Abstract
This post-hoc analysis of the SABINA III study evaluated the association of short-acting β2-agonist (SABA) prescriptions and self-reported over-the-counter (OTC) SABA purchase in the previous 12 months with asthma-related outcomes using multivariable regression models in 4556 patients (mean age, 48.9 years). Of the 2810 patients prescribed ≥3 SABA canisters, 776 (27.6%) also purchased ≥1 SABA OTC. This subset of 776 patients reported the highest disease burden; 73.2% had ≥1 severe exacerbation and 55.7% had uncontrolled asthma. Asthma-related outcomes worsened with any SABA OTC purchase, regardless of SABA prescriptions; disease burden was the highest in patients with ≥3 SABA prescriptions and ≥1 SABA OTC purchase vs 1–2 SABA prescriptions only (86% lower odds of having at least partly controlled asthma and 124% increased incidence of severe asthma (both P < 0.001). These findings emphasize the need to implement policy changes to restrict SABA purchase without prescriptions and ensure access to affordable asthma care.
Subject terms: Asthma, Health policy
Introduction
Asthma, one of the world’s fastest-growing diseases, affects approximately 339 million people globally, imposing a substantial burden on patients, healthcare systems, and societies as a whole1–3. Despite a global consensus on the goals of asthma management and the availability of a range of effective treatment options4, asthma remains poorly controlled in a substantial proportion of patients worldwide5–7. Additionally, avoidable asthma-related deaths continue to occur, particularly in low- and lower-middle-income countries, where undertreatment and poor access to inhaled corticosteroid (ICS) controller medications remain a challenge1,8,9.
A range of factors contribute to poor asthma control10; however, overprescription of short-acting β2-agonists (SABAs; prescription of ≥3 canisters in the previous 12 months)11 and insufficient provision of ICS-containing medication continue to remain key reasons for preventable disease worsening9. The high reliance on reliever inhalers may be attributable, in part, to the long-held paradox of asthma management, whereby for over 20 years, the Global Initiative for Asthma (GINA) recommended as-needed SABA as the preferred reliever for patients with mild asthma12,13. However, following the accumulating evidence that SABA overuse (≥3 canisters/year) is associated with an increased risk of exacerbations, hospitalizations, and mortality14–17, GINA has not recommended as-needed SABA without concomitant ICS for patients aged 12 years or older since 201913,18. Nevertheless, GINA updates have not been universally adopted, and even though SABAs have no inherent anti-inflammatory activity19, many patients remain psychologically attached to their reliever inhalers owing to their rapid symptom relief, unaware that frequent SABA usage could worsen asthma control20,21. While patients often recognize early signs of asthma worsening, their initial response is to increase SABA intake rather than adjust the dose of anti-inflammatory ICS-containing medication, and increase their ICS dose only at the peak of asthma worsening22,23, thereby contributing to preventable disease morbidity.
SABAs are relatively inexpensive inhaled medications24 and can be obtained without a prescription (over-the-counter [OTC]) in many parts of the world25; therefore, patients may preferentially rely on relievers instead of prescribed controller medications26, which further increases the potential for SABA overuse11. Indeed, findings from the SABA use IN Asthma (SABINA) International III study, which was conducted in 8351 patients from 24 countries across 5 continents, reported not only SABA overprescription ( ≥3 canisters in the previous 12 months) in 38% of patients, but that 18% of patients also purchased SABA OTC11, likely without physicians’ recommendation or knowledge. Furthermore, among patients who purchased SABA OTC, 76.8% had also received SABA prescriptions: 69.9% for ≥3 canisters and 35.8% for ≥10 canisters in the 12 months before the study visit11. Strikingly, purchase of SABA OTC was a prevalent phenomenon in many SABINA III countries, including the Philippines27, Russia28, and Australia25, where 31%, 30%, and 25% of patients purchased SABA OTC from the pharmacy without a prescription, respectively (Supplementary Fig. 1). In addition, high levels of SABA OTC purchase were also observed in many African countries. For instance, 39% of patients in Kenya29, 33% in Egypt25, and 27% in South Africa30 purchased SABA OTC. Notably, results from an Australian study of 720 patients, which analyzed data from SABINA III, the Optimum Patient Care Research Database Australia, and Optimum Patient Care Australia, reported that although 92.9% of patients receiving SABA prescriptions were overprescribed SABAs, 37.5% of those purchasing SABA OTC acquired ≥3 canisters annually31.
The purchase of SABA OTC may be compounded by systemic issues in healthcare systems across countries, socioeconomic and structural barriers that may restrict access to healthcare services, limited availability of drugs or a lack of access to affordable medications, inadequate healthcare reimbursement, and high rates of out-of-pocket expenditure for outpatient services1,26,32. This is of concern because OTC purchase of SABA is associated with low rates of consultation with family practitioners and specialists; low use of prescription-only medication, particularly ICS; and undertreatment of asthma33–35. With recent data emerging on the high prevalence of SABA OTC purchase globally11,26,36, an assessment of the clinical burden associated with this potentially unregulated access to SABA, which is currently lacking, is urgently required. Although the SABINA III study showed that SABA overprescription was associated with decreased odds of having at least partly controlled asthma (partly controlled plus well-controlled asthma) and an increased incidence rate of severe exacerbations, the analysis did not evaluate the clinical burden of unregulated purchase of SABA OTC11. Therefore, this post hoc analysis of the SABINA III study was undertaken to evaluate the association of SABA prescriptions and SABA OTC purchases with asthma-related health outcomes. As part of this analysis, the incremental impact of SABA OTC purchase on asthma-related health outcomes was also evaluated in patients already prescribed SABAs.
Results
Baseline patient and disease characteristics
This analysis included 4556 patients (mean age 48.9 years) from the SABINA III study who received SABA prescriptions with and without SABA OTC purchase (Table 1). Of these patients, 3,449 (75.7%) received SABA prescriptions only, with 1,107 (24.3%) both prescribed and purchased SABA OTC. A majority of patients were treated by specialists rather than by primary care physicians (81.4% and 18.6%, respectively). Half of all patients (50.6%) reported full healthcare reimbursement and 27.8% received no healthcare reimbursement.
Table 1.
Demographics and baseline clinical characteristics of the SABINA III population included in the analysis (N = 4556) categorized by SABA prescriptions and SABA OTC purchase.
| SABA prescriptions onlya (n = 3449) | SABA prescriptions + SABA OTC purchase (n = 1107) | SABA prescriptions (with and without SABA OTC purchase) (N = 4556) | |
|---|---|---|---|
| Practice type | |||
| Primary care | 683 (19.9) | 160 (14.5) | 843 (18.6) |
| Specialist care | 2751 (80.1) | 942 (85.5) | 3693 (81.4) |
| Total | 3434 | 1102 | 4536 |
| Age (years) | |||
| Mean (SD) | 49.9 (16.3) | 45.9 (15.6) | 48.9 (16.2) |
| Median (Min, Max) | 51.0 (12.0, 93.0) | 45.0 (12.0, 87.0) | 50.0 (12.0, 93.0) |
| 25th and 75th percentile | 39.0, 62.0 | 35.0, 57.0 | 37.0, 61.0 |
| Total | 3449 | 1107 | 4556 |
| Age groups (years) | |||
| 12–17 | 104 (3.0) | 31 (2.8) | 135 (3.0) |
| ≥18–54 | 1868 (54.2) | 724 (65.4) | 2592 (56.9) |
| ≥55 | 1477 (42.8) | 352 (31.8) | 1829 (40.1) |
| Total | 3449 | 1107 | 4556 |
| Sex | |||
| Female | 2421 (70.2) | 750 (67.8) | 3171 (69.6) |
| Male | 1028 (29.8) | 357 (32.2) | 1385 (30.4) |
| Total | 3449 | 1107 | 4556 |
| BMI (kg/m2) | |||
| Mean (SD) | 28.1 (6.2) | 28.7 (6.6) | 28.2 (6.3) |
| Median (Min, Max) | 27.1 (11.4, 58.7) | 27.7 (13.6, 57.0) | 27.3 (11.4, 58.7) |
| 25th and 75th percentile | 23.9, 31.2 | 24.2, 32.2 | 24.0, 31.6 |
| Total | 3449 | 1107 | 4556 |
| BMI groups (kg/m2) | |||
| <18.5 | 96 (2.8) | 43 (3.9) | 139 (3.1) |
| ≥18.5–24.9 | 1010 (29.3) | 283 (25.6) | 1293 (28.4) |
| ≥25–29.9 | 1235 (35.8) | 373 (33.7) | 1608 (35.3) |
| ≥30 | 1108 (32.1) | 408 (36.9) | 1516 (33.3) |
| Total | 3449 | 1107 | 4556 |
| Education level | |||
| Unknown | 126 (3.7) | 56 (5.1) | 182 (4.0) |
| Primary school | 579 (16.8) | 165 (14.9) | 744 (16.3) |
| Secondary school | 775 (22.5) | 224 (20.2) | 999 (21.9) |
| High school | 841 (24.4) | 297 (26.8) | 1138 (25.0) |
| University and/or post-university | 1127 (32.7) | 365 (33.0) | 1492 (32.8) |
| Total | 3448 | 1107 | 4555 |
| Healthcare insurance/medication funding | |||
| Unknown | 76 (2.2) | 29 (2.6) | 105 (2.3) |
| Not reimbursed | 869 (25.2) | 397 (35.9) | 1266 (27.8) |
| Partially reimbursed | 663 (19.2) | 213 (19.2) | 876 (19.2) |
| Fully reimbursed | 1840 (53.3) | 467 (42.2) | 2307 (50.6) |
| Total | 3448 | 1106 | 4554 |
| Tobacco smoking status history | |||
| Active smoker | 159 (4.6) | 92 (8.3) | 251 (5.5) |
| Former smoker | 432 (12.5) | 146 (13.2) | 578 (12.7) |
| Never smoker | 2856 (82.8) | 869 (78.5) | 3725 (81.8) |
| Total | 3447 | 1107 | 4554 |
Data are presented as n (%) unless otherwise specified.
BMI body mass index, Max maximum, Min minimum, OTC over-the-counter, SABA short-acting β2-agonist, SABINA SABA use IN Asthma, SD standard deviation.
aSABA prescriptions only do not include SABA OTC purchase.
Baseline patient and disease characteristics were comparable between the two subgroups; however, a few exceptions were observed (Tables 1 and 2). Compared with patients who were both prescribed and had purchased SABA OTC, a higher proportion of patients who received SABA prescriptions only reported full healthcare reimbursement (53.3% vs 42.2%, respectively) and ≥1 comorbidity (66.5% vs 61.7%, respectively). Additionally, a higher proportion of patients who were both prescribed and had purchased SABA OTC were treated in specialist care than those who received SABA prescriptions only (85.5% vs 80.1%, respectively).
Table 2.
Disease characteristics of the SABINA III population included in the analysis (N = 4556) categorized by SABA prescriptions and SABA OTC purchase.
| SABA prescriptions onlya (n = 3449) | SABA prescriptions + SABA OTC purchase (n = 1107) | SABA prescriptions (with and without SABA OTC purchase) (N = 4556) | |
|---|---|---|---|
| Asthma duration (years) | |||
| Mean (SD) | 17.5 (15.40) | 16.6 (13.20) | 17.3 (14.90) |
| Median (Min, Max) | 12.0 (1.0, 85.0) | 13.0 (1.0, 70.0) | 12.0 (1.0, 85.0) |
| 25th and 75th percentile | 6.0, 25.0 | 6.0, 24.0 | 6.0, 25.0 |
| Total | 3449 | 1107 | 4556 |
| GINA classification | |||
| Step 1 | 301 (8.7) | 126 (11.4) | 427 (9.4) |
| Step 2 | 714 (20.7) | 212 (19.2) | 926 (20.3) |
| Step 3 | 781 (22.6) | 220 (19.9) | 1001 (22.0) |
| Step 4 | 1136 (32.9) | 351 (31.7) | 1487 (32.6) |
| Step 5 | 517 (15.0) | 198 (17.9) | 715 (15.7) |
| Total | 3449 | 1107 | 4556 |
| Number of comorbidities | |||
| No comorbidities | 1157 (33.5) | 424 (38.3) | 1581 (34.7) |
| 1–2 comorbidities | 1643 (47.6) | 504 (45.5) | 2147 (47.1) |
| 3–4 comorbidities | 535 (15.5) | 153 (13.8) | 688 (15.1) |
| ≥5 comorbidities | 114 (3.3) | 26 (2.3) | 140 (3.1) |
| Total | 3449 | 1107 | 4556 |
Data are presented as n (%) unless otherwise specified.
GINA Global Initiative for Asthma, Max maximum, Min minimum, OTC over-the-counter, SABA short-acting β2-agonist, SABINA SABA use IN Asthma, SD standard deviation.
aSABA prescriptions only do not include SABA OTC purchase.
Asthma-related health outcomes by SABA OTC purchase and SABA prescription categories
Overall, 2810 patients (61.7%) were prescribed ≥3 SABA canisters; of these patients, 2034 (72.4%) did not purchase SABA OTC, while 776 (27.6%) also purchased ≥1 SABA canister OTC (Table 3).
Table 3.
OTC purchase of SABA canisters categorized by SABA prescription categories for the SABINA III population included in the analysis (N = 4556).
| SABA prescription → | 1–2 canisters (n = 1746) | ≥3 canisters (n = 2810) | ||
|---|---|---|---|---|
| SABA OTC purchase → | 0 canisters | ≥1 canisters | 0 canisters | ≥1 canisters |
| Number of patients, n (%) | 1415 (81.0) | 331 (19.0) | 2034 (72.4) | 776 (27.6) |
| Number of missing values | 0 | 0 | 0 | 0 |
| Total | 1415 | 331 | 2034 | 776 |
OTC over-the-counter, SABA short-acting β2-agonist, SABINA SABA use IN Asthma.
Level of asthma control assessed during the study visit
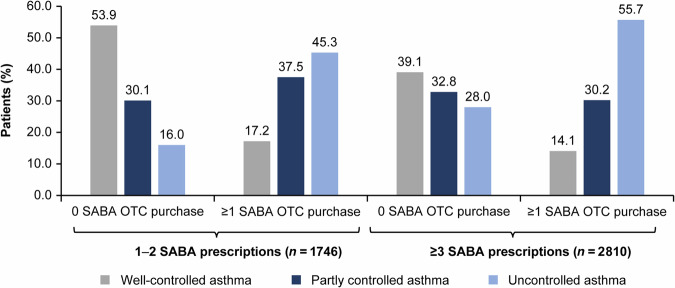
The highest percentage of patients with well-controlled asthma (53.9%) was observed in the subset with 1–2 SABA canister prescriptions with no SABA OTC purchase (Fig. 1). In contrast, the percentage of patients with uncontrolled asthma was the highest (55.7%) among those who were prescribed ≥3 SABA canisters and who also purchased ≥1 SABA canister OTC (Fig. 1). Across both SABA prescription groups (1–2 and ≥3 prescriptions), the proportion of patients with uncontrolled asthma increased with ≥1 vs 0 SABA OTC purchase (from 16.0% to 45.3% and 28.0% to 55.7%, respectively).
Fig. 1. Level of asthma control for the SABINA III population included in the analysis (N = 4556) by number of SABA prescriptions and number of SABA canisters purchased OTC.
Data missing for 15 patients with 1–2 SABA prescriptions and 0 SABA OTC purchase and 1 patient with ≥3 SABA prescriptions and ≥1 SABA OTC purchase. OTC over-the-counter, SABA short-acting β2-agonist, SABINA SABA use IN Asthma.
Severe asthma exacerbations during the previous 12 months
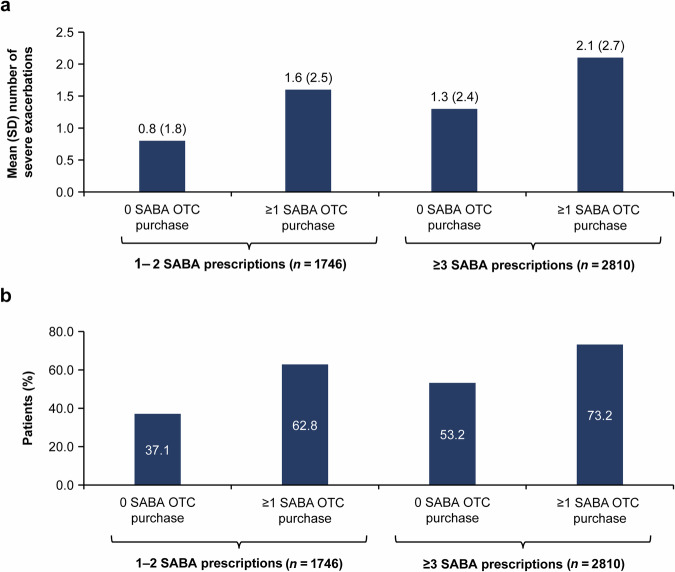
Patients who were prescribed 1–2 SABA canisters and did not purchase any SABA OTC reported the lowest mean (standard deviation [SD]) number of severe exacerbations (0.8 [1.8]; Fig. 2a) and the highest percentage of patients experiencing 0 exacerbations (62.8%; Fig. 2b). In contrast, the subset of patients who were prescribed ≥3 SABA canisters and who also purchased ≥1 SABA canister OTC reported the highest mean (SD) number of severe exacerbations (2.1 [2.7]; Fig. 2a) and the highest percentage of patients experiencing ≥1 severe exacerbation (73.2%; Fig. 2b). Regardless of SABA prescription (1–2 or ≥3 prescriptions), the proportion of patients with ≥1 severe exacerbation increased with ≥1 vs 0 SABA OTC purchase across both SABA prescription groups (from 37.1% to 62.8% and 53.2% to 73.2%, respectively).
Fig. 2. Severe asthma exacerbations in the previous 12 months by number of SABA prescriptions and number of SABA canisters purchased OTC for the SABINA III population included in the analysis (N = 4556).
a Mean (SD) number of severe exacerbations; b Proportion of patients experiencing ≥1 severe exacerbation. Data missing for 1 patient with ≥3 SABA prescriptions and 0 SABA OTC purchase. OTC over-the-counter, SABA short-acting β2-agonist, SABINA SABA use IN Asthma, SD standard deviation.
Association of SABA prescriptions and SABA OTC purchase with asthma-related health outcomes
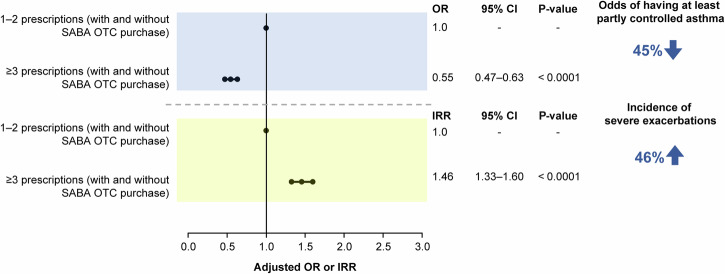
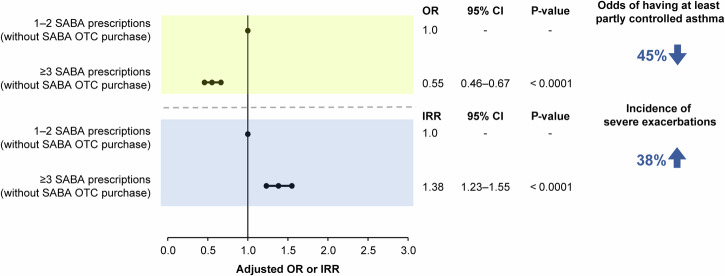
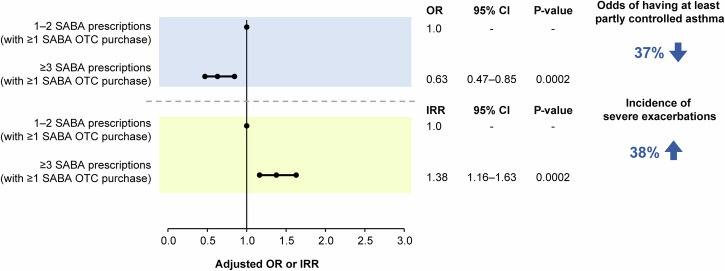
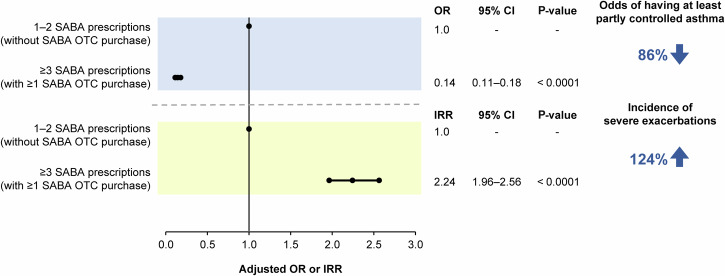
The disposition of patients included in the association analyses is shown in Supplementary Figs. 2–5. Findings from the prespecified association analyses revealed that prescription of ≥3 SABA canisters in the previous 12 months was consistently associated with poor asthma-related health outcomes. In patients prescribed ≥3 vs 1–2 SABA canisters, both with and without SABA OTC purchase, the odds of having at least partly controlled asthma decreased by 45% (adjusted odds ratio [OR], 0.55; 95% confidence interval [CI], 0.47–0.63; P < 0.0001), while the incidence of severe asthma exacerbations increased by 46% (adjusted incidence rate ratio [IRR], 1.46; 95% CI, 1.33–1.60; P < 0.0001; Fig. 3). Similar findings were observed when patients with SABA OTC purchase were excluded from the analyses, with prescription of ≥3 vs 1–2 SABA canisters associated with 45% lower odds of having at least partly controlled asthma (adjusted OR, 0.55; 95% CI, 0.46–0.67; P < 0.0001) and a 38% increased incidence of severe asthma exacerbations (adjusted IRR, 1.38; 95% CI, 1.23–1.55; P < 0.0001; Fig. 4). Likewise, when patients with ≥1 SABA OTC purchase were included in the analysis, prescription of ≥3 vs 1–2 SABA canisters was associated with 37% lower odds of having at least partly controlled asthma (adjusted OR, 0.63; 95% CI, 0.47–0.85; P = 0.0002) and a 38% increased incidence of severe asthma exacerbations (adjusted IRR, 1.38; 95% CI, 1.16–1.63; P = 0.0002; Fig. 5). However, asthma-related health outcomes were worse in patients with ≥3 SABA prescriptions and ≥1 SABA OTC purchase compared with those with only 1–2 SABA canister prescriptions in the previous 12 months, with 86% lower odds of having at least partly controlled asthma (adjusted OR, 0.14; 95% CI, 0.11–0.18; P < 0.0001) and a 124% increased incidence of severe asthma exacerbations (adjusted IRR, 2.24; 95% CI, 1.96–2.56; P < 0.0001; Fig. 6).
Fig. 3. Association of 1–2 vs ≥3 SABA prescriptions (both with and without SABA OTC purchase) with the level of asthma control assessed during the study visit and severe exacerbations in the 12 months before the study visit in the SABINA III study population included in this analysis (N = 4556).
CI confidence interval, IRR incidence rate ratio, OR odds ratio, OTC over-the-counter, SABA short-acting β2-agonist, SABINA SABA use IN Asthma.
Fig. 4. Association of 1–2 vs ≥3 SABA prescriptions (both without SABA OTC purchase) with the level of asthma control assessed during the study visit and severe exacerbations in the 12 months before the study visit in the SABINA III study population included in this analysis (N = 4556).
CI confidence interval, IRR incidence rate ratio, OR odds ratio, OTC over-the-counter, SABA short-acting β2-agonist, SABINA SABA use IN Asthma.
Fig. 5. Association of 1–2 vs ≥3 SABA prescriptions (both with ≥1 SABA OTC purchase) with the level of asthma control assessed during the study visit and severe exacerbations in the 12 months before the study visit in the SABINA III study population included in this analysis (N = 4556).
CI confidence interval, IRR incidence rate ratio, OR odds ratio, OTC over-the-counter, SABA short-acting β2-agonist, SABINA SABA use IN Asthma.
Fig. 6. Association of 1–2 SABA prescriptions (without SABA OTC purchase) vs ≥3 SABA prescriptions (with ≥1 SABA OTC purchase) with the level of asthma control assessed during the study visit and severe exacerbations in the 12 months before the study visit in the SABINA III study population included in this analysis (N = 4556).
CI confidence interval, IRR incidence rate ratio, OR odds ratio, OTC over-the-counter, SABA short-acting β2-agonist, SABINA SABA use IN Asthma.
Discussion
This post hoc analysis of the SABINA III study shows that, regardless of SABA prescriptions, asthma-related outcomes worsened with any SABA OTC purchase, rendering patients more vulnerable to poor disease control and severe exacerbations. Of concern, approximately two of every three patients were overprescribed SABAs in the previous 12 months; of these, approximately one-fourth also purchased ≥1 SABA canister OTC, highlighting the potential for overuse by patients receiving SABA from 2 sources. Unsurprisingly, therefore, asthma-related outcomes were worse in patients who were overprescribed SABAs and had purchased ≥1 SABA OTC, reporting a mean number of 2.1 severe exacerbations in the preceding 12 months, and 55.7% reporting uncontrolled asthma at baseline.
Overall, these findings provide valuable real-world insights into how patients with asthma across the globe manage their condition with SABA therapy. The fact that many patients who received SABA prescriptions also obtained SABA OTC suggests that those experiencing symptom flare-ups preferentially rely on SABAs instead of prescribed controller medications, favor rapid and easy access to SABAs to manage their asthma symptoms, and resort to purchasing SABA OTC instead of waiting for prescription refills21,26. The high prevalence of both SABA overprescription and SABA OTC purchase observed in this cohort may be explained by the fact that only half of the study population reported full healthcare reimbursement; indeed, it has been documented that patients without adequate healthcare insurance coverage are less likely to have access to effective treatment options for chronic illnesses32, which may potentially contribute to out-of-pocket expenditure on inexpensive treatment options such as SABAs.
In the overall study population, with and without SABA OTC purchase, ≥3 vs 1–2 SABA canister prescriptions were associated with 45% lower odds of having at least partly controlled asthma and a 46% increased incidence of severe asthma exacerbations. Notably, the association persisted even in patients without SABA OTC purchase, with prescription of ≥3 vs 1–2 SABA canisters being associated with 45% lower odds of having at least partly controlled asthma and a 38% increased incidence of severe asthma exacerbations. These findings confirm the association between SABA prescriptions and poor asthma outcomes previously reported in the SABINA I and II studies (conducted in the United Kingdom14 and Sweden16, respectively) and the SABINA III study11, and contribute to the growing evidence that SABA overuse in asthma needs to be addressed if further reductions in asthma morbidity and mortality are to be achieved. Owing to the lower number of patients among those who purchased ≥1 SABA canister OTC, the strength of association between prescription of ≥3 vs 1–2 SABA canisters and asthma-related outcomes was comparable to the other analyses in this study; however, patients who received ≥3 SABA prescriptions and purchased ≥1 SABA canister OTC reported significantly worse asthma-related outcomes compared with those who were only prescribed 1–2 SABA canisters (86% lower odds of having at least partly controlled asthma and a 124% increased incidence of severe asthma exacerbations).
Taken together, these findings clearly demonstrate the increased risk associated with purchase of SABA OTC without a prescription in patients who are already overprescribed reliever medication. Consequently, as part of the education process on reliever use, there is an urgent need to implement policies that regulate the purchase of SABA without a prescription, including online pharmacy stores, while ensuring that patients have access to affordable care and asthma medications and ensuring adequate provision for maintenance therapy.
Clinical implications
These results have important public health and policy implications, necessitating the implementation of interventions to break the cycle of SABA over-reliance. Restricting SABA OTC purchase, which may be considered an extreme measure37, requires an understanding of national/regional regulations and factors influencing SABA supply/demand in pharmacies38 and would need to be supported by a regulatory framework. Thus, a viable alternative may be the implementation of policies that only permit the purchase of SABA OTC when dispensed with ICS-containing medications, which would prevent SABA monotherapy, as also advised by GINA4.
As well-controlled asthma is associated with minimal reliever use, both SABA overprescription and SABA OTC purchase should be considered a sign of poorly controlled disease, potentially indicative of an impending or ongoing asthma exacerbation, and should prompt a detailed assessment of the patient39. However, even patients with 0 SABA prescriptions should undergo asthma reviews, particularly as results from Australia have reported that patients with 0 SABA prescriptions experienced 2.71 times more self-reported severe asthma exacerbations than those prescribed 1–2 SABA canisters, likely because they did not visit their primary care physician or have their level of asthma control assessed31. Furthermore, healthcare systems should develop frameworks to identify patients who are most likely to overuse their inhalers by implementing a system wherein healthcare practitioners (HCPs), including pharmacists, receive an “alert” to prescribe or dispense an ICS whenever a SABA is purchased OTC, thereby reducing SABA-only treatment. Such an approach could also prompt physicians to assess asthma control and the requirement for treatment optimization. Additionally, tools such as the Reliever Reliance Test40,41 and Asthma Slide Rule42 can help initiate conversations with patients to better understand their beliefs underpinning SABA over-reliance and define the acceptable threshold for SABA use before a detailed review is conducted.
Implementation of stewardship programs may promote appropriate reliever use and reduce SABA over-reliance, similar to how the Pan American Health Organization tackled antimicrobial resistance in 201843; however, to be truly effective, interventions will need to be accompanied by pharmacist-led education campaigns. Owing to their clinical expertise, pharmacists are uniquely positioned to play the role of a “SABA Guardian”9 and can educate patients about the potential harms associated with SABA overuse26, difference between controller and reliever medications44, and symptom-based use of a fast-acting β2-agonist–ICS combination instead of SABAs45, which is also recommended by GINA4. Additionally, suboptimal adherence to controller medications, which significantly affects disease control, remains common and affects people of all ages around the world46–49. This issue may be explained, in part, by patients’ perception about ICS-containing medications being ineffective, as they do not provide quick symptom relief compared with SABAs36. Therefore, in countries where SABAs are available without a prescription, pharmacists should play an active role in ensuring safe supply of relievers by reviewing medication utilization, monitoring adherence to asthma medications, addressing patient concerns about medications, and overseeing both OTC purchase and prescription trends50. Notably, the Asthma Right Care (ARC) program was piloted in four countries (Canada, Portugal, Spain, and the United Kingdom) by setting up a multi-national delivery team, including patients, pharmacists, general physicians, and nurses38,51,52. The ARC program was first introduced in Spain to encourage community pharmacists to play a proactive role in educating patients about the potential risks associated with SABA-only treatment and referring them to their physicians for further assessment of symptom control instead of dispensing relievers OTC38. Since its inception, the program has broadened the scope of community pharmacists, positioning them as integral members of the healthcare team instead of a retailer; this in turn has led to increased loyalty toward particular pharmacies and improved the likelihood of the ICS:SABA ratio being nearly 6:1 rather than 1:638.
This study has a few limitations. SABA OTC purchase data were based on patient recall and may therefore have been subject to recall and nonresponse bias53,54. Prescription data served as a surrogate proxy for medication usage or adherence and may not always reflect actual medication use; therefore, SABA use may have been overestimated or underestimated. Additionally, the study was not designed to assess the association between OTC SABA purchase and adherence to ICS-containing medications, which could be a topic of future research. Patients included in this analysis were predominantly treated under specialist care, thereby representing a “better case scenario.” Nevertheless, to our knowledge, this is the first analysis to assess the clinical burden associated with unregulated access to SABA medications, with results highlighting the key role that pharmacists, as accessible HCPs, can play in addressing both SABA overprescription and unregulated purchase of SABA OTC.
In summary, the results from this post hoc analysis of the SABINA III study in over 4500 patients showed the incremental disease-related burden linked with any SABA OTC purchase among patients already prescribed SABAs. Although prescription of SABAs in excess of current treatment recommendations (≥3 SABA canisters/year) was consistently associated with an increased risk of severe exacerbations and lower odds of having at least partly controlled asthma, disease-related burden was the highest in patients who were overprescribed SABAs and had purchased ≥1 SABA OTC. Overall, these findings highlight that unregulated access to SABA OTC represents a serious global health concern, necessitating a concerted effort from all HCPs and policymakers to reduce SABA over-reliance and prevent avoidable adverse outcomes in patients with asthma. Therefore, this is a call to action for HCPs and policymakers to implement evidence-based interventions supported by policy change, prioritize asthma control, promptly identify patients at risk of overusing relievers, promote HCP and patient education, implement SABA stewardship programs, and further define the role of pharmacists in asthma management.
Methods
Study design
The methodology for the SABINA III study has been published previously11. In brief, this was a multi-country, observational, cross-sectional study conducted in 24 countries, with patients recruited from March 2019 to January 2020. Retrospective data were obtained from existing medical records, and patient data, including prescriptions for asthma medications and an assessment of current asthma symptom control, were collected by physicians during a single study visit and entered into an electronic case report form (eCRF). At the study visit, physicians also enquired and recorded whether patients had experienced exacerbations that were not captured in their medical records. Data on SABA OTC purchases were based on patient recall and were obtained directly from patients at the study visit, which were subsequently entered into the eCRF by the investigator. Potential primary and specialist care study sites were selected using purposive sampling with the aim of obtaining a sample representative of asthma management within each participating country by a national coordinator, who also facilitated the selection of investigators.
The study was conducted in accordance with the study protocol, the Declaration of Helsinki, and local ethics committee approvals. The study was approved by the ethics committee and institutional review board of each participating country. All patients or their legal guardians provided signed informed consent in accordance with regulations set by the local ethics review committee.
Patient population
At each site, patients aged ≥12 years with a documented diagnosis of asthma, ≥3 prior consultations with their HCP, and having medical records containing data for ≥12 months before the study visit were enrolled. Patients with a diagnosis of other chronic respiratory diseases, such as chronic obstructive pulmonary disease, or with an acute or chronic condition that, in the opinion of the investigator, would limit their ability to participate in the study were excluded. As SABA OTC purchase is prohibited in South Korea55 and Taiwan56 and strictly regulated in Singapore and cannot usually be bought without prescription57, patients from these three countries included in the SABINA III study were excluded from this analysis. For the purpose of this analysis, patients were categorized into two groups: SABA prescriptions only (i.e., no SABA OTC purchase) and those with both SABA prescriptions and SABA OTC purchases.
Study variables
Patients were categorized by SABA canister prescriptions (1–2 and ≥3 canisters) during the 12 months before the study visit, and overprescription was defined as prescription of ≥3 SABA canisters/year. For consistency, across the entire SABINA program, one SABA canister was assumed to contain 150 inhalations58.
Secondary variables included practice type (primary or specialist care), investigator-classified asthma severity (guided by GINA 2017; treatment steps 1–2: mild asthma; treatment steps 3–5: moderate-to-severe asthma)59, and asthma duration. Other variables included healthcare insurance (not reimbursed, partially reimbursed, or fully reimbursed), education level (primary school, secondary school, high school, or university and/or post-university education), body mass index (BMI), number of comorbidities (0, 1–2, 3–4, or ≥5), and tobacco smoking status (active, former, or never smoker).
Outcomes
Asthma symptom control was evaluated according to the GINA 2017 assessment of asthma control and categorized as well-controlled, partly controlled, or uncontrolled59. Severe exacerbations in the 12 months before the study visit were based on the American Thoracic Society/European Respiratory Society recommendations60 and defined as a deterioration in asthma resulting in hospitalization or emergency room treatment, the need for intravenous corticosteroids or oral corticosteroids for ≥3 days, or a single intramuscular corticosteroid dose.
Statistical analysis
All analyses were performed at the population level and were aggregated by countries. The association of SABA prescriptions (1–2 vs ≥3 canisters) and SABA OTC purchases with the odds of achieving at least partly controlled asthma (uncontrolled asthma as the reference; expressed as OR) at the study visit and the incidence rate of severe exacerbations (expressed as IRR) in the previous 12 months were analyzed using logistic regression and negative binomial models, respectively. Patients with 0 SABA prescriptions were excluded from the association analysis as it was not possible to determine the reliever medication used; this group was highly heterogeneous and included patients with no prescriptions for relievers and those with prescriptions for low-dose ICS/formoterol as reliever or alternative relievers. The regression models were adjusted for prespecified covariates and potential confounders based on the literature and modeling data from the SABINA I study14. Covariates included age (continuous), country, sex, BMI (continuous), education level, healthcare insurance, practice type, investigator-classified asthma severity, asthma duration (continuous), number of comorbidities, and tobacco smoking status.
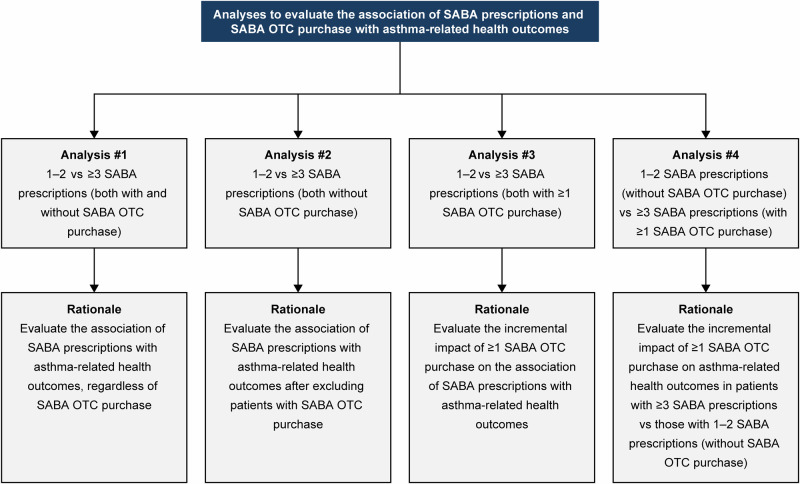
To better understand the association of SABA prescriptions and SABA OTC purchases with asthma-related health outcomes, four association analyses were conducted (Fig. 7). All statistical tests were two-sided and at a 5% level of significance and were performed using the statistical software R (version 3.6.0).
Fig. 7. Description of four analyses to evaluate the association of SABA prescriptions and SABA OTC purchase with asthma-related health outcomes in the SABINA III population (N = 4556).
OTC over-the-counter, SABA short-acting β2-agonist, SABINA SABA use IN Asthma.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
AstraZeneca funded all the SABINA studies and was involved in designing the study, developing the study protocol, conducting the study, and performing the analyses. AstraZeneca was given the opportunity to review the manuscript before submission and funded medical writing support. Writing and editorial support was provided by Praveen Kaul, PhD, of Cactus Life Sciences (part of Cactus Communications), Mumbai, India, in accordance with Good Publication Practice (GPP 2022) guidelines (https://www.acpjournals.org/doi/10.7326/M22-1460) and fully funded by AstraZeneca.
Author contributions
D.P. contributed to data collection, data analysis, data interpretation, and writing and editing of the manuscript. W.J.M. contributed to data collection, data analysis, data interpretation, and writing and editing of the manuscript. M.J.H.I.B. designed the study and contributed to data collection, data analysis, data interpretation, and writing and editing of the manuscript. R.M.B.-A. contributed to data collection, data analysis, data interpretation, and writing and editing of the manuscript. H.-C.W. contributed to data collection, data analysis, data interpretation, and writing and editing of the manuscript. D.V.D. contributed to data collection, data analysis, data interpretation, and writing and editing of the manuscript. A.K. contributed to data collection, data analysis, data interpretation, and writing and editing of the manuscript. M.P.G. contributed to data collection, data analysis, data interpretation, and writing and editing of the manuscript. A.A.Z. contributed to data collection, data analysis, data interpretation, and writing and editing of the manuscript. H.F. contributed to data collection, data analysis, data interpretation, and writing and editing of the manuscript. D.A.-Z. contributed to data collection, data analysis, data interpretation, and writing and editing of the manuscript. All authors approved the manuscript and have agreed to be accountable for all aspects of the work.
Data availability
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available, outlining further details: https://vivli.org/ourmember/astrazeneca/. The datasets supporting the conclusions of this article are available from the corresponding author on a reasonable request.
Competing interests
D.P. has advisory board membership with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Viatris, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals, and Thermo Fisher Scientific Inc.; consultancy agreements with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Viatris, Mundipharma, Novartis, Pfizer, Teva Pharmaceuticals, and Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Viatris, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals, Theravance, and the UK National Health Service; has received payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Viatris, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, and Teva Pharmaceuticals; has received payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Circassia, Mundipharma, Novartis, Teva Pharmaceuticals, and Thermo Fisher Scientific Inc.; has received funding for patient enrollment or completion of research from Novartis; has stock/stock options from AKL Research and Development Ltd., which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); is a 5% shareholder in Timestamp, which develops adherence monitoring technology; is a peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation programme and Health Technology Assessment; and was an expert witness for GlaxoSmithKline. M.J.H.I.B. was an employee of AstraZeneca at the time this study was conducted. W.J.M. has served as a study investigator for AstraZeneca, GlaxoSmithKline, and Novartis. He has also served on speakers’ bureaus for AstraZeneca. R.M.B.-A. has participated in clinical trials for AstraZeneca, GlaxoSmithKline, Novartis, and Chiesi. H.-C.W. has no conflicts to declare. D.V.D. has received funding and honorarium from AstraZeneca as a principal investigator for the present study. A.K. has no conflicts to declare. M.P.G. has served on speakers’ bureaus for AstraZeneca, Roche, Novartis, Novamed, Bayer, Boehringer Ingelheim, Scandinavia Pharma, and Janssen. A.A.Z. has no conflicts to declare. H.F. is an employee of AstraZeneca. D.A.-Z. has served on advisory boards and reports speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Glenmark, Janssen, Johnson & Johnson, Novartis, Orion, Pfizer, Scope, Teva, Thornton & Ross, Trudell, and Viatris.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41533-024-00397-4.
References
- 1.Global Asthma Network (GAN). The Global Asthma Report. http://www.globalasthmareport.org (2018).
- 2.Chen, C. et al. Prevalence, economic burden, and neurophenotype of asthma. Explor. Res. Hypothesis Med.8, 359–365 (2023). [Google Scholar]
- 3.Wang, Z. et al. Global, regional, and national burden of asthma and its attributable risk factors from 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Respir. Res.24, 169 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Initiative for Asthma. Global strategy for asthma management and prevention. http://ginasthma.org/ (2023).
- 5.Price, D., Fletcher, M. & van der Molen, T. Asthma control and management in 8000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim. Care Respir. Med.24, 14009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maspero, J. F. et al. Insights, attitudes, and perceptions about asthma and its treatment: findings from a multinational survey of patients from Latin America. World Allergy Organ J.6, 19 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price, D. et al. Time for a new language for asthma control: results from REALISE Asia. J. Asthma Allergy8, 93–103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Asthma. https://www.who.int/news-room/fact-sheets/detail/asthma (2024).
- 9.Levy, M. L. The national review of asthma deaths: what did we learn and what needs to change? Breathe11, 14–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haughney, J. et al. Achieving asthma control in practice: understanding the reasons for poor control. Respir. Med.102, 1681–1693 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Bateman, E. D. et al. Short-acting β2-agonist prescriptions are associated with poor clinical outcomes of asthma: the multi-country, cross-sectional SABINA III study. Eur. Respir. J.59, 2101402 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Initiative for Asthma. Global strategy for asthma management and prevention. http://ginasthma.org/ (2018).
- 13.Reddel, H. K. et al. GINA 2019: a fundamental change in asthma management: treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents. Eur. Respir. J.53, 1901046 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Bloom, C. I. et al. Asthma-related health outcomes associated with short-acting β2-agonist inhaler use: an observational UK Study as part of the SABINA Global Program. Adv. Ther.37, 4190–4208 (2020). [DOI] [PubMed] [Google Scholar]
- 15.FitzGerald, J. M., Tavakoli, H., Lynd, L. D., Al Efraij, K. & Sadatsafavi, M. The impact of inappropriate use of short acting beta agonists in asthma. Respir. Med.131, 135–140 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Nwaru, B. I. et al. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur. Respir. J.55, 1901872 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanford, R. H., Shah, M. B., D’Souza, A. O., Dhamane, A. D. & Schatz, M. Short-acting β-agonist use and its ability to predict future asthma-related outcomes. Ann. Allergy Asthma Immunol.109, 403–407 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Global Initiative for Asthma. Global strategy for asthma management and prevention. http://ginasthma.org/ (2019).
- 19.O’Byrne, P. M., Jenkins, C. & Bateman, E. D. The paradoxes of asthma management: time for a new approach? Eur. Respir. J.50, 1701103 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Blakeston, S., Harper, G. & Zabala Mancebo, J. Identifying the drivers of patients’ reliance on short-acting β2-agonists in asthma. J. Asthma58, 1094–1101 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Loh, Z. C. et al. Perceptions, attitudes, and behaviors of asthma patients towards the use of short-acting β2-agonists: a systematic review. PLoS ONE18, e0283876 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Partridge, M. R., van der Molen, T., Myrseth, S. E. & Busse, W. W. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm. Med.6, 13 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tattersfield, A. E. et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. Am. J. Respir. Crit. Care Med.160, 594–599 (1999). [DOI] [PubMed] [Google Scholar]
- 24.Stolbrink, M. et al. The availability, cost, and affordability of essential medicines for asthma and COPD in low-income and middle-income countries: a systematic review. Lancet Glob. Health10, e1423–e1442 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farouk, H. et al. Over-the-counter (OTC) short-acting β2-agonist (SABA) purchase and asthma outcomes in SABINA III. Eur. Respir. J.60, 3434 (2022). [Google Scholar]
- 26.Loh, Z. C. et al. Over-the-counter use of short-acting beta-2 agonists: a systematic review. J. Pharm. Policy Pr.16, 119 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz, D. V. et al. Short-acting β2-agonist prescription patterns in patients with asthma in the Philippines: results from SABINA III. Acta Med. Philipp.57, 12–24 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avdeev, S. et al. SABA overuse in Russia—burden and possible causes: an analysis of the Russian population in the SABINA III (SABA use IN Asthma) study. J. Asthma Allergy15, 371–379 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakaya, J., Mecha, J. & Beekman, M. Over-prescription of short-acting β2-agonists remains a serious health concern in Kenya: results from the SABINA III study. BMC Prim. Care24, 141 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, C. et al. Over-prescription of short-acting β2-agonists for asthma in South Africa: results from the SABINA III study. Afr. J. Thorac. Crit. Care Med. 28, 10.7196/AJTCCM.2022.v28i4.220 (2022). [DOI] [PMC free article] [PubMed]
- 31.Price, D. et al. The association between short-acting β2-agonist over-prescription, and patient-reported acquisition and use on asthma control and exacerbations: data from Australia. Adv. Ther.41, 1262–1283 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vialle-Valentin, C. E., Serumaga, B., Wagner, A. K. & Ross-Degnan, D. Evidence on access to medicines for chronic diseases from household surveys in five low- and middle-income countries. Health Policy Plan30, 1044–1052 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibson, P. et al. Association between availability of non-prescription beta 2 agonist inhalers and undertreatment of asthma. BMJ306, 1514–1518 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henry, D. A., Sutherland, D. & Francis, L. The use of non-prescription salbutamol inhalers by asthmatic patients in the Hunter Valley, New South Wales. Newcastle Retail Pharmacy Research Group. Med. J. Aust.150, 445–449 (1989). [PubMed] [Google Scholar]
- 35.Reddel, H. K., Ampon, R. D., Sawyer, S. M. & Peters, M. J. Risks associated with managing asthma without a preventer: urgent healthcare, poor asthma control and over-the-counter reliever use in a cross-sectional population survey. BMJ Open7, e016688 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azzi, E. A. et al. Understanding reliever overuse in patients purchasing over-the-counter short-acting beta(2) agonists: an Australian community pharmacy-based survey. BMJ Open9, e028995 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amirav, I. & Newhouse, M. T. Asthma and COVID-19: in defense of evidence-based SABA. J. Asthma Allergy13, 505–508 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The International Primary Care Respiratory Group (IPCRG). Asthma Right Care case study. How Spanish pharmacists have improved their role in asthma management from supplying SABA without restriction to prescription-only dispensing. https://www.ipcrg.org/sites/ipcrg/files/content/attachments/2021-09-13/SABA%20OTC%20Case%20Study%20-%20FINAL.pdf (2021).
- 39.Royal College of Physicians. Why Asthma Still Kills? National Review of Asthma Deaths. https://www.rcplondon.ac.uk/projects/outputs/why-asthma-still-kills (2016).
- 40.Asthma and allergy association. Do you use your blue reliever inhaler 3 or more times a week? https://www.rateyourreliance.sg/ (2024).
- 41.Horne, R. Reliever reliance test. https://www.ipcrg.org/sites/ipcrg/files/content/attachments/2023-12-04/Reliever%20Reliance%20Test%20-%20English.pdf (2024). [DOI] [PMC free article] [PubMed]
- 42.Primary Care Respiratory Society. Online Asthma Slide Rule. https://www.pcrs-uk.org/resource/online-asthma-slide-rule (2024).
- 43.Pan American Health Organization. Florida International University. Recommendations for implementing antimicrobial stewardship programs in Latin America and the Caribbean: manual for public health decision-makers https://iris.paho.org/handle/10665.2/49645 (2018).
- 44.de Oliveira, M. A., Faresin, S. M., Bruno, V. F., de Bittencourt, A. R. & Fernandes, A. L. Evaluation of an educational programme for socially deprived asthma patients. Eur. Respir. J.14, 908–914, (1999). [DOI] [PubMed] [Google Scholar]
- 45.Kaplan, A. et al. Effective asthma management: is it time to let the AIR out of SABA? J. Clin. Med.9, 921 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engelkes, M., Janssens, H. M., de Jongste, J. C., Sturkenboom, M. C. & Verhamme, K. M. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur. Respir. J.45, 396–407 (2015). [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization. Adherence to long-term therapies : evidence for action. https://iris.who.int/handle/10665/42682 (2003).
- 48.Murphy, J. et al. Prevalence and predictors of adherence to inhaled corticosteroids in young adults (15-30 years) with asthma: a systematic review and meta-analysis. J. Asthma58, 683–705 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Desai, M. & Oppenheimer, J. J. Medication adherence in the asthmatic child and adolescent. Curr. Allergy Asthma Rep.11, 454–464 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Zeitouni, M. O. et al. Challenges and recommendations for the management of asthma in the Middle East and Africa. Ann. Thorac. Med.17, 71–80 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The International Primary Care Respiratory Group (IPCRG). Asthma Right Care. https://www.ipcrg.org/asthmarightcare (2024).
- 52.The International Primary Care Respiratory Group (IPCRG). Asthma Right Care Strategy. https://www.ipcrg.org/asthmarightcare/asthma-right-care-strategy (2024).
- 53.Wang, X. & Cheng, Z. Cross-sectional studies: strengths, weaknesses, and recommendations. Chest158, S65–S71 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Roche, N. et al. Quality standards for real-world research. Focus on observational database studies of comparative effectiveness. Ann. Am. Thorac. Soc.11, S99–S104 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Korean Legislation Research Institute. Pharmaceutical Affairs Acts. Sejong-si (KR): Korean Legislation Research Institute, c2017. https://elaw.klri.re.kr/eng_service/lawView.do?hseq=40196&lang=ENG (2017).
- 56.Shen, S. Y. et al. SABA prescriptions and asthma management practices in patients treated by specialists in Taiwan: Results from the SABINA III study. J. Formos. Med. Assoc.121, 2527–2537 (2022). [DOI] [PubMed] [Google Scholar]
- 57.Tan, N. C., Tay, I. H., Ngoh, A. & Tan, M. Factors influencing family physicians’ drug prescribing behaviour in asthma management in primary care. Singap. Med. J.50, 312–319 (2009). [PubMed] [Google Scholar]
- 58.Cabrera, C. S. et al. SABINA: global programme to evaluate prescriptions and clinical outcomes related to short-acting β2-agonist use in asthma. Eur. Respir. J.55, 1901858 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Global Initiative for Asthma. Global strategy for asthma management and prevention. http://ginasthma.org/ (2017).
- 60.Reddel, H. K. et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am. J. Respir. Crit. Care Med.180, 59–99 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available, outlining further details: https://vivli.org/ourmember/astrazeneca/. The datasets supporting the conclusions of this article are available from the corresponding author on a reasonable request.