Bm14 Ab | 323 | 25.1 | 260 | 15.3 | 65 | 44.8 | (42.5-47.0) | 60 | 27.6 | (18.9-38.3) | 258 | 24.5 | (19.8-29.9) | 200 | 14.8 | (11.7-18.6) | <0.001 | 0.001 | 0.02 |
| Bm33 Ab | 825 | 53.9 | 834 | 48.0 | 134 | 69.2 | (62.8-74.9) | 163 | 63.9 | (51.3-74.8) | 691 | 53.5 | (47.1-59.7) | 671 | 47.4 | (40.5-54.4) | 0.37 | 0.01 | 0.08 |
| Wb123 Ab | 545 | 37.5 | 442 | 26.5 | 91 | 55.4 | (49.1-61.5) | 93 | 40.1 | (30.6-50.4) | 454 | 37.0 | (30.7-43.9) | 349 | 25.9 | (20.2-32.7) | <0.001 | 0.02 | 0.045 |
| Any Abs | 957 | 61.5 | 932 | 53.9 | 146 | 73.3 | (68.3-77.8) | 171 | 67.9 | (53.6-79.5) | 811 | 61.2 | (54.9-67.2) | 761 | 53.4 | (46.1-60.6) | 0.09 | 0.02 | 0.16 |
| Any Ag or Ab | 960 | 61.6 | 932 | 53.9 | 148 | 75.3 | (68.7-81.0) | 171 | 67.9 | (53.6-79.5) | 812 | 61.2 | (54.9-67.2) | 761 | 53.4 | (46.1-60.6) | 0.08 | 0.01 | 0.16 |
Ag: Antigen; Ab: Antibody; CI: Confidence Interval; PSU: Primary Sampling Unit.
P-value 1: Testing for significant differences between sex in all participants included in the study; P-value 2: Testing for significant differences between in prevalence between male participants in randomly and purposively selected PSUs; P-value 3: Testing for significant differences in prevalence between female participants in randomly and purposively selected PSUs.
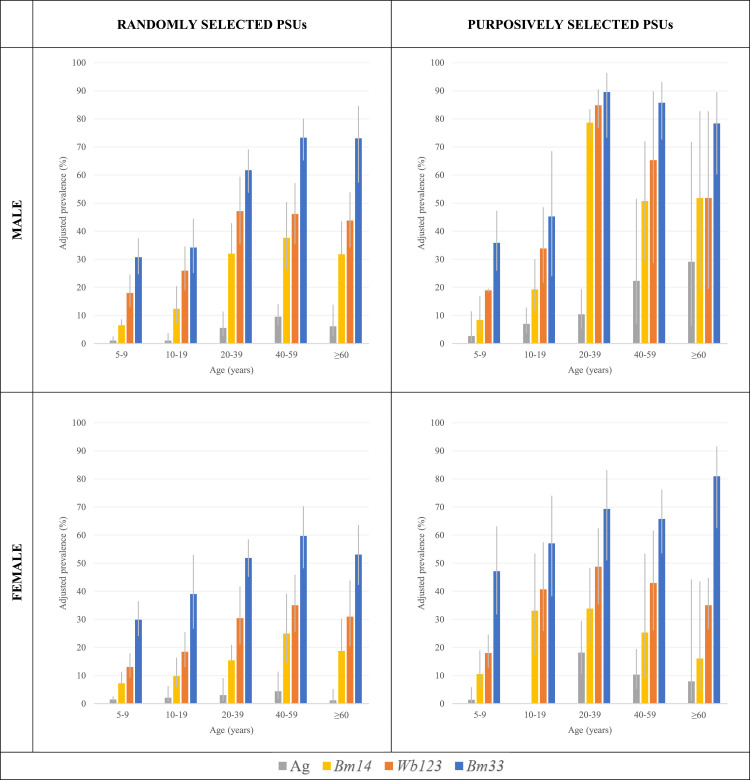
The lowest prevalence of Ag and all 3 Ab were among participants aged 5-9 years irrespective of gender or PSU selection. For males and females from randomly selected PSUs, the highest prevalence of Bm14 Ab (37.7% & 25.0%), Bm33 Ab (73.3% & 59.7%), and Ag (9.6% & 4.4%) were seen among 40-59-year-olds, respectively. The highest prevalence of Wb123 Ab was seen among 40-59-year-old females (35.0%) and 20-39-year-old males (47.2%). Among males from purposively selected PSUs, seroprevalence of Bm33 Ab (89.6%), Wb123 Ab (84.8%), and Bm14 Ab (78.7%) was highest among males aged 20-39 years, though Ag prevalence (29.1%) was highest among ≥60-year-olds. Among females from purposive PSUs, Wb123 Ab (48.8%) and Ag (18.2%) were highest among 20-39-year-olds, Bm14 Ab (33.1%) among 10-19-year-olds, and Bm33 Ab (80.9%) among ≥60-year-olds (Figure 1)
Figure 1.
Prevalence of antigen and antibody among males from randomly (top left) and purposively selected PSUs (top right), and females from randomly (bottom left) and purposively selected PSUs (bottom right) adjusted for survey design, Samoa 2018.
Patterns of poly-seropositivity with Ag and Ab by age and PSU selection
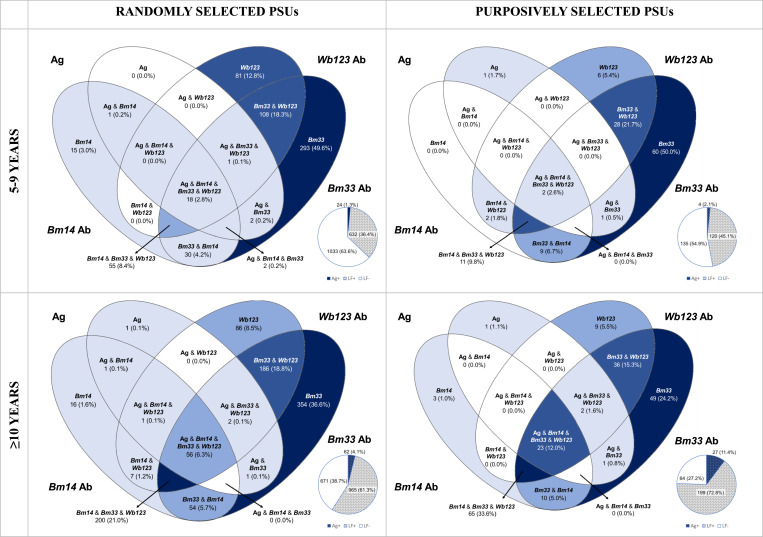
For all 1892 LF-positive participants, patterns of poly-seropositivity differed by age group (5-9 years vs ≥10 years) and PSU selection (randomly selected vs purposively selected). Overall, 0% of 5-9-year-olds from randomly selected PSUs tested positive to Ag only, 64.0% (n=389) to a single Ab, 23.0% (n=140) to 2 Ab, 0.5% (n=3) to Ag and one other Ab, 0.5% (n=3) to Ag and 2 Ab, 9.0% (n=55) to all 3 Ab, and 3.0% (n=18) to all seromarkers. For ≥10-year-olds from randomly selected PSUs, 0.1% (n=1) tested positive to Ag only, 47.3% (n=456) to a single Ab, 25.6% (n=247) to 2 Ab, 0.2% (n=2) to Ag and one other Ab, 0.3% (n=3) to Ag and 2 Ab, and 20.7 (n=200) to all 3 Ab, and 5.8% (n=56) to all seromarkers. Among 5-9-year-olds from purposively selected PSUs, 0.8% (n=1) tested positive to Ag only, 55.0% (n=66) to a single Ab, 32.5% (n=39) to 2 Ab, 0.8% (n=1) to Ag and one other Ab, 0% to Ag and 2 Ab, 9.2% (n=11) to all 3 Ab, and 1.7% (n=2) to all seromarkers. Lastly, 0.5% (n=1) of ≥10-year-olds from purposively selected PSUs tested positive to Ag only, 30.7% (n=61) to a single Ab, 23.1% (n=46) to 2 Ab, 0.5% (n=1) to Ag and one other Ab, 1.0% (n=2) to Ag and 2 Ab, 32.7% (n=65) to all 3 Ab, and 11.6% (n=23) to all seromarkers. Figure 2 presents a Venn diagram of poly-seropositivity of all LF-positive participants by age and PSU selection, adjusted for survey design.
Figure 2.
Venn diagrams to represent the prevalence among 1892 LF-positive participants by age and PSU selection, adjusted for survey design, Samoa 2018. Pie charts indicate the total individuals testing LF-positive (hashed slice) and LF-negative (white slice). The blue slice indicates the total LF-positive individuals who tested Ag-positive.
Significantly more participants aged 5-9 years from randomly selected PSUs were seropositive for Bm33 Ab only (P<0.001) and Wb123 Ab only (P=0.04) compared to ≥10-year-olds in randomly selected PSUs. However, significantly fewer participants aged 5-9 years in randomly selected PSUs were seropositive for all 3 Abs only (P<0.001) and for all Abs and Ag (P=0.05) compared to ≥10-year-olds in randomly selected PSUs. In purposively selected PSUs, significantly more 5-9-year-olds were seropositive for Bm33 Ab only (P<0.001), but fewer seropositives for all 3 Abs only (P<0.001) and all Abs and Ag (P=0.01). Significantly more 5-9-year-olds in randomly selected PSUs were seropositive for Wb123 Ab only (P=0.04) compared to 5-9-year-olds in purposively selected PSUs. Significantly more participants aged ≥10 years in purposively selected PSUs were seropositive for all 3 Ab only (P=0.004) and all Ab and Ag (P=0.03), and fewer were seropositive for Bm33 Ab only (P=0.01) when compared to those aged ≥10 years in randomly selected PSUs (Supplementary Table 2).
Seroprevalence by region and randomly selected PSU
Seroprevalence to seromarkers did not differ significantly by region (Supplementary Figure 3 and Supplementary Table 3). The highest seroprevalence of Bm14 Ab (23.6%), Wb123 Ab (36.6%), and Bm33 Ab (54.9%) were seen in participants from NWU. Among participants aged ≥10 years, 26.7%, 39.8%, and 59.0% of participants were seropositive to Bm14 Ab, Wb123 Ab, and Bm33 Ab, respectively in NWU. The highest seroprevalence among 5-9-year-old participants was seen in ROU, where 10.3%, 20.2%, and 36.9% of participants were Bm14 Ab, Wb123 Ab, and Bm33 Ab seropositive, respectively. The highest Ag prevalence among participants was found in AUA (4.4%), for participants aged 5-9 years in ROU (1.8%), and for participants aged ≥10 years in AUA (5.1%). Ag-positive participants were found in 28/35 PSUs, ranging from 1 to 13 positive participants per PSU. PSU-level Ag prevalence ranged from 0.2% to 20.0%. A higher proportion of participants aged ≥10 years were Ag-positive compared to those aged 5-9 years (range 0.0-18.7% vs 0.0-9.9%). Ab-positive participants were identified in all 35 PSUs. Participants aged 5-9 years were seropositive for Bm14 Ab in 31/35 PSUs, for Wb123 Ab in 34/35 PSUs, and for Bm33 Ab in 35/35 PSUs (Supplementary Figure 4).
Associations between seroprevalence in 5-9-year-olds and overall prevalence
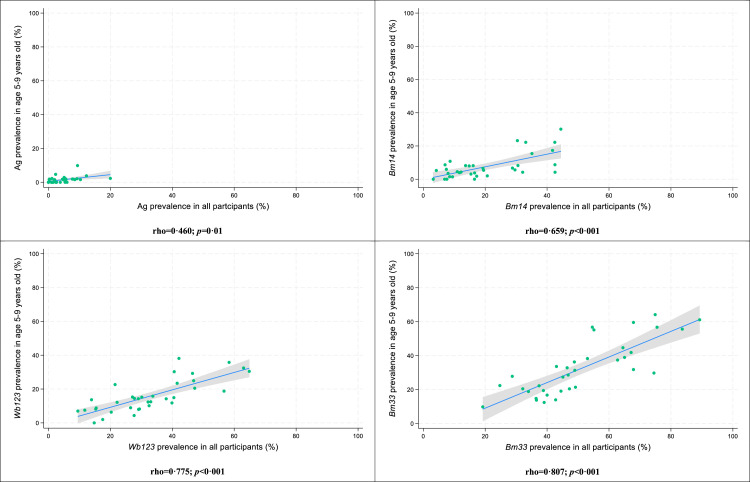
Significant associations but poor correlation was seen between prevalence at the PSU level among participants aged 5-9 years and participants ≥5 years (Figure 3). Correlation was strongest for Bm33 Ab (Spearman's rho=0.807), followed by Wb123 Ab (Spearman's rho=0.775), Bm14 Ab (Spearman's rho=0.659), and Ag (Spearman's rho=0.460).
Figure 3.
Correlation plots between antigen and antibody prevalence in participants aged 5-9 years and prevalence in all participants at the PSU level, Samoa 2018. Shaded areas represent 95% confidence intervals; green circles represent individual PSUs.
Risk factor analysis
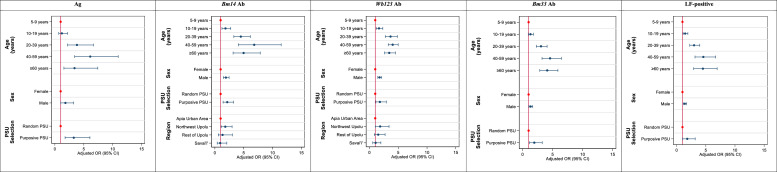
For all markers, male sex, older age groups, and residents of purposive PSUs were significantly more likely to be seropositive. The strongest association with male sex was seen for Bm14 Ab, with 1.91 (95% CI: 1.48-2.47; P<0.001) the odds of testing positive compared to females. The strongest association with age was seen for Bm14 Ab; 10-19-year-olds, 20-39-year-olds, 40-59-year-olds, and ≥60-year-olds had 1.84 (95% CI: 1.24-2.74; P=0.004), 4.50 (95% CI: 3.29-6.15; P<0.001), 6.83 (95% CI: 4.05-11.50; P<0.001), and 4.99 (95% CI: 3.16-7.88; P<0.001) the odds of seropositivity compared to 5-9-year-olds. For all seromarkers, 40-59-year-olds had the highest odds of seropositivity compared to 5-9-year-olds (P<0.001 for all markers). The strongest association with PSU selection was for Ag positivity; participants from purposively selected PSUs had 3.27 (95% CI: 1.76-6.07; P<0.001) the odds of testing Ag-positive compared to those from randomly selected PSUs. Residents of NWU were significantly more likely to test positive for Bm14 Ab (aOR: 1.81, 95% CI: 1.10-2.99; P=0.02) and Wb123 Ab (aOR: 1.85, 95% CI: 1.01-3.40; P=0.046) compared to other regions (Figure 4 and Supplementary Table 4).
Figure 4.
Summary of demographic and behavioural factors, and their associations with seropositivity for Bm14 antibody, Wb123 antibody, Bm33 antibody, antigen and any antigen or antibody (LF-positive) using multivariable logistic regression, Samoa 2018. An odds ratio (OR) of 1.0 indicates no association between the exposure and outcome (seropositivity). An OR>1 indicates higher odds of association between the exposure and outcome. An OR<1 indicates lower odds of association between the exposure and outcome. The reference group is indicated by the red circles.
Intra-cluster correlation
Clustering of seropositive participants was significantly higher in households (ICC: Ag0.45 [95% CI: 0.30, 0.61], Bm14 Ab0.32 [95% CI: 0.23, 0.42], Bm33 Ab0.31 [95% CI: 0.24, 0.39], Wb123 Ab0.29 [95% CI: 0.22, 0.38]) compared to PSUs or regions (Supplementary Figure 5). Clustering at the household level in purposively selected PSUs was higher than in randomly selected PSUs for Wb123 Ab (ICC: 0.30 [95% CI: 0.16, 0.51]) and Bm33 Ab (ICC: 0.35 [95% CI: 0.18, 0.56]), but was higher in randomly selected PSUs compared to purposively selected PSUs for Ag (ICC: 0.44 [95% CI: 0.27, 0.63]) and Bm14 Ab (ICC: 0.29 [95% CI: 0.20, 0.40]) (Supplementary Figure 6).
Discussion
Our study found that antifilarial Abs provided a more sensitive indicator of transmission than Ag and were useful for identifying high-risk populations and hotspots. Among all participants, 20.3%, 32.3%, and 51.0% tested seropositive to Bm14 Ab, Wb123 Ab, and Bm33 Ab, respectively, whilst only 3.7% were Ag-positive. Poly-seropositivity for Ag and Ab was found to differ by age groups (aged 5-9 years and ≥10 years) and PSU selection (randomly selected and purposively selected PSUs). For all seromarkers, we found significant clustering at the household level compared to the PSU or regional level.
Our findings are similar to other reporting higher rates of Ab seroprevalence compared to Ag [5,20,21], suggesting that monitoring Ag alone may not accurately reflect transmission intensity, particularly in low prevalence settings. We also found significantly higher seroprevalence of Ag and Ab among participants aged ≥10 years compared to 5-9 years, and stronger correlation estimates of Ab prevalence in 5-9-year-olds with the overall population compared to Ag, suggesting Ab seroprevalence in 5-9-year-olds could be a good predictor of LF transmission at the community level. In American Samoa, Ab surveillance in school-based TAS found indications of ongoing LF transmission over a year earlier than Ag surveillance. Further, Ag prevalence estimates were seen to differ significantly between a school-based TAS of 6-7-year-olds and a community-based TAS of people aged ≥8 years, with estimates of 0.7% and 6.2%, respectively [21]. The implications of these findings are twofold; firstly, they suggest that measuring Ag prevalence alone in TAS may not be sufficiently sensitive for making programmatic decisions about stopping MDA or determining whether LF elimination targets have been reached. Secondly, they suggest that school-based TAS are not a good indicator of Ag prevalence in the community, or an accurate identifier of foci of ongoing transmission [1].
Such observations draw into question the appropriateness of monitoring LF prevalence among school-aged children to determine whether the critical threshold of <1% Ag prevalence has been reached. Whilst school-based TAS are more cost-effective and logistically efficient, they may not be accurately reflecting transmission in the community or be sufficiently sensitive to identify foci of ongoing LF transmission. Though our study suggests Ab prevalence in children aged 5-9 years may better correlate with prevalence in older age groups, overall Ab prevalence in still significantly lower in younger age groups, and there is a risk that residual pockets of ongoing transmission may be missed if prevalence is solely measured in younger age groups. As reported frequently [10,22], our study found significantly higher Ag and Ab seroprevalence among males compared to females. High seroprevalence in adult males suggest that they may be a reservoir of infection for LF, and future surveillance programmes should consider a targeted surveillance approach that includes older males to identify possible hotspots of infection [23]. It has been suggested that community-based Ab surveys of adults and older children may be more sensitive than school-based TAS of 6-7-year-old children for determining elimination thresholds [10]. Monitoring Ab seroprevalence may be a better measure of transmission for LF elimination programmes; however, further research is needed to better understand Ab kinetics and determine whether there are IgG thresholds that can be used to differentiate between active and past LF infection as well as targets for elimination.
In the 2018 SaMELFS study, the Samoan Ministry of Health purposively selected 5 PSUs as ‘suspected LF hotspots’. As has been previously reported [14], Ag prevalence was higher in these purposively selected PSUs compared to randomly selected PSUs. This study confirms similar Ab seroprevalence patterns, with significantly higher seropositivity to all 3 Ab in purposively selected PSUs. Our findings support those of Lau et al. [14], which emphasise the importance of incorporating local knowledge and co-design in the development of LF monitoring and surveillance strategies.
Our finding of intense clustering of positive seromarkers at the household level, supported by similar reports of higher ICC values for Mf, Ag, Bm14 Ab, Wb123 Ab, and Bm33 Ab at the household level in American Samoa [3], suggest that LF infections and exposure are more intensely clustered within households. Our findings can further inform LF surveillance strategies wherein testing and treating household members (and possibly near neighbours) of any LF-positive individuals could be an effective means to eliminate pockets of residual LF transmission [3,14]. To date, treatment protocols have been based on Ag-positivity; however, to achieve the endgame of LF elimination, treatment of individuals and household members who are seropositive to Ag and Ab may be required. One important caveat to consider is the ambiguity around whether Ab-seropositivty is a marker or current LF infection or recent exposure, and the implications around treating individuals who may be ‘false-positives’. However, as MDA is given to whole populations irrespective of LF-positivity, such a method could be considered as a targeted MDA strategy for high-risk populations. This is the first report of LF Ab results from a large nationwide population representative serosurvey in Samoa, the results of which will act as a baseline of Ab seroprevalence to enable the understanding of Ab behaviour pre- and post-MDA, with Ab seroprevalence results 6-months following MDA soon to be available. An important limitation is that this study was conducted 7-11 weeks following MDA distribution, thus Mf prevalence would have been rapidly cleared from the population following treatment, preventing a comparison of Mf prevalence and Ab seroprevalence. However, we are confident that Ab seroprevalence reported reflects the pre-MDA epidemiology and can serve as a baseline for Ab prevalence in Samoa, given evidence of Ab persisting in the blood after treatment [24]. Further, adjustments were made to account for sampling design due to the inherent risk of selection bias when a cluster sampling methodology is adopted. Standardizing for participant age and sex accounted for the convenience survey of 5-9-year-olds. Whilst all efforts were made to mediate selection bias in the analysis of the study data and increase the accuracy of the results, there is still a risk that our results may have over- or underestimated the prevalence of seromarkers.
Our findings are particularly relevant as the WHO Neglected Tropical Diseases Roadmap 2030 is rolled out globally, and as the WHO methodology to undertake TAS in IDA areas is being revised. Our results question whether using an Ag prevalence of <1% in 6-7-year-old children is an appropriate measure to determine whether a country has achieved elimination of LF, given that (i) significantly lower prevalence of both Ag and Ab among this demographic has been reported in several studies to date, and (ii) Ag prevalence is significantly lower than Ab seroprevalence rates, implying the lower sensitivity of Ag to detect LF exposure and infection. Further, whilst an individual can be infected with LF with only one bite, not all exposures will result in a viable worm infection, and it takes time for worm infections to establish. Therefore, young children are less likely to have detectable LF markers. Incorporating Ab surveillance into LF elimination programmes could be advantageous, given that Ab against filarial Ag can indicate infection prior to Ag or Mf detection [12]. Studies in Tanzania and Indonesia have used Ab prevalence over time as a means to measure the impact of MDA [25] and for stopping MDA decision-making [26].
A key limitation in LF surveillance is the absence of diagnostic tests that can identify active LF infections, and thus can signal sustained transmission. Rapid diagnostic tests for antifilarial Abs are in development; utilizing these tools in field surveys could provide more timely results to indicate active or recent LF transmission. However, there are concerns about low specificity of Abs due to persistent host responses to infection following clearance of the original infection. Our analysis of Abs 6-months post-MDA in Samoa will further elucidate Ab behaviour in Samoa.
Conclusion
In conclusion, testing for Ab seroprevalence is a promising surveillance tool that can be adopted by countries aiming to eliminate LF. To fully understand how Ab surveillance can be used by LF programmes, further research is needed to establish a seroprevalence threshold to signal ongoing LF transmission and determine a threshold to indicate validation of elimination using Ab seroprevalence. Future cohort studies that monitor Ab levels of participants at baseline and following consecutive MDA rounds are required to elucidate the kinetics of Ab and Ag behaviour to determine whether monitoring Ab prevalence is an appropriate measure of LF transmission and infection.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Ethical Approval
Ethics approvals were granted by the Samoan Ministry of Health and The Australian National University Human Research Ethics Committee (protocol 2018/341). The study was conducted in close collaboration with the Samoa Ministry of Health, the WHO country office in Samoa, and the Samoa Red Cross. Written informed consent was obtained from adult participants. For participants aged <18 years, verbal assent was obtained from the child and formal written consent was obtained from a parent or guardian. This activity was reviewed by CDC, deemed not research, and was conducted consistent with applicable federal law and CDC policy.
Data availability
Regarding data availability, all relevant data are within the paper. We are unable to provide individual-level antigen prevalence data and demographic data because of the potential for breaching participant confidentiality. The communities in Samoa are very small, and individual-level data such as age, sex, and village of residence could potentially be used to identify specific persons. For researchers who meet the criteria for access to confidential data, the data are available on request from the Human Ethics Officer at the University of Queensland, email: humanethics@research.uq.edu.au.
Funding
This work received financial support from the Coalition for Operational Research on Neglected Tropical Diseases (OPP1053230, CL, https://www.ntdsupport.org/cor-ntd), which is funded at The Task Force for Global Health primarily by the Bill & Melinda Gates Foundation, by the United States Agency for International Development through its Neglected Tropical Diseases Program, and with United Kingdom Aid from the British people (OPP1053230, CL). CLL was supported by an Australian National Health and Medical Research Council (www.nhmrc.gov.au) Fellowship (Grant number 1193826). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
We would like to thank all the staff at the Samoa Ministry of Health who supported the many different aspects of the study. We sincerely thank Tautala Maula, the general secretary of the Samoa Red Cross, and her team for their enthusiastic and untiring support with fieldwork, village visits, and laboratory work; this survey would not have been possible without their hard work and dedication. We greatly appreciate all the support and advice provided by Rasul Baghirov and Lepaitai Hansell at the WHO country office in Samoa. We thank Patricia Graves for her assistance with data analysis and interpretation.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2024.107194.
Appendix. Supplementary materials
References
- 1.Sheel M., Sheridan S., Gass K., Won K., Fuimaono S., Kirk M., et al. Identifying residual transmission of lymphatic filariasis after mass drug administration: comparing school-based versus community-based surveillance - American Samoa, 2016. PLoS Negl Trop Dis. 2018;12(7) doi: 10.1371/journal.pntd.0006583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cadavid Restrepo A.M., Martin B.M., Fuimaono S., Clements A.C.A., Graves P.M., Lau C.L. Spatial predictive risk mapping of lymphatic filariasis residual hotspots in American Samoa using demographic and environmental factors. PLoS Negl Trop Dis. 2023;17(7) doi: 10.1371/journal.pntd.0010840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau C.L., Sheel M., Gass K., Fuimaono S., David M.C., Won K.Y., et al. Potential strategies for strengthening surveillance of lymphatic filariasis in American Samoa after mass drug administration: reducing ‘number needed to test’ by targeting older age groups, hotspots, and household members of infected persons. PLOS Negl Trop Dis. 2021;14(12) doi: 10.1371/journal.pntd.0008916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graves P.M., Sheridan S., Fuimaono S., Lau C.L. Demographic, socioeconomic and disease knowledge factors, but not population mobility, associated with lymphatic filariasis infection in adult workers in American Samoa in 2014. Paras Vect. 2020;13(1):125. doi: 10.1186/s13071-020-3996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau C.L., Sheridan S., Ryan S., Roineau M., Andreosso A., Fuimaono S., et al. Detecting and confirming residual hotspots of lymphatic filariasis transmission in American Samoa 8 years after stopping mass drug administration. PLoS Negl Trop Dis. 2017;11(9) doi: 10.1371/journal.pntd.0005914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Global programme to eliminate lymphatic filariasis. Wkly Epidemiol Rec. 2010;85:365–372. [PubMed] [Google Scholar]
- 7.World Health Organization, “Global programme to eliminate lymphatic filariasis: progress report, 2022,” in “Weekly Epidemiolog Record,” 2023, vol. 98 (41), 489-501. Available: https://iris.who.int/handle/10665/373357
- 8.World Health Organization, “Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030,” 2020.
- 9.NTD Modelling Consortium Lymphatic Filariasis Group The roadmap towards elimination of lymphatic filariasis by 2030: insights from quantitative and mathematical modelling. Gates Open Res. 2019;3:1538. doi: 10.12688/gatesopenres.13065.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao R.U., Samarasekera S.D., Nagodavithana K.C., Punchihewa M.W., Ranasinghe U.S.B., Weil G.J. Systematic sampling of adults as a sensitive means of detecting persistence of lymphatic filariasis following mass drug administration in Sri Lanka. PLoS Negl Trop Dis. 2019;13(4) doi: 10.1371/journal.pntd.0007365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Won K.Y., Robinson K., Hamlin K.L., Tufa J., Seespesara M., Wiegand R.E., et al. Comparison of antigen and antibody responses in repeat lymphatic filariasis transmission assessment surveys in American Samoa. PLoS Negl Trop Dis. 2018;12(3) doi: 10.1371/journal.pntd.0006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pastor A.F., Silva M.R., Dos Santos W.J.T., Rego T., Brandão E., de-Melo-Neto O.P., et al. Recombinant antigens used as diagnostic tools for lymphatic filariasis. Parasit Vectors. 2021;14(1):474. doi: 10.1186/s13071-021-04980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graves P.M., Joseph H., Coutts S.P., Mayfield H.J., Maiava F., Leong-Lui T.A.A., et al. Control and elimination of lymphatic filariasis in Oceania: Prevalence, geographical distribution, mass drug administration, and surveillance in Samoa, 1998–2017. Adv Parasitol. 2021;114:27–73. doi: 10.1016/bs.apar.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Lau C.L., Meder K., Mayfield H.J., Kearns T., McPherson B., Naseri T., et al. Lymphatic filariasis epidemiology in Samoa in 2018: Geographic clustering and higher antigen prevalence in older age groups. PLoS Negl Trop Dis. 2020;14(12) doi: 10.1371/journal.pntd.0008927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization The PacELF way: towards the elimination of lymphatic filariasis from the Pacific, 1999-2005. https://apps.who.int/iris/bitstream/handle/10665/208189/9290612150_eng.pdf
- 16.Goodhew E.B., Priest J.W., Moss D.M., Zhong G., Munoz B., Mkocha H., et al. CT694 and pgp3 as serological tools for monitoring trachoma programs. PLoS Negl Trop Dis. 2012;6(11):e1873. doi: 10.1371/journal.pntd.0001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lammie P.J., Moss D.M., Brook Goodhew E., Hamlin K., Krolewiecki A., West S.K., et al. Development of a new platform for neglected tropical disease surveillance. Int J Parasitol. 2012;42(9):797–800. doi: 10.1016/j.ijpara.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Samoa Bureau of Statistics, "2016 census," 2016. Accessed: 19 September 2023. Available: https://sbs.gov.ws/populationanddemography.
- 19.Galbraith S., Daniel J.A., Vissel B. A study of clustered data and approaches to its analysis. J Neurosci. 2010;30(32):10601–10608. doi: 10.1523/jneurosci.0362-10.2010. Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickson B.F.R., Masson J.J.R., Mayfield H.J., Aye K.S., Htwe K.M., Roineau M., et al. Bayesian network analysis of lymphatic filariasis serology from myanmar shows benefit of adding antibody testing to post-MDA surveillance. Trop Med Infect Dis. 2022;7(7):113. doi: 10.3390/tropicalmed7070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadavid Restrepo A.M., Gass K., Won K.Y., Sheel M., Robinson K., Graves P.M., et al. Potential use of antibodies to provide an earlier indication of lymphatic filariasis resurgence in post-mass drug ad ministration surveillance in American Samoa. Int J Infect Dis. 2022;117:378–386. doi: 10.1016/j.ijid.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao R.U., Nagodavithana K.C., Samarasekera S.D., Wijegunawardana A.D., Premakumara W.D., Perera SN., et al. A comprehensive assessment of lymphatic filariasis in Sri Lanka six years after cessation of mass drug administration. PLoS Negl Trop Dis. 2014;8(11):e3281. doi: 10.1371/journal.pntd.0003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao R.U., Samarasekera S.D., Nagodavithana K.C., Goss C.W., Punchihewa M.W., Dassanayaka T.D.M., et al. Comprehensive assessment of a hotspot with persistent bancroftian filariasis in coastal Sri Lanka. Am J Trop Med Hyg. 2018;99(3):735–742. doi: 10.4269/ajtmh.18-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greene S.E., Huang Y., Curtis K.C., King C.L., Fischer P.U., Weil G.J. IgG4 antibodies to the recombinant filarial antigen Wb-Bhp-1 decrease dramatically following treatment of lymphatic filariasis. PLoS Negl Trop Dis. 2023;17(6) doi: 10.1371/journal.pntd.0011364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simonsen P.E., Pedersen E.M., Rwegoshora R.T., Malecela M.N., Derua Y.A., Magesa S.M. Lymphatic filariasis control in Tanzania: effect of repeated mass drug administration with ivermectin and albendazole on infection and transmission. PLoS Negl Trop Dis. 2010;4(6):e696. doi: 10.1371/journal.pntd.0000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dewi R.M., Tuti S., Ganefa S., Anwar C., Larasati R., Ariyanti E., et al. Brugia Rapid™ antibody responses in communities of Indonesia in relation to the results of ‘transmission assessment surveys’ (TAS) for the lymphatic filariasis elimination program. Paras Vect. 2015;8(1):499. doi: 10.1186/s13071-015-1093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Regarding data availability, all relevant data are within the paper. We are unable to provide individual-level antigen prevalence data and demographic data because of the potential for breaching participant confidentiality. The communities in Samoa are very small, and individual-level data such as age, sex, and village of residence could potentially be used to identify specific persons. For researchers who meet the criteria for access to confidential data, the data are available on request from the Human Ethics Officer at the University of Queensland, email: humanethics@research.uq.edu.au.