Abstract
Seawater electrolysis using renewable electricity offers an attractive route to sustainable hydrogen production, but the sluggish electrode kinetics and poor durability are two major challenges. We report a molybdenum nitride (Mo2N) catalyst for the hydrogen evolution reaction with activity comparable to commercial platinum on carbon (Pt/C) catalyst in natural seawater. The catalyst operates more than 1000 hours of continuous testing at 100 mA cm−2 without degradation, whereas massive precipitate (mainly magnesium hydroxide) forms on the Pt/C counterpart after 36 hours of operation at 10 mA cm−2. Our investigation reveals that ammonium groups generate in situ at the catalyst surface, which not only improve the connectivity of hydrogen-bond networks but also suppress the local pH increase, enabling the enhanced performances. Moreover, a zero-gap membrane flow electrolyser assembled by this catalyst exhibits a current density of 1 A cm−2 at 1.87 V and 60 oC in simulated seawater and runs steadily over 900 hours.
Subject terms: Electrocatalysis, Electrocatalysis, Hydrogen energy
Efficient catalysts for seawater electrolysis are crucial for sustainable hydrogen production but struggle with slow kinetics and low durability. Here, the authors report a molybdenum nitride catalyst that in situ generates ammonium groups, enhancing both performance and stability in natural seawater.
Introduction
Water electrolysis is the most promising green hydrogen technology that uses renewable electricity to produce H2 fuel without carbon emissions1. Current low-temperature water electrolysis devices, including alkaline electrolyser and proton exchange membrane (PEM) electrolyser, rely on high-purity water feeds2,3; this, however, will raise severe issue of freshwater resource shortage when large-scale commercialization of these devices becomes prevalent4. Direct seawater splitting is potentially an ideal solution to this issue, as the oceans account for 96.5% of the water reserves on Earth5,6. However, the complex components of natural seawater possess manifold challenges with respect to the efficiency and durability of this technology4,6,7, including (i) dramatic pH fluctuations nearby the electrode surfaces, (ii) physical blockages at the cathode due to the formation of insoluble precipitates and (iii) competitive reactions between the oxygen evolution reaction (OER) and chloride electro-oxidation chemistry at the anode.
Over the past years, the majority of research efforts have centered on avoiding the unwanted chloride oxidations on the anode side, and an important progress has been achieved8–10. By contrast, less attention has been given to the salt deposition issue at the cathode that causes active site blocking and corrosion. Despite natural seawater is approximately neutral (pH ~7.5–8.4)11, the local pH in the vicinity of cathode increases greatly during electrolysis, resulting in precipitation of seawater cations (mainly Mg2+ and Ca2+) in the form of hydroxides6,12. Indirect electrolysis of seawater coupled with external pre-desalination and purification processes can remove major seawater ions, but this approach needs additional energy input, rendering it less cost effective13. Introducing chemical additives, such as KOH (US$800 per tonne)7, into seawater could allow these cations to precipitate out via hydroxide formation, which is then filtered before electrolysis14,15. However, this greatly complicates the electrolysis process and adds the cost issue. A recently developed membrane-based seawater electrolyser has enabled 100% ion-blocking efficiency via an in situ self-driven water purification design16. The approach, although side-reaction-free and corrosion-free, requires 2.25 V to reach a current density of 0.4 A cm−2. This performance was comparable to alkalized seawater electrolysers with metrics of >2 V to achieve 0.4 A cm−2 to 0.7 A cm−2 (23–80 °C)17–19, while remaining inferior to that of PEM freshwater electrolysers those can attain 1 A cm−2 at 1.55 V to 2.1 V (80 °C)20–22.
In addition, compared with alkalized water, the kinetics of both anodic OER and cathodic hydrogen evolution reaction (HER) become sluggish under neutral seawater environments, which requires larger overpotentials to attain desired current densities and therefore reduces the overall electrolysis efficiency23–25. Herein, we address these challenges using a molybdenum nitride (Mo2N) catalyst that synthesized via a simple and rapid nitrogen plasma irradiation method. During natural seawater electrolysis, we find that ammonium (NH4+) cations generate in situ on the catalyst surface, which can be hydrogen-bonded with hydroxyl (OH-) groups from the interfacial H2O dissociation and consequently prevents the hydroxide formation. Meanwhile, these NH4+ groups also greatly improve the connectivity of hydrogen-bond networks in the electric double layer (EDL), lowering the hydrogen transfer barrier and enhancing the HER energetics. As a result, the catalyst demonstrates enhanced activity and long-term stability as the cathode in a seawater electrolyser.
Results
Preparation of metal nitride catalysts
Transition metal nitrides (TMNs) are a family of functional materials that synergize the properties of high electrical conductivity, good electrochemical stability, and corrosion resistance and are used widely for electrolysis26,27. Recently, we reported that nickel nitride (Ni3N) and zirconium nitride (ZrN) were applied as high-performance electrocatalysts for hydrogen oxidation and oxygen reduction, respectively, in a fuel cell28. We reasoned that the unique features of TMNs would offer catalysts that can survive the harsh conditions encountered in seawater. To examine the potential, we prepared a series of TMNs, including ScN, TiN, Ni3N, YN, ZrN, Mo2N, and InN, by means of plasma-enhanced chemical vapor deposition (PECVD). This synthetic method enables ionization of N2 to generate energetic nitrogen plasmas, which can substantially enhance the reactivity between nitrogen and metal precursors, yielding high-purity TMNs29 (see Methods; Supplementary Fig. 1; Supplementary Table 1). Taken Mo2N as an example, the X-ray diffraction (XRD) patterns (Fig. 1a) showed that the metallic Mo precursor was successfully converted into Mo2N phase. Scanning transmission electron microscopy (STEM) image and STEM with energy-dispersive spectroscopy (STEM-EDS) element maps exhibited particulate morphology of Mo2N with homogeneous distribution of Mo and N (Fig. 1b and Supplementary Fig. 2a, b). High-resolution transmission electron microscope (HRTEM) image (Fig. 1c) depicted continuous lattice fringes with a spacing of 0.24 nm, corresponding to Mo2N (11-1) planes. The N 1 s X-ray photoelectron spectroscopy (XPS) (Supplementary Fig. 2c) and the Mo K-edge extended X-ray absorption fine structure (EXAFS) (Supplementary Figs. 2d, e and Supplementary Table 2) analyses both revealed Mo-N bond in the sample. Raman spectroscopy measurements (Supplementary Fig. 2f) showed that a broad band <350 cm−1 arose from the Mo precursor after nitrogen plasma irradiation, which are in good agreement with previous report of Raman modes of Mo2N30,31. Besides Mo2N, the evidences of obtaining other pure TMNs, i.e., ScN, TiN, Ni3N, YN, ZrN, and InN, are presented in Supplementary Figs. 3–8 and Supplementary Table 3.
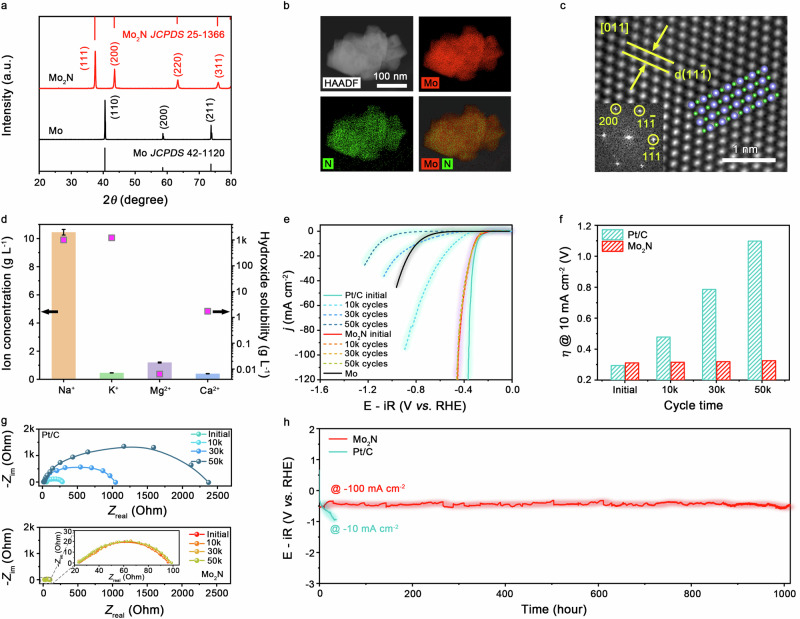
Fig. 1. Electrocatalytic performance of Mo2N in natural seawater.
a XRD patterns of Mo and Mo2N. b STEM-EDX elemental mapping of Mo2N, showing the homogeneous distribution of Mo (red) and N (green), respectively. c HRTEM image of Mo2N. Insets show the corresponding FFT pattern and atomic model viewed along the same direction. d The concentration of typical ions in natural seawater and the solubility of the corresponding hydroxides. Error bars are based on the standard deviation of three independent measurements. e HER polarization curves of Mo, Mo2N, and commercial Pt/C catalysts before and after different potential cycles in natural seawater at room temperature (100% iR correction, where R was determined to be 23.7 ± 0.2 Ω). Catalyst loading: ~1.0 mg cm−2. Sweep rate: 5 mV s−1. Rotation rate: 1600 rpm. f The increase in overpotential at 10 mA cm−2 for Mo2N and Pt/C catalysts before and after different potential cycles. g EIS Nyquist plots of Mo2N and Pt/C catalysts before and after different potential cycles. Inset shows the enlarged EIS Nyquist plots of Mo2N. h Chronopotentiometry curves of Mo2N and Pt/C catalysts performed in natural seawater.
Electrocatalytic performance in natural seawater
We assessed the HER activity of these TMN catalysts using the rotating disk electrode (RDE) method in real seawater (pH 7.84 ± 0.02; Gulf of Mexico, Alabama, USA). As a benchmark, commercial Pt/C catalyst (20 weight % Pt on Vulcan XC72R carbon) was also examined. Inductively coupled plasma atomic emission spectrometry (ICP-AES) analysis showed that the seawater Mg2+ and Ca2+ concentrations were about 49.12 and 9.90 mmol L−1 (Fig. 1d; Supplementary Table 4), respectively. Such high concentrations of the two harmful seawater cations would cause massive precipitations of Mg(OH)2 and Ca(OH)2, blocking the catalytic active sites. We screened all above TMN catalysts for HER and found that Mo2N shows the optimal activity (Supplementary Fig. 9). For example, the Mo2N catalyst required an overpotential of mere 311 ± 3 mV at 10 mA cm−2 (Fig. 1e and Supplementary Figs. 10 and 11). The potential is considerably smaller than that of 806 ± 9 mV for commercial Mo catalyst and approaches that of 294 ± 4 mV for the Pt/C benchmark. The trend agrees well with our electrochemically active surface area (ECSA)-normalized HER polarizations (Supplementary Figs. 12 and 13). This result put Mo2N among the most active non-precious metal catalysts documented in the aqueous high-salinity electrolytes (Supplementary Table 5). In situ Raman measurements show no sign of surface oxidation of Mo2N during the HER (Supplementary Fig. 14). The H2 evolved from seawater over Mo2N was detected and quantified by gas chromatography (GC) at 10 mA cm−2, which yielded a Faradaic efficiency of 99.9%, comparable to that of ~100% over Pt/C reference (Supplementary Fig. 15).
We also performed accelerated stability tests through potential cycling between −0.2 V to −0.4 V versus reversible hydrogen electrode (RHE) at 200 mV s−1 in Ar-saturated natural seawater. As Fig. 1e shows, the Pt/C catalyst underwent an enormous degradation after potential cycles. The degradation is expected, owing to the hydroxide formation that blocked Pt sites. However, we were surprised to observe that Mo2N subjected to the same aging measurement can largely retain its activity (Fig. 1e). Figure 1f shows that only 14-mV additional overpotential was required to reach 10 mA cm−2 over Mo2N catalyst after 50,000 potential cycles. By stark contrast, the Pt/C catalyst resulted in a 478-mV overpotential penalty at 10 mA cm−2 after 10,000 cycles, which further climbed to 1098 mV after 50,000 cycles. Moreover, electrochemical impedance spectroscopy (EIS) measurements (Fig. 1g) revealed that the charge transfer resistance (Rct) increases slightly (~4.57 Ohm) over Mo2N after 50,000 cycles, whereas a significant Rct increase by 2.3 kOhm was found over Pt/C under the same condition, suggesting constrained electron transfer from Pt/C surface to the reactant caused by hydroxide precipitates.
The Mo2N catalyst was further subjected to a galvanostatic stability test at 100 mA cm−2 (Fig. 1h). Continuous operation over 1000 hours was demonstrated with no clear sign of catalyst deactivation in seawater. This observation was in marked comparison to that of Pt/C catalyst, which showed fast deterioration in performance even holding the electrode at 10 mA cm−2. After chronopotentiometry experiment, the morphology, composition and electronic states of Mo2N were well maintained (Supplementary Figs. 16 and 17). We also compare the performance metrics of the Mo2N catalyst with those reported previously in terms of overpotential at 10 mA cm−2 and operating lifetime. The metrics obtained on Mo2N outperform previous noble and non-noble catalysts those assessed under similar conditions (Supplementary Table 5).
Mechanistic investigation
To elucidate the source of high HER activity and, in particular, the unexpected stability, the real-time surface microenvironment and chemistry of Mo2N and other reference catalysts were investigated in detail by in situ surface-enhanced infrared adsorption spectroscopy (SEIRAS) (Supplementary Fig. 18). In previous research, Chen and co-workers, through a combination of computational simulation and in situ SEIRAS, demonstrated that interfacial water configuration and the connectivity of H-bond networks in the EDL govern the HER activity32. Figure 2a exhibits the SEIRAS data acquired on different catalysts in Ar-saturated natural seawater at −0.05 V versus RHE. All the samples showed a notable vibration band in the range of 1544.64–1724.83 cm−1, which can be assigned to the H-O-H bending vibration band of interface water32–34. Gaussian fitting and deconvolution of the band revealed two distinct components corresponding to strongly H-bonded water (s-HB-H2O at 1675.36 cm−1) and weakly H-bonded water (w-HB-H2O at 1632.42 cm−1), respectively33,34. Noticeably, a band centered at ~1531.57 cm−1 was observed for the TMN catalysts, attributed to the H-N-H bending vibration of NH4+ (ref35.), and its intensity increases following the order of Mo2N > Ni3N > TiN > ZrN > ScN0.98 > InN > YN (Fig. 2a). For metallic Mo, this band was absent. Therefore, we infer that surface lattice nitrogen would react with H adsorbed on TMNs during HER, forming NH4+ species. The calculated N 2p projected density of states (PDOS) of the TMNs showed that their p-band centers vary with respect to the Fermi level (EF) (Supplementary Fig. 19 and Supplementary Data 1). As the N 2p states move away from the EF, the anti-bonding states below the EF exhibit less nitrogen character, resulting in reduced covalency of metal-nitrogen bonds and, consequently, activated surface nitrogen36,37. Our PDOS calculations show a trend of Mo2N > Ni3N > TiN > ZrN > ScN0.98 > InN > YN. This trend correlated with the trend of the spectroscopic measurement of NH4+ signals of the seven TMNs, among which Mo2N yielded the most NH4+ species owing to its lowest covalency between Mo and N (Fig. 2b).
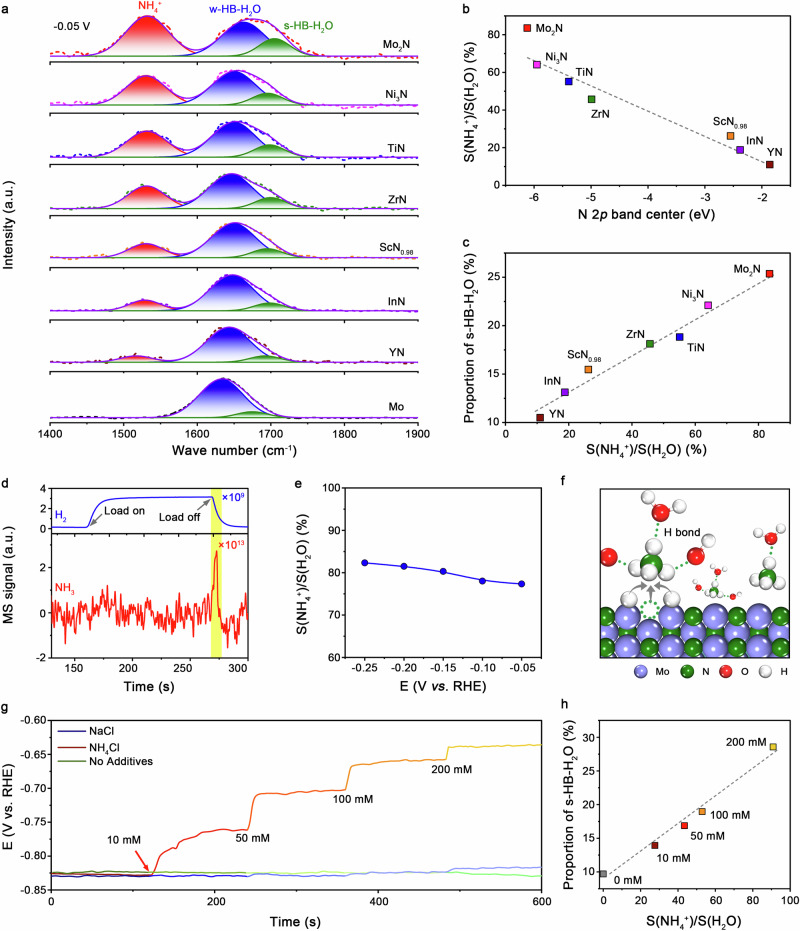
Fig. 2. In situ NH4+ formation at the catalyst surface.
a In situ SEIRAS spectra of different metal nitrides at −0.05 V in Ar-saturated natural seawater. b The proportion of NH4+ species in the EDL versus N 2p band center for different metal nitrides. c The proportion of s-HB-H2O versus the proportion of NH4+ species in the EDL for different metal nitrides. d Online electrochemical DEMS measurements over Mo2N catalyst in natural seawater. e The proportion of NH4+ species in the EDL of the Mo2N catalysts as a function of applied potentials. The proportion of NH4+ in the EDL were extracted from the in situ SEIRAS spectra in Supplementary Fig. 23. f Schematic of the NH4+ formation on the Mo2N surface. g Chronopotentiometry curves of Mo operated at −10 mA cm−2 in natural seawater with different concentration of NH4Cl and NaCl, respectively. The pH of the bulk seawater was fixed at 7.84 by adding NaOH. h The proportion of s-HB-H2O versus the proportion of NH4+ species in the EDL for the Mo catalyst operated at −0.05 V in Ar-saturated natural seawater with different concentrations of NH4Cl. The proportion of s-HB-H2O and the proportion of NH4+ were extracted from the in situ SEIRAS spectra in Supplementary Fig. 24.
The normalized integrated NH4+ peak area relative to that of interfacial H2O was plotted as a function of the proportion of s-HB-H2O for each TMN catalyst. Figure 2c reveals a positive correlation between NH4+ and s-HB-H2O, implying that NH4+ benefits the formation of interfacial water with strong H-bond interactions (Supplementary Fig. 20). The direct evidence for the existence of NH4+ is provided by operando differential electrochemical mass spectroscopy (DEMS) measurements in natural seawater (Supplementary Fig. 21). Holding the electrode at −10 mA cm−2 yielded a considerable mass-to-charge signal (m/z) for H2 (m/z = 2), which attenuated rapidly once the applied current was removed (Fig. 2d). Immediately afterwards, a sharp signal of NH3 (m/z = 17) appeared. Under negative electric field, the in situ formed NH4+ species were attracted in the EDL, which accumulated and were released to generate NH3 when the electrode surface turned electrically neutral. The formation of NH4+ can be further confirmed by the 1H nuclear magnetic resonance (1H NMR) spectra and ion chromatography (Supplementary Fig. 22 and Supplementary Table 6). Additionally, the NH4+ population on Mo2N surface increases linearly with increasing the applied negative bias (Fig. 2e and Supplementary Fig. 23).
In natural seawater, the high concentration of alkaline cations (Fig. 1d) makes them easily be migrated to catalyst surface at negative bias owing to the electrostatic effect. The enriched cations in the EDL would cause the loss of their partial solvation, promoting w-HB-H2O molecules and thus raising the barrier of hydrogen transfer at electrochemical interface33,38,39. Over Mo2N catalyst, NH4+ groups are generated in situ at the surface at negative potential and can interact with H2O and hydroxyl in the EDL35,40,41, giving rise to increased connectivity of H-bond networks and, consequently, more s-HB-H2O molecules (Fig. 2f). We also performed operando electrochemical impedance spectroscopy (EIS) at different overpotentials in Ar-saturated seawater to probe hydrogen adsorption resistance and hydrogen adsorption pseudo-capacitance for Mo and Mo2N catalysts42,43 (Supplementary Fig. 25). Our experimental results indicate that Mo2N has greatly lower hydrogen adsorption resistance and higher adsorbed hydrogen than that for Mo at all biases examined, indicating that NH4+ species in the Stern layer improve H-bond networks for better hydrogen transfer and adsorption. To validate the enhancement of HER by NH4+ species, we carried out galvanostatic measurements of metallic Mo at 10 mA cm−2 with the addition of NH4Cl. Figure 2g shows that the overpotential decreased from about 761.8 to 637.4 mV with increasing the NH4+ amount from 10 to 200 mM. Accordingly, in situ SEIRAS analyses showed that the s-HB-H2O populations at −0.05 V increased from 13.9% to 28.6% (Fig. 2h and Supplementary Fig. 24). Without NH4+ addition, no reduced overpotential was observed. Moreover, the addition of NaCl instead of NH4Cl also cannot bring overpotential reduction. The critical role of NH4+ in making s-HB-H2O, which shows positive correlation with the connectivity of H-bond networks, and thus enhancing HER energetics was also evidenced by the linear sweep voltammetry measurements with and without NH4Cl addition (Supplementary Fig. 26).
Origin of anti-precipitation capability
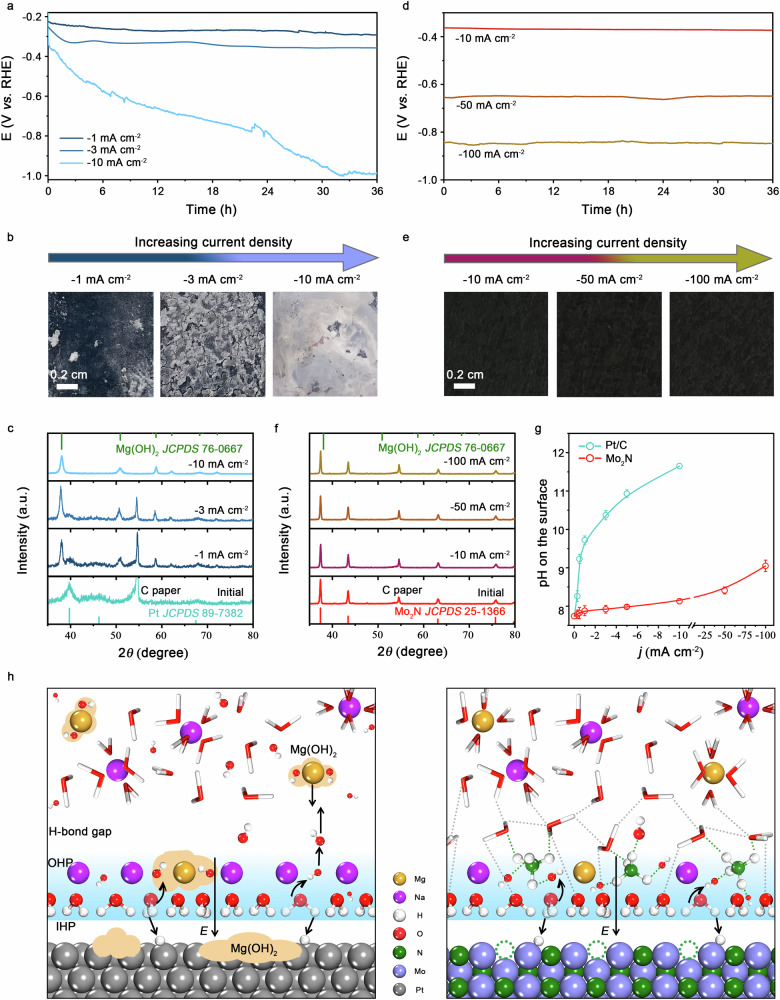
To track the evolution of the surface product and corresponding phase not observable by RDE measurements, the Mo2N and Pt/C dispersions were sprayed uniformly onto carbon papers at a loading of 1 mg cm−2, which we then used for galvanostatic experiments by holding the electrodes at different current densities. The Pt/C electrode showed slow degradation at −1 and −3 mA cm−2, and enormous degradation at −10 mA cm−2 (Fig. 3a). The initial and spend electrodes were visualized by a series of photographs, showing that the electrodes were gradually covered with white precipitates as the applied current increases (Fig. 3b). XRD patterns exhibited a majority Mg(OH)2 phase, and no diffraction peaks from Ca(OH)2 could be observed (Fig. 3c). This is not unexpected, as the high seawater Mg2+ concentration and the low solubility of Mg(OH)2 (Fig. 1d). These results are in marked contrast to Mo2N electrode on which Mg(OH)2 formation was completely prohibited even up to −100 mA cm−2 (Fig. 3d–f). We note that, as the current density increases, the diffraction corresponding to Mo2N (111) facets shifted gradually to higher angles (Supplementary Fig. 27), which indicates the formation of nitrogen vacancies44, consistent with our electron paramagnetic resonance (EPR) measurements45 (Supplementary Fig. 28). Additionally, only surface lattice nitrogen on Mo2N can migrate out to form NH4+, while lattice nitrogen from inner Mo2N is inactive (Supplementary Figs. 29 and 30). In general, Mg(OH)2 would form when solution pH is greater than 9.5 (ref12.). We speculate that the in situ formed NH4+ groups play an important role in confining the locally generated OH- groups during HER through hydrogen-bond interaction. To support this view, an IrOx-modified rotating RDE (RRED) measurement was employed to quantitatively probe pH on Mo2N and Pt/C electrode surfaces in natural seawater46 (Supplementary Figs. 31, 32). As Fig. 3g shows, the pH value on Pt/C surface exhibited a sharp increase (from 8.26 to 11.65) with increasing the applied current density from −0.3 to −10 mA cm−2. The surface pH on Mo2N also increased, but in a much slower manner, which reached mere 9.05 at even −100 mA cm−2, thus avoiding Mg(OH)2 formation. Density functional theory (DFT) simulations predict that hydrogen of the NH4+ is poised to form strong hydrogen-bond interactions with H2O and OH- groups (Supplementary Fig. 33 and Supplementary Data 1), consistent with previous reports40,41. As a result, OH- generated from the water dissociation during HER was constrained by NH4+ in the Stern layer, suppressing the pH increase.
Fig. 3. Anti-precipitation capability of Mo2N catalyst.
a, d Chronopotentiometry curves of commercial Pt/C (a) and Mo2N (d) catalysts operated at different current density in natural seawater. b, e Photographs of Pt/C (b) and Mo2N (e) catalysts on the carbon paper after electrolysis at different current densities for 36 hours in natural seawater. c, f XRD patterns of Pt/C (c) and Mo2N (f) catalysts on the carbon paper after electrolysis at different current densities for 36 hours in natural seawater. g Local pH on the surface of Pt/C and Mo2N catalysts as a function of current density in natural seawater. Error bars are based on the standard deviation of three independent measurements. h Schematic of the NH4+ formation on the Mo2N surface that prevents precipitate formation, whereas Mg(OH)2 precipitate formed on the surface of Pt/C catalyst.
From our analysis, the electrocatalytic HER performance of Mo2N in natural seawater can be understood from the proposed mechanism shown in Fig. 3h. Under negative bias, surface nitrogen on Mo2N was activated and reacted with the adsorbed H that generated during the HER process, forming NH4+ species. These NH4+ groups were confined in the EDL via electrostatic interaction and strongly H-bonded to hydroxyl and interfacial water, which improved the connectivity of H-bond networks, facilitating high-efficiency proton transfer in the interfacial region. On one hand, this more connected H-bond network with reduced hydrogen transfer barrier can greatly enhance the HER kinetics. What’s more, the H-bonded OH- groups were confined in the Stern layer and can barely diffuse out of the EDL, thus suppressing the rise of pH and, consequently, the formation of hydroxide precipitates. We notice that the attack from Cl- toward the Mo2N cathode is negligible owing to the weak binding strength between Cl and Mo2N surface (Supplementary Figs. 34 and 35 and Supplementary Data 1).
Performance in a practical electrolyser
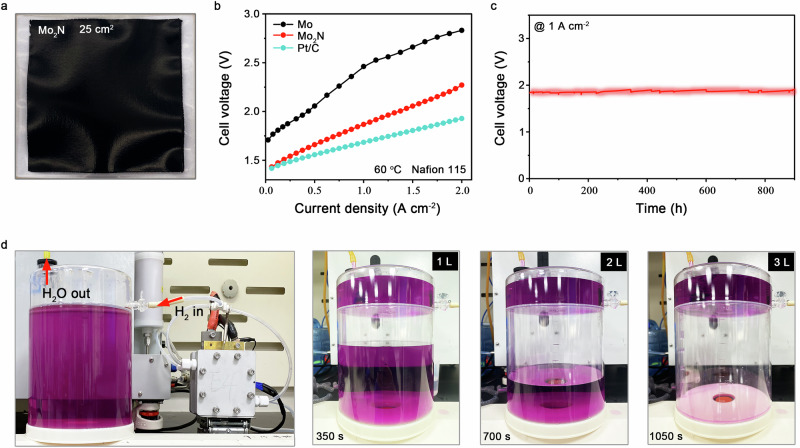
To further corroborate the outstanding performance described above, we incorporated Mo2N catalyst (1.0 mg cm−2) in the cathode of a 25-cm2 membrane electrode assembly (Fig. 4a) and tested it in a practical water electrolyser at 60 °C (see Methods, Supplementary Fig. 36). At the anode, commercial IrO2 was used as catalyst with a loading of 1.0 mgIr cm−2. Considering that IrO2 can suffer from chloride attack in natural seawater electrolysis47, simulated seawater with 1.19 g L−1 MgSO4 and 0.40 g L−1 CaSO4 was used as the feed. Figure 4b shows the current-voltage polarization curve of Mo2N catalyst in the electrolyser, which required a voltage of 1.87 V to reach the industrially relevant current density of 1 A cm−2. By comparison, the electrolyser with Pt/C cathode displayed a cell voltage of 1.68 V at 1 A cm−2, showing only 190 mV advantage over the nonprecious Mo2N catalyst. When operating at 1 A cm−2, the energy efficiency of our electrolyser with Mo2N cathode arrived at 65.9%, corresponding to an energy consumption of 50.0 kWh kg−1 H2 (see Supplementary materials for calculation details).
Fig. 4. Electrolyser performance.
a Photograph of Mo2N catalyst on Nafion 115 membrane. Catalyst loading: 1.0 mg cm−2. b Current-voltage polarizations of the zero-gap membrane flow electrolyser using Mo, Mo2N, and commercial Pt/C as the cathodic catalysts. c, Chronopotentiometry measurement of the electrolyser at 1 A cm−2 and 60 °C for Mo2N cathodic catalyst. d, Photograph of the zero-gap membrane flow electrolyser with Mo2N cathode, showing that 3 L H2 was obtained after 1050 s at 1 A cm−2 and 60 °C.
We also performed long-term stability measurement of the electrolyser with Mo2N cathode at the current density of 1 A cm−2 and 60 °C. Figure 4c shows that the device run steadily over 900 hours with no appreciable voltage increase. The performance metrics of Mo2N are superior when compared with previous non-noble cathodes tested in pure water and seawater (Supplementary Table 7). By contrast, Pt/C cathode underwent a notable decay with a degradation rate of 2.16 mV h−1, originating from the formation of Mg(OH)2 precipitate (Supplementary Fig. 37). During electrolysis, the cations, such as Na+, will replace with H+ and cause the lack of H+ at cathode. Consequently, the reduction of H2O happens at cathode, leading to a steep pH increase48. Furthermore, we determined experimentally the H2 production rate of about 10.45 L h−1 from the Mo2N-drove electrolyser at 1 A cm−2 (Fig. 4d), corresponding to a H2 cost of US$1 per kilogram (see Methods for calculation details), half that of the US Department of Energy target of US$2 per kilogram49. No NH3 impurity can be detected by the gas chromatography–mass spectrometry in the as-produced H2 (Supplementary Fig. 38).
Discussion
In conclusion, high-quality Mo2N catalyst with enhanced HER activity and stability in seawater electrolysis was synthesized through a convenient and scalable plasma-enhanced CVD route. The catalyst can generate NH4+ groups in situ on its surface, which limits local pH increase in the vicinity of the electrode, thus preventing the formation of hydroxide precipitates during seawater electrolysis. Meanwhile, these NH4+ species also greatly improve the connectivity of the H-bond networks in the EDL region, leading to the enhanced HER energetics. We have showed of how ammonium groups formed at the catalyst surface can improve catalytic activity and simultaneously avoid hydroxide formation. We foresee that these findings will stimulate further tuning of catalyst microenvironments for highly efficient seawater splitting.
Methods
Material synthesis
Scandium powder (Sc, 99.9 %), titanium powder (Ti, 99.99 %), nickel powder (Ni, 99.99 %), yttrium powder (Y, 99.9 %), zirconium powder (Zr, 99.5 %), molybdenum powder (Mo, 99.5 %) and indium powder (In, 99.99 %) were purchased from Innochem. The dinitrogen (N2, 99.999%) was purchased from Nanjing Special Gas Factory Co., Ltd, China. All the chemicals were used as received without further purification.
The metal nitrides were prepared by the plasma-enhanced chemical vapor deposition. Briefly, the metal powder was subjected to the N2 plasma treatment in the chamber of the radio frequency-plasma enhanced chemical vapor deposition (RF-PECVD) systems under a pressure of 0.02 Torr. After the temperature of the chamber reached the synthetic temperature, the radio frequency-plasma discharge was conducted at 300 W and 13.56 MHz for 30 min. The synthetic temperature used for each nitride, as well as their structural information were summarized in Supplementary Table 1.
Material characterization
The study employed Transmission Electron Microscopy (TEM) analysis, High-Resolution Transmission Electron Microscopy (HRTEM) measurements, and Energy Dispersive Spectroscopy (EDS) mappings using a FEI Talos F200X. This instrument is equipped with a Super X-EDS system, which comprises four systematically arranged windowless silicon drift detectors, operating at 200 kV. High-resolution HAADF images were obtained using the JEM-ARM 200 F Atomic Resolution Analytical Microscope, which operates with an acceleration voltage of 200 kV. Scanning electron microscope (SEM) images were captured utilizing the Zeiss Supra 40 apparatus.
X-ray powder diffraction (XRD) data were acquired using a Japan Rigaku DMax-γA rotation anode X-ray diffractometer, calibrated with graphite monochromatized Cu-K radiation. The Raman spectra was conducted using a LABRAM-HR confocal laser micro-Raman spectrometer, equipped with a 532 nm wavelength. X-ray photoelectron spectroscopy (XPS) data were collected using an ESCALAB-MKII x-ray photoelectron spectrometer, with Mg Kα radiation serving as the excitation source (Mg Ka = 1253.6 eV). The XAFS spectra were gathered at the 1W1B station of the Beijing Synchrotron Radiation Facility. The ion chromatography data were derived from the ICS-3000 instrument.
Density functional theory calculation
The Vienna Ab-initio Simulation Package (VASP) software50, a plane-wave code, was utilized to carry out all the spin-polarized density functional theory (DFT) calculations. These calculations were conducted within the Generalized Gradient Approximation (GGA) framework, employing the Perdew-Burke-Ernzerhof (PBE) formulation51. The projected augmented wave (PAW) potentials52 were selected to characterize the ionic cores and incorporate valence electrons. This was achieved using a plane wave basis set, with a kinetic energy cutoff of 500 eV. Partial occupancies of the Kohn-Sham orbitals were permitted using the Gaussian smearing technique, with a width of 0.02 eV. Electronic energy was deemed self-consistent when the energy change fell below 10−6 eV. Hubbard U corrections were implemented for the transition metal d-electrons. The U–J parameters for Sc (3.28), Ti (5.61), Ni (3.80), Cu (3.08), Y (1.98), Zr (2.61), and Mo (3.28) atoms were subsequently adopted.
Electrochemical measurement
All electrochemical measurements were conducted utilizing a standard three-electrode cell at ambient temperature, interfaced with a VSP-300 potentiostat (BioLogic, France). The Ag/AgCl electrode, saturated in 3.5 M KCl, served as the reference electrode, while the graphite rod functioned as the counter electrode. The potential documented in this study were normalized relative to the RHE, employing a standard RHE calibration equation: ERHE = EAg/AgCl + (0.196 + 0.059 pH) V. The working electrode, a rotating disk electrode (RDE), was fabricated using glassy carbon (PINE, with a diameter of 5.00 mm and a disk area of 0.196 cm2). The RDE was meticulously polished using α-Al2O3 powder of progressively decreasing sizes (ranging from 1.0 to 50 nm). Subsequently, it was ultrasonically cleansed with deionized water and absolute ethanol. The catalyst powder, weighing 5 mg, was dispersed in 1 ml of a 1:3 v/v isopropanol/deionized water (DIW) solution, containing 40 μL of a 5 wt% Nafion solution. This mixture was ultrasonicated to generate a homogeneous ink.
For the electrochemical measurements of the hydrogen evolution reaction (HER), a volume of 40 µl of catalyst ink was deposited on a glassy carbon electrode, yielding a catalyst loading of approximately 1.0 mg cm−2. Prior to conducting the HER measurements, 200 mL of freshly electrolytes (seawater with a pH of 7.84 ± 0.02, sourced from the Gulf of Mexico, specifically the Gulf Stream off Dauphin Island, Alabama, and supplied by Sigma-Aldrich under the product code S9148) were purged with pure argon for a duration of 30 minutes. The polarization curves of HER were documented at a rate of 5 mV s−1 and 1600 r.p.m (to eliminate the formation of H2 bubbles in situ) under ambient conditions. The accelerated stability measurements were conducted through potential cycling between −0.2 V and −0.4 V relative to RHE, with a sweep rate of 200 mV s−1. Subsequently, the electrode underwent polarization curves with a sweep rate of 5 mV s−1.
The electrochemical impedance spectroscopy (EIS) measurement was executed under a constant overpotential of 300 mV, spanning a frequency range from 100 kHz to 100 mHz. The sinusoidal voltage amplitude was set at 5 mV. The polarization curves were re-configured to represent overpotential (η) against the logarithm of current (log j) to generate Tafel plots for the evaluation of the HER kinetics of the examined catalysts. The Tafel slope (b) was derived by fitting the linear section of the Tafel plots to the Tafel equation (η=blog(j)+a). The exchange current density (j0) was subsequently calculated from the Tafel curves employing an extrapolation technique.
The working electrode, comprised of Mo2N-modified carbon paper with a catalyst loading of approximately 1.0 mg cm−2, was utilized to perform chronopotentiometry experiments at a constant current density of 100 mA cm−2. To gauge the double-layer capacitance, cyclic voltammograms were taken at various sweep rates within the potential range of −0.05 to 0.05 V relative to RHE, under ambient temperature conditions. All polarization curves were adjusted with iR compensation, derived from the resistance of the solution.
The process of CO stripping was initiated by maintaining the electrode potential at 0.1 V relative to RHE for a duration of 10 minutes, allowing for the adsorption of CO on the catalyst surface. Subsequently, an Ar purge was conducted for 30 minutes to eliminate any residual CO present in the electrolyte. The CO stripping current was then determined using cyclic voltammetry within a potential range of 0 to 1.2 V, with a sweep rate of 20 mV s−1.
The ECSA of the catalyst is determined using the double-layer capacitance, calculated from the equation:
| 1 |
The specific capacitance, denoted as Cs, refers to the capacitance of a catalyst or an atomically smooth, planar surface of a material, per unit area, under identical electrolyte conditions. A general value of Cs = 0.030 mF cm−2 is commonly adopted, based on the typical reported data53.
NMR measurement
In the context of 1H nuclear magnetic resonance (1H NMR) measurement, a carbon fiber paper modified with Mo2N served as the working electrode (catalyst loading: ~1.00 mg cm−2; area: 10 cm2) and 15 mL Ar-saturated natural seawater was used as the electrolyte. Subsequently, HER was conducted at −10 mA cm−2 for a duration of 10 minutes, and the above operation was repeated twenty times with a newly prepared Mo2N-modified carbon fiber paper before collecting the electrolyte. The pH of the post-reaction electrolyte was subsequently adjusted to 2 through the addition of HCl. Then, 500 μL of electrolyte was combined with 100 μL of D2O, which contained 50 ppm (m/m) of dimethyl sulfoxide (DMSO) as the internal standard. Uniform spectral acquisition parameters were employed across all measurements to guarantee thorough relaxation and accurate quantification. The 1H NMR signal, with water suppression mode, was recorded utilizing a Bruker 400 MHz system.
Zero-gap membrane flow electrolyser measurement
The preparation of the catalyst ink involves dispersing the catalyst and a 5 wt% Nafion solution in ethanol. This mixture is subsequently subjected to ultrasonication to achieve a homogeneous ink. For the creation of the catalyst-coated membrane (CCM), the anode (IrO2) and cathode (Mo2N) catalysts are sprayed onto sheets of polytetrafluoroethylene (PTFE)-coated glass fiber fabric, respectively. Subsequently, the cathode catalysts, supported on PTFE-coated glass fiber fabric and Nafion 115, and the anode catalysts, supported on PTFE-coated glass fiber fabric, are subjected to hot pressing. This process is conducted at a temperature of 150 °C for a duration of 5 minutes, applying a pressure of 10 tons. Following the cooling process, the PTFE-coated glass fiber fabric was meticulously removed to achieve the CCM with an electrode area of 25 cm2. The final catalyst loadings were set at 1.0 mgIr cm−2 for the anode and 1.0 mg Mo2N cm−2 for the cathode. The prepared CCM was subsequently stored in distilled water for further measurements.
To evaluate the performance of the zero-gap membrane flow electrolyser, a titanium felt coated with platinum was utilized as the porous transport layer (PTL) at the anode. In contrast, a titanium felt without platinum coating served as the PTL at the cathode. The zero-gap membrane flow electrolyser was operated at a temperature of 60 °C. Simulated seawater, composed of 1.19 g L−1 MgSO4 and 0.40 g L−1 CaSO4, served as the reactant for the constructed zero-gap membrane flow electrolyser. The electrolyser was supplied via a peristaltic pump circulation system.
Hydrogen production rate
The drainage technique is employed to accumulate hydrogen over a specified timeframe in order to ascertain the rate of hydrogen production. Briefly, fill the cylinder with water and position it in the sink with its mouth submerged below the water level. Subsequently, place the gas outlet beneath the bottle’s mouth. As the water drains from the bottle, it is simultaneously replaced by gas. The rate of H2 production is ~10.45 L h−1 when the zero-gap membrane flow electrolyser operates at a current density of 1 A cm−2 and a temperature of 60 °C.
Energy efficiency calculation
The calculation of energy efficiency for a zero-gap membrane flow electrolyser is predicated on the subsequent equation:
| 2 |
The value of 1.23 V represents the theoretical energy of the products, while Ucell denotes the cell voltage (V) necessary to deliver a current density of 1 A cm−2. The Faradaic efficiency (FE) for the conversion of H2O to H2, quantified here, is 99.95%.
Energy consumption calculation
The energy consumption of the zero-gap membrane flow electrolyzer is determined using the subsequent equation:
| 3 |
Icell refers to the current delivered (A), t represents the operational time (h), and m denotes the mass of hydrogen produced over a period of t.
Hydrogen production cost calculation
The electricity cost is calculated based on the energy consumption demonstrated above:
| 4 |
where energy consumption is calculated at 1 A cm−2, with the electricity bill being $0.02/Kw h.
Local pH on the catalyst surface
In this study, we utilized a rotating ring-disk electrode (RRDE), modified with IrOx, to measure the local pH on the catalyst surfaces. First, we prepared the electrodeposition solution of IrOx in accordance with Yamanaka’s method46. Subsequently, we electrodeposited the IrOx film onto the Pt ring electrode of the RRDE using cyclic voltammetry. Next, the pH dependence of the open circuit potential (Eocp) in seawater was quantified utilizing an IrOx ring electrode. The correlation between Eocp and the pH value of the ring electrode (pHring) is established through the subsequent equation:
| 5 |
The values of a and b were derived from the linear regression analysis conducted on the relationship between Eocp and the pH values of the bulk seawater (Supplementary Fig. 32). Subsequently, we conducted a measurement of local pH on the catalyst surface, varying the current density. We applied different constant current densities to the catalyst-coated disk electrode immersed in seawater. Simultaneously, we recorded the Eocp using an IrOx ring electrode. The pH value of the IrOx ring electrode was determined using the Eocp and Eq. (5). To ascertain the pH value of the catalyst-coated disk electrode, the pH value of the IrOx ring electrode was utilized in the subsequent equation:
| 6 |
The concentrations of H+ ions on the ring electrode, disk electrode, and the bulk seawater are denoted as , and , respectively. And the concentrations of OH− on the ring electrode, disk electrode and the bulk seawater are denoted as , and , respectively. In addition, the value of ND = 0.275 represents the collection efficiency.
In situ SEIRAS experiment
Firstly, it is necessary to prepare the gold film for SEIRAS. The process begins with washing the silicon prism using an aqua regia solution. Subsequently, the prism is polished with a 0.05 μm Al2O3 slurry, and then it undergoes ultrasound treatment in acetone and deionized water, respectively. Following this, the prism is dried and immersed in a NH4F solution for 120 seconds. The final step involves submerging the silicon prism in a mixture of a gold-plated solution and a 2 wt % HF aqueous solution at a temperature of 55 °C for 7 minutes, thereby chemically depositing the gold film. Following deposition, the gold film on the silicon prism was rinsed with deionized water (DIW). Subsequently, the working electrodes were prepared by applying ink onto the gold film. A catalyst powder of 10 mg was dispersed in 1 mL of isopropanol. The mixture was subjected to ultrasonication to create a homogeneous ink. Then, 200 μL of the catalyst ink was evenly pipetted onto the gold film. Lastly, the working electrode was allowed to dry naturally.
In situ SEIRAS tests were conducted using a spectroelectrochemical cell with a three-electrode configuration. A graphite rod served as the counter electrode, while a saturated Ag/AgCl acted as the reference electrode. The cell was incorporated into a NICOLET iS50 FTIR spectrometer, which featured a liquid nitrogen-cooled MCT detector. Throughout the in situ SEIRAS test, a continuous flow of Ar was maintained within the electrolyte. Furthermore, each spectrum was composed of 32 individual beams, each with a resolution of 4 cm−1.
In situ DEMS measurements
In situ DEMS measurements were carried on a Linglu QMG 250 device, which was fitted with a high vacuum chamber linked to a molecular pump. An electrochemical cell was linked to this vacuum chamber through a cold trap, which was cooled using dry ice. This configuration was specifically designed to capture water vapor for introduction into the mass spectrometer. For the process of gas-liquid separation, the working electrode was constructed by depositing gold onto a 50 μm thick, porous polytetrafluoroethylene (PTFE) membrane. Characterized by a pore size of 0.2 μm and a porosity of 85%, this membrane served as the foundation for the electrode. Subsequently, catalyst ink was applied to a gold-coated membrane, with a loading of roughly 1.0 mg cm−2. A saturated Ag/AgCl electrode functioned as the reference electrode, while a graphite rod served as the counter electrode. The experiments were conducted in seawater, with the electrolyte saturated by Ar bubbling prior to electrochemical measurements. A mass spectrometer monitored the gaseous products, of varying molecular weights, generated during the HER in real-time.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
This work is supported by the National Basic Research Program of China (Grant 2018YFA0702001, M.-R.G.), the National Natural Science Foundation of China (Grants 22225901, 21975237, 22175162, M.-R.G.; 22275047, Y.-R.Z.), the Fundamental Research Funds for the Central Universities (GrantWK2340000101, M.-R.G.), the USTC Research Funds of the Double First-Class Initiative (Grant YD2340002007, M.-R.G.; YD9990002017, X.-L.Z.), the Open Funds of the State Key Laboratory of Rare Earth Resource Utilization (Grant RERU2022007, M.-R.G.), the China Postdoctoral Science Foundation (Grants 2022M723032, X.-L.Z.), the Natural Science Foundation Youth Project of Anhui Province (2308085QB37, X.-L.Z.), and the China National Postdoctoral Program for Innovative Talents (BX20230340, X.-L.Z.).
Author contributions
M.-R.G. supervised the project. X.-L.Z. and Y.-R.Z. performed the experiments, collected and analyzed the data. L.S. performed the HRTEM measurements. P.-C.Y. and S.-P.S. performed the SEM measurements. P.-P.Y., L.-P.C., and Z.-Z.W. helped with electrochemical data collection and analysis. M.-R.G. and X.-L.Z. co-wrote the manuscript. All authors discussed the results and commented on the manuscript. All authors discussed the results and commented on the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiao-Long Zhang, Peng-Cheng Yu, Shu-Ping Sun.
Contributor Information
Ya-Rong Zheng, Email: yrzh@hfut.edu.cn.
Min-Rui Gao, Email: mgao@ustc.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-53724-1.
References
- 1.Turner, J. A. Sustainable hydrogen production. Science305, 972–974 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Rashid, M., Al Mesfer, M. K., Naseem, H. & Danish, M. Hydrogen production by water electrolysis: a review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. Int. J. Eng. Adv. Technol.4, 80–93 (2015). [Google Scholar]
- 3.Chatenet, M. et al. Water electrolysis: from textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev.51, 4583–4762 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dresp, S., Dionigi, F., Klingenhof, M. & Strasser, P. Direct electrolytic splitting of seawater: opportunities and challenges. ACS Energy Lett.4, 933–942 (2019). [Google Scholar]
- 5.Khan, M. A. et al. Seawater electrolysis for hydrogen production: a solution looking for a problem? Energ Environ Sci14, 4831–4839 (2021). [Google Scholar]
- 6.Tong, W. et al. Electrolysis of low-grade and saline surface water. Nat. Energy5, 367–377 (2020). [Google Scholar]
- 7.Jin, H. et al. Emerging materials and technologies for electrocatalytic seawater splitting. Sci. Adv.9, eadi7755 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang, N. et al. Strong-proton-adsorption co-based electrocatalysts achieve active and stable neutral seawater splitting. Adv. Mater.35, e2210057 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Xu, W. et al. Ag nanoparticle‐induced surface chloride immobilization strategy enables stable seawater electrolysis. Adv. Mater.35, e202306062 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Kang, X. et al. A corrosion-resistant RuMoNi catalyst for efficient and long-lasting seawater oxidation and anion exchange membrane electrolyzer. Nat. Commun.14, 3607 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu, H., Wan, J., Goodsite, M. & Jin, H. Advancing direct seawater electrocatalysis for green and affordable hydrogen. One Earth6, 267–277 (2023). [Google Scholar]
- 12.KAPP, E. M. The precipitation of calcium and magnesium from sea water by sodium hydroxide. Biol. Bull.55, 453–458 (1928). [Google Scholar]
- 13.Farràs, P., Strasser, P. & Cowan, A. J. Water electrolysis: direct from the sea or not to be? Joule5, 1921–1923 (2021). [Google Scholar]
- 14.Ros, C. et al. Facing seawater splitting challenges by regeneration with Ni−Mo−Fe bifunctional electrocatalyst for hydrogen and oxygen evolution. ChemSusChem14, 2872–2881 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Li, P. et al. Common-ion effect triggered highly sustained seawater electrolysis with additional NaCl production. Research2020, 2872141 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie, H. et al. A membrane-based seawater electrolyser for hydrogen generation. Nature612, 673–678 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Wu, L. et al. Heterogeneous bimetallic phosphide Ni2P-Fe2P as an efficient bifunctional catalyst for water/seawater splitting. Adv. Funct. Mater.31, 2006484 (2021). [Google Scholar]
- 18.Liu, H. et al. High-performance alkaline seawater electrolysis with anomalous chloride promoted oxygen evolution reaction. Angew. Chem. Int. Ed.62, e202311674 (2023). [DOI] [PubMed] [Google Scholar]
- 19.Kuang, Y. et al. Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels. Proc. Natl. Acad. Sci. USA.116, 6624–6629 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chong, L. et al. La- and Mn-doped cobalt spinel oxygen evolution catalyst for proton exchange membrane electrolysis. Science380, 609–616 (2023). [DOI] [PubMed] [Google Scholar]
- 21.Stiber, S. et al. A high-performance, durable and low-cost proton exchange membrane electrolyser with stainless steel components. Energy Environ. Sci.15, 109–122 (2022). [Google Scholar]
- 22.Shi, Z. et al. Enhanced acidic water oxidation by dynamic migration of oxygen species at the Ir/Nb2O5−x catalyst/support interfaces. Angew. Chem. Int. Ed.61, e202212341 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Cheng, F. et al. Synergistic action of Co-Fe layered double hydroxide electrocatalyst and multiple ions of sea salt for efficient seawater oxidation at near-neutral pH. Electrochim. Acta251, 336–343 (2017). [Google Scholar]
- 24.Kuai, C. et al. Phase segregation reversibility in mixed-metal hydroxide water oxidation catalysts. Nat. Catal.3, 743–753 (2020). [Google Scholar]
- 25.Liu, E. et al. Unifying the hydrogen evolution and oxidation reactions kinetics in base by identifying the catalytic roles of hydroxyl-water-cation adducts. J. Am. Chem. Soc.141, 3232–3239 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Alexander, A.-M. & Hargreaves, J. S. J. Alternative catalytic materials: carbides, nitrides, phosphides and amorphous boron alloys. Chem. Soc. Rev.39, 4388–4401 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Niewa, R. & DiSalvo, F. J. Recent developments in nitride chemistry. Chem. Mater.10, 2733–2752 (1998). [Google Scholar]
- 28.Zhang, X.-L. et al. Plasma-assisted synthesis of metal nitrides for an efficient platinum-group-metal-free anion-exchange-membrane fuel cell. Nano Lett.23, 107–115 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Wang, Z. et al. Catalyst preparation with plasmas: how does it work? ACS Catal.8, 2093–2110 (2018). [Google Scholar]
- 30.Shebanova, O., Soignard, E. & McMillan, P. F. Compressibilities and phonon spectra of high-hardness transition metal-nitride materials. High Press. Res.26, 87–97 (2006). [Google Scholar]
- 31.Liu, H.-X. et al. Ptn–Ov synergistic sites on MoOx/γ-Mo2N heterostructure for low-temperature reverse water–gas shift reaction. Nat. Commun.13, 5800 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, P. et al. Hydrogen bond network connectivity in the electric double layer dominates the kinetic pH effect in hydrogen electrocatalysis on Pt. Nat. Catal.5, 900–911 (2022). [Google Scholar]
- 33.Huang, B. et al. Cation- and pH-dependent hydrogen evolution and oxidation reaction kinetics. JACS Au1, 1674–1687 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ataka, K., Yotsuyanagi, T. & Osawa, M. Potential-dependent reorientation of water molecules at an electrode/electrolyte interface studied by surface-enhanced infrared absorption spectroscopy. J. Phys. Chem.100, 10664–10672 (1996). [Google Scholar]
- 35.Pankewitz, T., Lagutschenkov, A., Niedner-Schatteburg, G., Xantheas, S. S. & Lee, Y. T. Infrared spectrum of NH4+(H2O): evidence for mode specific fragmentation. J. Chem. Phys.126, 074307 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Grimaud, A. et al. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem.9, 457–465 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Peng, J. et al. Design principles for transition metal nitride stability and ammonia generation in acid. Joule7, 150–167 (2023). [Google Scholar]
- 38.Alfarano, S. R. et al. Stripping away ion hydration shells in electrical double-layer formation: Water networks matter. Proc. Natl. Acad. Sci. USA.118, e2108568118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serva, A., Scalfi, L., Rotenberg, B. & Salanne, M. Effect of the metallicity on the capacitance of gold–aqueous sodium chloride interfaces. J. Chem. Phys.155, 044703 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Perra, D., Drenchev, N., Chakarova, K., Cutrufello, M. G. & Hadjiivanov, K. Remarkable acid strength of ammonium ions in zeolites: FTIR study of low-temperature CO adsorption on NH4FER. RSC Adv.4, 56183–56187 (2014). [Google Scholar]
- 41.de Lima, G. F., Duarte, H. A. & Pliego, J. R. Dynamical discrete/continuum linear response shells theory of solvation convergence test for NH4+ and OH- ions in water solution using DFT and DFTB methods. J. Phys. Chem. B114, 15941–15947 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Li, J. et al. A fundamental viewpoint on the hydrogen spillover phenomenon of electrocatalytic hydrogen evolution. Nat. Commun.12, 3502 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, B. et al. A strongly coupled Ru–CrOx cluster–cluster heterostructure for efficient alkaline hydrogen electrocatalysis. Nat. Catal.7, 441–451 (2024). [Google Scholar]
- 44.Yang, B., Huang, T., Dou, Y. & Kong, W. Stoichiometry ratio-induced structural and electronic properties changes of TaN thin films prepared by reactive magnetron sputtering. Mater. Sci. Semicond. Proc.170, 107979 (2024). [Google Scholar]
- 45.Lv, C. et al. Defect engineering metal-free polymeric carbon nitride electrocatalyst for effective nitrogen fixation under ambient conditions. Angew. Chem. Int. Ed.57, 10246–10250 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Yokoyama, Y. et al. In situ local pH measurements with hydrated iridium oxide ring electrodes in neutral pH aqueous solutions. Chem. Lett.49, 195–198 (2020). [Google Scholar]
- 47.Khatun, S. et al. New age chloride shielding strategies for corrosion resistant direct seawater splitting. Chem. Commun.59, 4578–4599 (2023). [DOI] [PubMed] [Google Scholar]
- 48.Zhang, L., Jie, X., Shao, Z.-G., Wang, X. & Yi, B. The dynamic-state effects of sodium ion contamination on the solid polymer electrolyte water electrolysis. J. Power Sources241, 341–348 (2013). [Google Scholar]
- 49.Bender, G. & Dinh, H. N. National Renewable Energy Lab.(NREL) (2020).
- 50.Kresse, G. & Hafner, J. Ab initiomolecular dynamics for liquid metals. Phys. Rev. B47, 558–561 (1993). [DOI] [PubMed] [Google Scholar]
- 51.Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett.77, 3865 (1996). [DOI] [PubMed] [Google Scholar]
- 52.Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B50, 17953–17979 (1994). [DOI] [PubMed] [Google Scholar]
- 53.McCrory, C. C. L., Jung, S., Peters, J. C. & Jaramillo, T. F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc.135, 16977–16987 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Source data are provided with this paper.