Abstract
Background
The incidence of breast cancer among young Asian women is increasing, yet they remain underrepresented in global data. We analyzed the epidemiology and outcomes of Asian patients with breast cancer <40 years old across different subtypes to identify their clinical unmet needs.
Patients and methods
Female patients aged ≥20 years diagnosed with early breast cancer were analyzed from the prospective cohort of the Asian Breast Cancer Cooperative Group (ABCCG). For comparison, data from the Surveillance, Epidemiology, and End Results Program (SEER) cancer registry were used. Patients were categorized into three age groups: young (<40 years), alleged premenopausal mid-age (40-49 years), and alleged postmenopausal (aged ≥50 years). Multivariable Cox proportional hazards models for survival were adjusted for subtypes, histologic grade, T stage, nodal status, and study centers.
Results
A total of 45 021 patients with breast cancer from Asian study centers, 496 332 SEER-White patients, and 18 279 SEER-Asian patients were included in the analysis. The median age at diagnosis was younger in the Asian cohort (51 years) compared with SEER-Whites (62 years) and SEER-Asians (58 years; P < 0.0001). In the young-age group, hormone receptor-positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) breast cancer was more prevalent among Asians and SEER-Asians compared with SEER-Whites (61.2% and 59.8% versus 54.7%). In the Asian population, young patients with HR+/HER2− breast cancer exhibited significantly inferior overall survival than the mid-age group (6-year overall survival 94.4% versus 96.6%; mid-age to young-age group hazard ratio 0.62; P < 0.001). Similarly, young patients in SEER-Whites showed an earlier decline in survival compared with the mid-age group (89.1% versus 94.0%; P < 0.001).
Conclusion
ABCCG-Asian patients with breast cancer <40 years old with HR+/HER2− subtypes were more likely to have worse survival outcomes than their mid-age counterparts. Our study highlights the poorer prognosis of young patients and underscores the need for a tailored therapeutic approach, such as ovarian function suppression, particularly considering ethnic factors.
Key words: HR+/HER2− breast cancer, young, Asian, prevalence, survival
Highlights
-
•
Asians in the ABCCG cohort were diagnosed with early breast cancer at younger ages than SEER-Whites and SEER-Asians.
-
•
Young Asian patients in the ABCCG cohort had a higher frequency of HR+/HER2− breast cancer.
-
•
Asian patients <40 years old with HR−/HER2− breast cancer had higher mortality than the mid-age group.
Introduction
Breast cancer is the most common cancer in women worldwide. Guidelines recommend breast cancer screening for women after the age of 40 years (NCCN Guidelines Version 1.2022, Breast Cancer Screening and Diagnosis).1 The incidence of breast cancer in young females under 40 years, however, has been increasing in the United States, according to the Surveillance, Epidemiology, and End Results (SEER) database.2,3 The incidence of breast cancer has also continuously increased in Asia, especially in young women <40 years of age.4, 5, 6, 7
Breast cancer consists of heterogeneous subtypes, each with distinct clinical courses and treatments. The clinical subtypes are classified based on the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), while the molecular subtypes further divided these subtypes into luminal A, luminal B, normal breast-like, HER2-positive, and basal-like.8,9 Recent data have indicated that ER-positive breast cancer increases with age in Americans, with the probability of ER positivity being significantly higher at 40-49 years of age in Asians.10 Young female patients with breast cancer are expected to have more aggressive types.11 However, the survival outcomes of young Asian female patients with breast cancer across different subtypes have not been sufficiently investigated. In this study, the characteristics and survival of young Asian patients with early-stage breast cancer were analyzed.
Patients and methods
Study design and patients
Female patients aged ≥20 years, diagnosed with early breast cancer between 1 January 2005 and 31 December 2015, were enrolled from the prospective cohort of five academic institutions within the Asian Breast Cancer Cooperative Group (ABCCG): Cancer Institute Hospital of Japanese Foundation for Cancer Research, National Taiwan University Hospital, Samsung Medical Center, Seoul National University Hospital (SNUH), and Singapore General Hospital/National Cancer Centre Singapore.10 As a validation set, we used SEER 18 cancer registry data and extracted information from four ethnic groups of patients with a primary diagnosis of stage I-III unilateral breast cancer: Whites [including Hispanic (SEER-Whites) individuals], Chinese, Japanese, and Korean (SEER-Asian).
Early breast cancer was defined as pathologic stage I-III according to the 5th, 6th, or 7th edition of the TNM (tumor–node–metastasis) classification by the American Joint Committee on Cancer. Clinical staging was used for patients who received neoadjuvant therapy. Pathologic diagnosis and immunohistochemistry (IHC) for ER, PR, and HER2 status were confirmed by the pathologic reports of surgical and percutaneous biopsies, following the American Society of Clinical Oncology/College of American Pathologists (ASCO-CAP) recommendations.12, 13, 14 It should be noted that in 2010, the definition of ER and PR positivity was revised from 10% to 1%.14 HER2 status was defined according to the 2007 and 2014 ASCO-CAP guidelines and HER2 testing was conducted in pathology laboratories accredited by CAP or the national pathology accreditation body. Patients without clinical or pathological data were excluded.
Statistical analyses
Categorical variables were summarized with frequencies (numbers) and percentages (rates). Continuous variables were represented by median values and ranges. Differences were assessed using the Mann–Whitney U test for continuous variables and Pearson’s χ2 or Fisher’s exact test for categorical variables. A multivariable Cox proportional hazards model was used to analyze survival, adjusted for age, hormone receptor status, HER2 status, histologic grade, T stage, nodal status, and study centers.
Overall survival (OS) was defined as the time from breast cancer diagnosis to death from any cause. OS curves were estimated using the Kaplan–Meier method. For patients who were alive at the last follow-up, survival was censored at the date of the latest follow-up when no death was confirmed. A log-rank test P-value <0.05 was considered statistically significant. Patients diagnosed between 2005 and 2015 were censored at the maximum follow-up time of patients diagnosed between 2010 and 2015, which was 6 years.
Ethics approval and consent to participate
The study was approved by the SNUH Institutional Review Board (institutional review board number H1509-047-702) and the respective ethics committees in the participating institutions. Clinical data were anonymized and deidentified before analysis.
Results
Ethnic difference in the epidemiology of breast cancer
A total of 45 007 patients were diagnosed with stage I to III breast cancer in Asian regions, including the Republic of Korea (n = 17 376), Singapore (n = 11 237), Taiwan (n = 8771) and Japan (n = 7623), from 2005 to 2015. In the SEER data, 496 332 SEER-White patients and 18 279 SEER-Asian patients were included. In our ABCCG data, the median age at diagnosis was 51 years, which is younger than the corresponding age of 62 years in SEER-Whites and 58 years in SEER-Asians (P < 0.0001). The proportion of infiltrating ductal carcinoma was higher in ABCCG-Asians (84.88%) compared with SEER-Whites (72.05%) and SEER-Asians (77.55%). Most patients were diagnosed with stage II or III breast cancer, with 60% being node-negative. While the proportions of patients with hormone receptor-positive and HER2-positive (HR+/HER2+) breast cancers were similar across all populations, HR+/HER2− breast cancers were more common among SEER-Whites (75.89%) and SEER-Asians (73.38%), compared with ABCCG-Asians (65.75%). Triple-negative breast cancer (TNBC) and HER2-positive breast cancers were more common in ABCCG-Asians (14.32% and 9.38%, respectively) than in SEER-Whites (10.21% and 3.96%, respectively) and SEER-Asians (10.0% and 5.74%, respectively; Table 1).
Table 1.
Epidemiology of patients with early breast cancer stage I-III
| Epidemiology | Total (N = 559 632) | SEER-White (N = 496 332) | SEER-Asian (N = 18 279) | ABCCG-Asian (N = 45 021) | |
|---|---|---|---|---|---|
| Age at diagnosis | Patients, n | 559 618 | 496 332 | 18 279 | 45 007 |
| Median (range) | 61 (2-117) | 62 (2-117) | 58 (20-108) | 51 (17-97) | |
| Histologic grade | Patients, n | 547 267 | 496 332 | 18 279 | 32 656 |
| Grade I, n (%) | 123 225 (22.52) | 113 675 (22.90) | 3967 (21.70) | 5583 (17.10) | |
| Grade II, n (%) | 228 224 (41.7) | 206 437 (41.59) | 7606 (41.61) | 14 181 (43.43) | |
| Grade III, n (%) | 159 330 (29.11) | 141 037 (28.42) | 5402 (29.55) | 12 891 (39.48) | |
| Others (unknown), n (%) | 36 488 (6.67) | 35 183 (7.09) | 1304 (7.13) | 1 (0.00) | |
| Histologic subtype | Patients, n | 559 632 | 496 332 | 18 279 | 45 021 |
| Invasive ductal carcinoma, n (%) | 409 981 (73.26) | 357 590 (72.05) | 14 176 (77.55) | 38 215 (84.88) | |
| Invasive lobular carcinoma, n (%) | 49 912 (8.92) | 47 089 (9.49) | 983 (5.38) | 1840 (4.09) | |
| Others, n (%) | 99 739 (17.82) | 91 653 (18.47) | 3120 (17.07) | 4966 (11.03) | |
| Stage | Patients, n | 420 634 | 365 765 | 13 567 | 41 302 |
| I, n (%) | 172 390 (40.98) | 149 972 (41) | 5713 (42.11) | 16 705 (40.45) | |
| II, n (%) | 184 128 (43.77) | 160 757 (43.95) | 6151 (45.34) | 17 220 (41.69) | |
| III, n (%) | 64 116 (15.24) | 55 036 (15.05) | 1703 (12.55) | 7377 (17.86) | |
| T | Patients, n | 536 730 | 477 178 | 17 637 | 41 915 |
| 0, n (%) | 1414 (0.26) | 807 (0.17) | 28 (0.16) | 579 (1.38) | |
| 1, n (%) | 339 556 (63.26) | 304 167 (63.74) | 11 170 (63.33) | 24 219 (57.78) | |
| 2, n (%) | 154 106 (28.71) | 134 238 (28.13) | 5237 (29.69) | 14 631 (34.91) | |
| 3, n (%) | 26 963 (5.02) | 24 275 (5.09) | 815 (4.62) | 1873 (4.47) | |
| 4, n (%) | 14 691 (2.74) | 13 691 (2.87) | 387 (2.19) | 613 (1.46) | |
| N | Patients, n | 544 934 | 483 538 | 17 831 | 43 565 |
| 0, n (%) | 382 240 (70.14) | 343 010 (70.94) | 13 007 (72.95) | 26 223 (60.19) | |
| 1, n (%) | 118 346 (21.72) | 102 684 (21.24) | 3608 (20.23) | 12 054 (27.67) | |
| 2, n (%) | 28 586 (5.25) | 24 466 (5.06) | 811 (4.55) | 3309 (7.60) | |
| 3, n (%) | 15 762 (2.89) | 13 378 (2.77) | 405 (2.27) | 1979 (4.54) | |
| Breast subtype | Patients, n | 305 881 | 258 102 | 9902 | 37 877 |
| HR+/HER2−, n (%) | 228 049 (74.55) | 195 877 (75.89) | 7266 (73.38) | 24 906 (65.75) | |
| HR+/HER2+, n (%) | 31 110 (10.17) | 25 661 (9.94) | 1078 (10.89) | 4371 (11.54) | |
| HR–/HER2+, n (%) | 14 339 (4.69) | 10 218 (3.96) | 568 (5.74) | 3553 (9.38) | |
| Triple-negative, n (%) | 32 383 (10.59) | 26 346 (10.21) | 990 (10) | 5047 (13.32) | |
ABCCG, Asian Breast Cancer Cooperative Group; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; SEER, Surveillance, Epidemiology, and End Results Program.
Differences in subtype distribution by age groups
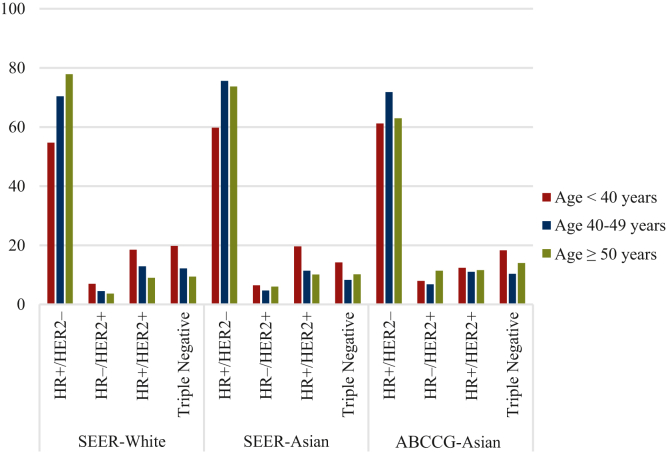
The distribution of subtypes, however, differed significantly in younger Asian patients <40 years of age. In the ABCCG population, the proportion of HR+/HER2− breast cancers was significantly higher than in SEER-Whites but similar to SEER-Asians (61.2% versus 54.7% versus 59.8%, respectively; Figure 1, Table 2). In this same age group, the proportions of patients with TNBC were similar between ABCCG-Asians and SEER-Whites but higher than in SEER-Asians (18.3% and 19.8% versus 14.2%, respectively). The proportions of patients with HER2-positive breast cancer were similar across the younger age groups. In the 40-49 age group, the proportion of HR+/HER2− breast cancer was highest in SEER-Asians and similar between ABCCG-Asians and SEER-Whites. Among patients aged ≥50 years, the proportion of TNBC increased in ABCCG-Asians, while the proportion of HR+/HER2− breast cancer decreased. For patients aged ≥50 years, the SEER-Whites had a higher proportion of HR+/HER2− breast cancer and a lower proportion of TNBC than other age groups (Figure 1, Table 2).
Figure 1.
Distribution of breast cancer subtypes by age groups. Percentages of breast cancer patients with HR+/HER2−, HR+/HER2+, HR−/HER2+, and HR−/HER2− status in the SEER-White, SEER-Asian, and ABCCG-Asian cohorts are shown across three age groups: young (<40 years), alleged premenopausal mid-age (40-49 years), and alleged postmenopausal age (≥50 years). ABCCG, Asian Breast Cancer Cooperative Group; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; SEER, Surveillance, Epidemiology, and End Results Program.
Table 2.
Distribution of breast cancer subtypes by age groups
| Distribution | Total | age: <40 years | age: 40-49 years | age: ≥50 years | |
|---|---|---|---|---|---|
| SEER-White | Patients, n | 496 332 | 21 010 | 75 997 | 399 325 |
| HR+/HER2−, n (%) | 195 877 (75.87) | 5792 (54.7) | 26 232 (70.4) | 163 853 (77.9) | |
| HR+/HER2+, n (%) | 25 661 (9.94) | 1963 (18.5) | 4810 (12.9) | 18 888 (9.0) | |
| HR–/HER2+, n (%) | 10 218 (3.96) | 736 (7.0) | 1684 (4.5) | 7798 (3.7) | |
| Triple-negative, n (%) | 26 346 (10.21) | 2098 (19.8) | 4559 (12.2) | 19 689 (9.4) | |
| SEER-Asian | Patients, n | 18 279 | 1081 | 4032 | 13 166 |
| HR+/HER2−, n (%) | 7266 (73.38) | 324 (59.8) | 1611 (75.6) | 5331 (73.7) | |
| HR+/HER2+, n (%) | 1078 (10.89) | 106 (19.6) | 242 (11.4) | 730 (10.1) | |
| HR–/HER2+, n (%) | 568 (5.74) | 35 (6.5) | 101 (4.7) | 432 (6.0) | |
| Triple-negative, n (%) | 990 (10.00) | 77 (14.2) | 176 (8.3) | 737 (10.2) | |
| ABCCG-Asian | Patients, n | 45 007 | 5890 | 14 873 | 24 244 |
| HR+/HER2−, n (%) | 24 887 (65.7) | 2956 (61.2) | 9073 (71.8) | 12 857 (63.0) | |
| HR+/HER2+, n (%) | 4354 (11.5) | 600 (12.4) | 1393 (11.0) | 2361 (11.6) | |
| HR–/HER2+, n (%) | 3570 (9.43) | 387 (8.0) | 858 (6.8) | 2324 (11.4) | |
| Triple-negative, n (%) | 5066 (13.37) | 885 (18.3) | 1317 (10.4) | 2864 (14.0) | |
ABCCG, Asian Breast Cancer Cooperative Group; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; SEER, Surveillance, Epidemiology, and End Results Program.
Survival
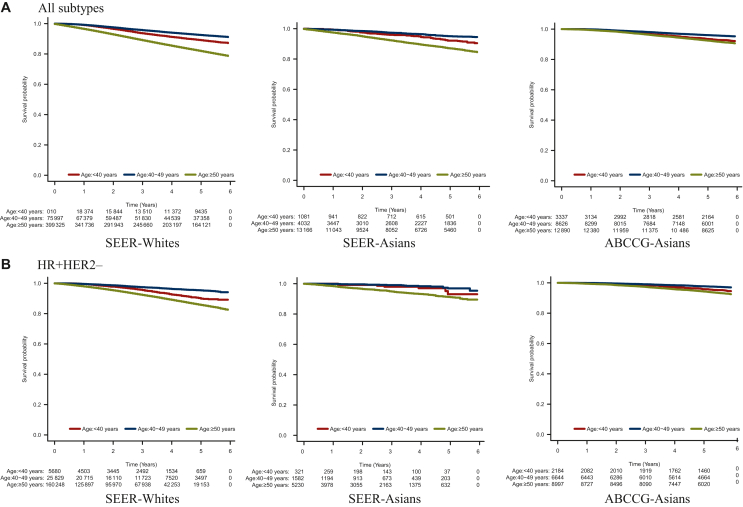
Survival was analyzed in 24 361 patients from the ABCCG data, 195 877 SEER-Whites, and 7266 SEER-Asians. In all populations, the mid-age group of 40-49 years showed the best survival, while the age group of ≥50 years showed the worst survival (Figure 2).
Figure 2.
6-Year survival rates for SEER-Whites, SEER-Asians, and ABCCG-Asians. Survival rates for patients with breast cancer with (A) all subtypes and (B) HR+/HER2− were compared across three age groups: young (<40 years), alleged premenopausal mid-age (40-49 years), and alleged postmenopausal age (≥50 years) in SEER-Whites, SEER-Asians, and ABCCG-Asians. ABCCG, Asian Breast Cancer Cooperative Group; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; SEER, Surveillance, Epidemiology, and End Results Program.
Further analyses by subtype in each age group showed distinct trends among ABCCG-Asians, SEER-Whites, and SEER-Asians. Interestingly, in ABCCG-Asian patients, the mid-age group with HR+/HER2− breast cancer had significantly superior OS than the young-age group of <40 years (6-year OS 96.6% versus 94.4%; adjusted hazard ratio 0.62, 95% confidence interval 0.48-0.80; P < 0.001; Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103732, Table 3). The poor prognosis for younger ABCCG-Asian patients with HR+/HER2− breast cancer remained evident at 15 years of follow-up (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.103732). Similarly, younger SEER-White patients experienced an early decline in the survival curve compared with those in the mid-age group (89.1% versus 94.0%; P < 0.001). By contrast, younger SEER-Asian patients had survival rates equivalent to those in the mid-age group (92.4% versus 95.0%; P = 0.448; Table 3). While patients with HR+/HER2+ breast cancer showed similar survival rates in both the young-age and mid-age groups across all populations at 6 years, ABCCG-Asian data indicated that the survival of the young-age group was numerically lower at the 15-year follow-up (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.103732). In contrast to HR+/HER2− breast cancer, patients with HER2-positive or TNBC showed relatively similar OS in both the young-age and mid-age groups across all populations. Patients aged ≥50 years across all subtypes had the worst prognosis in both ABCCG-Asians and SEER-Whites. Comparison of OS by subtype revealed that the ABCCG-Asian patients with HR+/HER2− breast cancer and TNBC had a better prognosis than SEER-Whites and SEER-Asians with HR+/HER2− breast cancer (hazard ratio 0.22 versus 0.819 and 0.891, respectively) and TNBC (0.826 versus 0.713 and 0.751, respectively).
Table 3.
Prognosis of early breast cancer by age group, subtypes, and ethnicity
| Classification | Versus age <40 (reference) | Adjusted hazard ratio | 95% CI | P-value | |
|---|---|---|---|---|---|
| HR+/HER2− | SEER-White | Age 40-49 | 0.65 | 0.57-0.76 | <0.001 |
| Age ≥50 | 2.36 | 2.08-2.67 | <0.001 | ||
| SEER-Asian | Age 40-49 | 0.71 | 0.30-1.71 | 0.45 | |
| Age ≥50 | 3.49 | 1.64-7.42 | 0.001 | ||
| ABCCG-Asian | Age 40-49 | 0.62 | 0.48-0.80 | <0.001 | |
| Age ≥50 | 1.29 | 1.04-1.61 | 0.02 | ||
| HR+/HER2+ | SEER-White | Age 40-49 | 1.01 | 0.72-1.41 | 0.96 |
| Age ≥50 | 3.93 | 2.96-5.21 | <0.001 | ||
| SEER-Asian | Age 40-49 | 0.91 | 0.08-10.03 | 0.94 | |
| Age ≥50 | 6.82 | 0.93-49.80 | 0.06 | ||
| ABCCG-Asian | Age 40-49 | 0.87 | 0.51-1.50 | 0.62 | |
| Age ≥50 | 1.96 | 1.22-3.16 | 0.01 | ||
| HR–/HER2+ | SEER-White | Age 40-49 | 1.36 | 0.91-2.04 | 0.13 |
| Age ≥50 | 3.23 | 2.27-4.60 | <0.001 | ||
| SEER-Asian | Age 40-49 | 0.69 | 0.17-2.84 | 0.61 | |
| Age ≥50 | 1.10 | 0.33-3.63 | 0.88 | ||
| ABCCG-Asian | Age 40-49 | 0.92 | 0.57-1.49 | 0.74 | |
| Age ≥50 | 1.01 | 0.66-1.55 | 0.96 | ||
| Triple-negative | SEER-White | Age 40-49 | 0.99 | 0.85-1.16 | 0.92 |
| Age ≥50 | 1.64 | 1.43-1.87 | <0.001 | ||
| SEER-Asian | Age 40-49 | 0.65 | 0.31-1.35 | 0.25 | |
| Age ≥50 | 0.94 | 0.51-1.74 | 0.85 | ||
| ABCCG-Asian | Age 40-49 | 0.85 | 0.65-1.11 | 0.22 | |
| Age ≥50 | 0.82 | 0.65-1.04 | 0.10 | ||
Adjusted with age, histologic grade, T stage, N stage, HR status, HER2 status, and study centers.
ABCCG, Asian Breast Cancer Cooperative Group; CI, confidence interval; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; SEER, Surveillance, Epidemiology, and End Results Program.
Discussion
Our data revealed significant findings: (i) ABCCG-Asians were diagnosed with early breast cancer at younger ages than SEER-Whites and SEER-Asians, (ii) young ABCCG-Asian patients had a higher frequency of HR+/HER2− breast cancer, (iii) and patients <40 years old had higher mortality rates than those in mid-age groups. Consistent with previous data, early breast cancer was more likely to be diagnosed at younger ages among Asians. The incidence of breast cancer peaked at age 50 years and declined with older age, contrasting with the increasing incidence seen with age in Western countries.5 Our age- and subtype-specified analysis also showed that HR+/HER2− breast cancer was more frequent in the ABCCG-Asians than SEER-Whites in younger age groups. Furthermore, the frequency of HR+/HER2− breast cancer in SEER-Asians was more similar to SEER-Whites than to ABCCG-Asians, suggesting possible environmental influences. The increase in estrogen-related disease, including breast and gynecologic cancers, has been noted15 and is attributed to a Westernized lifestyle, which may result in higher body mass index, earlier age at menarche, delayed age at first birth, and lower parity.16,17 Decreased fertility rates have also been associated with birth cohort effects due to national family planning campaigns in Asian countries, which have led to lower fertility rates.18, 19, 20 In addition, the higher breast density observed in Asian women compared with White women, particularly among premenopausal women, is thought to be an independent factor contributing to the reduced sensitivity of mammography in detecting breast diseases.21,22
Meanwhile, the varying frequency of HR+/HER2− breast cancer in the young-age group among SEER-Asians and SEER-Whites suggests a role for racial and biological differences.10 Genetically, ER-positive breast tumors in Asian patients have a higher prevalence of TP53 somatic mutations.23 In addition, premenopausal Asian patients, compared with those in The Cancer Genome Atlas, have been reported to present with a higher frequency of luminal B subtypes, decreased ESR1 expression, and more BRCA1 or BRCA2 germline pathogenic mutations.24 Increased tumor-infiltrating lymphocytes and decreased transforming growth factor-beta signaling have also been noted in these patients, suggesting that the tumor microenvironment may have ethnic distinctions.
Among younger Asian patients <40 years of age, there was a higher frequency of HR+/HER2− breast cancer; in addition, our data showed that these younger patients had a worse prognosis compared with their mid-age counterparts. In the Supplementary Material S1, available at https://doi.org/10.1016/j.esmoop.2024.103732, the 15-year follow-up data reveal that patients with HR+/HER2− breast cancer experienced a rapid decline in survival at later timepoints. Furthermore, the young-age group showed a more pronounced decline in survival at earlier timepoints than the mid-age group, which helps explain the observed worse prognosis. It has been noted that younger patients with luminal types of breast cancer have a worse prognosis, whereas those with HER2-positive or TNBC do not show the same trend.25,26 In trials such as HERA, NCCTG N9831, and NSABP B-31, age was not a significant risk factor for survival in HR-negative HER2-positive breast cancer.27,28 The recurrence score from the 21-gene breast cancer assay (Oncotype DX, Genomic Health) indicated that high RS was most prevalent among women <40 years of age, regardless of nodal status.29 Updated data from the SOFT and TEXT trials suggested that younger patients who remained premenopausal after adjuvant chemotherapy benefitted more from additional ovarian suppression.30 Unfortunately, the patients in this study were diagnosed before these findings were available, and thus, they might not have received adequate ovarian suppression. Consequently, it is important to recognize that young Asian patients with HR+/HER2− breast cancer are more likely to be at high risk of recurrence, and the prompt integration of updated guidelines into clinical practice is warranted.
This study has limitations due to its retrospective nature and reliance on national registry data. Detailed information on adjuvant hormonal treatments, including tamoxifen, aromatase inhibitors, and ovarian function suppression, as well as chemotherapy and targeted therapies, was not collected. In addition, specific treatment regimens used for metastatic disease, which could have influenced patients’ survival, were not known. Another limitation is the exclusion of patients who lacked clinical information. Despite these limitations, our data are consistent with previous reports from East Asian populations.
Conclusion
Asian patients with breast cancer <40 years with HR+/HER2− subtypes, but not those with HER2-positive or TNBC subtypes, were more likely to experience worse survival outcomes than their mid-age counterparts. Our study underscores the need for extended monitoring and the development of effective strategies specifically for this breast cancer subtype in young patients to enhance their survival.
Acknowledgments
Funding
None declared.
Disclosure
KHL reports receiving honoraria from AstraZeneca, Eli Lilly, Novartis, and Pfizer. YHP reports consultancy/advisory role for AstraZeneca, Pfizer, Eisai, Roche, Daiichi Sankyo, Eli Lilly, and Novartis Pharmaceuticals; patents and royalties from Hanmi; honoraria from AstraZeneca, Pfizer, Eisai, Roche, Daiichi Sankyo, and Novartis; grant/research funding from AstraZeneca, Merck, Pfizer, Novartis, Alteogen, and Roche. CHL reports receiving honoraria from AstraZeneca, Daiichi Sankyo, Merck, Eli Lilly, Novartis, Roche, and Pfizer. YSL reports grants and personal fees from Novartis, Merck Sharp & Dohme; and personal fees from Pfizer, Roche, Eisai, and Eli Lilly. TU reports receiving honoraria from Chugai Pharmaceutical, Eisai, Astra Zeneca, Novartis Pharma; and a grant from Eli Lilly. YSY reports receiving travel support and/or honoraria from AstraZeneca, Eisai, Lilly/DKSH, Novartis, Pfizer, Roche, MSD, Inivata, and Specialised Therapeutics; and research support from MSD. VKMT reports personal fees from Bertis, Roche, and AstraZeneca. WY reports receiving honoraria from AstraZeneca, Daiichi Sankyo, Merck Sharp & Dohme, Eli Lilly, Novartis, Roche, Pfizer, and Fosun. YN reports receiving grant funding and/or honoraria from AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Fuji Film Toyama Chemistry, Gardant, Ono, Mundi, Novartis, Pfizer, Roche, Shionogi, Taiho, and Takeda. HBL is a member of the board of directors of, and has stock and ownership interests at, DCGen Co., Ltd., and has received grants from Devicor, Inc. and personal fees from Alvogen, Boryung, Lilly, Novartis, Roche, and Takeda., Ltd. WH is a member on the board of directors of, and has stock and ownership interests at, DCGen, Co., Ltd. SAI reports received research grant from AstraZeneca, Daewoong Pharmaceutical, Eisai, Roche, and Pfizer (for institution); and personal consultation fees from AstraZeneca, Eisai, GSK, Hanmi, Lilly, MSD, Idience, Novartis, Roche, and Pfizer; and her research group has received drugs from Eisai and Boryung. All remaining authors have declared no conflicts of interest.
Data sharing
The datasets used and/or analyzed during this study are available from the corresponding authors on reasonable request.
Contributor Information
K.-H. Lee, Email: kyunghunlee@snu.ac.kr.
S.-A. Im, Email: moisa@snu.ac.kr.
Supplementary data
References
- 1.Duffy S.W., Tabar L., Olsen A.H., et al. Absolute numbers of lives saved and overdiagnosis in breast cancer screening, from a randomized trial and from the Breast Screening Programme in England. J Med Screen. 2010;17(1):25–30. doi: 10.1258/jms.2009.009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo F., Kuo Y.F., Shih Y.C.T., Giordano S.H., Berenson A.B. Trends in breast cancer mortality by stage at diagnosis among young women in the United States. Cancer. 2018;124(17):3500–3509. doi: 10.1002/cncr.31638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H.J., Kim S., Freedman R.A., Partridge A.H. The impact of young age at diagnosis (age <40 years) on prognosis varies by breast cancer subtype: a U.S. SEER database analysis. Breast. 2022;61:77–83. doi: 10.1016/j.breast.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keramatinia A., Mousavi-Jarrahi S.H., Hiteh M., Mosavi-Jarrahi A. Trends in incidence of breast cancer among women under 40 in Asia. Asian Pac J Cancer Prev. 2014;15(3):1387–1390. doi: 10.7314/apjcp.2014.15.3.1387. [DOI] [PubMed] [Google Scholar]
- 5.Sung H., Rosenberg P.S., Chen W.Q., et al. Female breast cancer incidence among Asian and Western populations: more similar than expected. J Natl Cancer Inst. 2015;107(7) doi: 10.1093/jnci/djv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin H.R., Joubert C., Boniol M., et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Causes Control. 2010;21(11):1777–1785. doi: 10.1007/s10552-010-9604-8. [DOI] [PubMed] [Google Scholar]
- 7.Matsuno R.K., Anderson W.F., Yamamoto S., et al. Early- and late-onset breast cancer types among women in the United States and Japan. Cancer Epidemiol Biomark Prev. 2007;16(7):1437–1442. doi: 10.1158/1055-9965.EPI-07-0108. [DOI] [PubMed] [Google Scholar]
- 8.Sørlie T., Perou C.M., Tibshirani R., et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldhirsch A., Winer E.P., Coates A.S., et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin C.-H., Yap Y.S., Lee K.H., et al. Contrasting epidemiology and clinicopathology of female breast cancer in Asians vs the US population. JNCI: Journal of the National Cancer Inst. 2019;111(12):1298–1306. doi: 10.1093/jnci/djz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H.L., Zhou M.Q., Tian W., Meng K.X., He H.F. Effect of age on breast cancer patient prognoses: a population-based study using the SEER 18 database. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0165409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rakha E.A., Starczynski J., Lee A.H., Ellis I.O. The updated ASCO/CAP guideline recommendations for HER2 testing in the management of invasive breast cancer: a critical review of their implications for routine practice. Histopathology. 2014;64(5):609–615. doi: 10.1111/his.12357. [DOI] [PubMed] [Google Scholar]
- 13.Wolff A.C., Hammond M.E., Hicks D.G., et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 14.Hammond M.E., Hayes D.F., Dowsett M., et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6(4):195–197. doi: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin C.-H., Chen Y.C., Chiang C.J., et al. The emerging epidemic of estrogen-related cancers in young women in a developing Asian country. Int J Cancer. 2012;130(11):2629–2637. doi: 10.1002/ijc.26249. [DOI] [PubMed] [Google Scholar]
- 16.Youn H.J., Han W. A review of the epidemiology of breast cancer in Asia: focus on risk factors. Asian Pac J Cancer Prev. 2020;21(4):867–880. doi: 10.31557/APJCP.2020.21.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter P. “Westernizing” women's risks? Breast cancer in lower-income countries. N Engl J Med. 2008;358(3):213–216. doi: 10.1056/NEJMp0708307. [DOI] [PubMed] [Google Scholar]
- 18.Minami Y., Tsubono Y., Nishino Y., Ohuchi N., Shibuya D., Hisamichi S. The increase of female breast cancer incidence in Japan: emergence of birth cohort effect. Int J Cancer. 2004;108(6):901–906. doi: 10.1002/ijc.11661. [DOI] [PubMed] [Google Scholar]
- 19.Fan L., Strasser-Weippl K., Li J.J., et al. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–e289. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 20.Leong S.P., Shen Z.Z., Liu T.J., et al. Is breast cancer the same disease in Asian and Western countries? World J Surg. 2010;34(10):2308–2324. doi: 10.1007/s00268-010-0683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajaram N., Mariapun S., Eriksson M., et al. Differences in mammographic density between Asian and Caucasian populations: a comparative analysis. Breast Cancer Res Treat. 2017;161(2):353–362. doi: 10.1007/s10549-016-4054-y. [DOI] [PubMed] [Google Scholar]
- 22.Hollingsworth A.B. Redefining the sensitivity of screening mammography: a review. Am J Surg. 2019;218(2):411–418. doi: 10.1016/j.amjsurg.2019.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan J.-W., Zabidi M.M.A., Ng P.S., et al. The molecular landscape of Asian breast cancers reveals clinically relevant population-specific differences. Nat Commun. 2020;11(1):6433. doi: 10.1038/s41467-020-20173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kan Z., Ding Y., Kim J., et al. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun. 2018;9(1):1725. doi: 10.1038/s41467-018-04129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryu J.M., Yu J., Kim S.I., et al. Different prognosis of young breast cancer patients in their 20s and 30s depending on subtype: a nationwide study from the Korean Breast Cancer Society. Breast Cancer Res Treat. 2017;166(3):833–842. doi: 10.1007/s10549-017-4472-5. [DOI] [PubMed] [Google Scholar]
- 26.Partridge A.H., Hughes M.E., Warner E.T., et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. 2016;34(27):3308–3314. doi: 10.1200/JCO.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 27.Chumsri S., Serie D.J., Li Z., et al. Effects of age and immune landscape on outcome in HER2-positive breast cancer in the NCCTG N9831 (Alliance) and NSABP B-31 (NRG) trials. Clin Cancer Res. 2019;25(14):4422–4430. doi: 10.1158/1078-0432.CCR-18-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Partridge A.H., Gelber S., Piccart-Gebhart M.J., et al. Effect of age on breast cancer outcomes in women with human epidermal growth factor receptor 2-positive breast cancer: results from a herceptin adjuvant trial. J Clin Oncol. 2013;31(21):2692–2698. doi: 10.1200/JCO.2012.44.1956. [DOI] [PubMed] [Google Scholar]
- 29.Iles K., Roberson M.L., Spanheimer P., et al. The impact of age and nodal status on variations in oncotype DX testing and adjuvant treatment. NPJ Breast Cancer. 2022;8(1):27. doi: 10.1038/s41523-022-00394-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pagani O., Walley B.A., Fleming G.F., et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer: long-term follow-up of the combined TEXT and SOFT trials. J Clin Oncol. 2023;41(7):1376–1382. doi: 10.1200/JCO.22.01064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.