Highlights
-
•
Subcortical volumes were previously shown to be associated with cognitive function.
-
•
This exploratory study focuses on the role of subcortical structures in CADASIL.
-
•
Among other regions, the thalamus and the putamen were associated with the Mini-Mental State Examination scores, vascular cognitive impairment.
-
•
Subcortical volumes should probably included into a disease-specific signature for cognition in CADASIL.
Keywords: CADASIL, Subcortical volumes, Thalamus, Putamen, Vascular cognitive impairment
Abstract
Background
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) represents the most common heritable cause of vascular dementia. Subcortical volumes might be proxies of brain reserve capacity and reflective of cognitive function. We explored the impact of subcortical volumes on cognition in CADASIL patients.
Methods
We included 90 patients with pathogenic NOTCH3 variants from the Taiwan Associated Genetic and Nongenetic Small Vessel Disease cohort. They underwent MRI sessions at baseline. The volumes of the putamen, caudate, pallidum, thalamus, cerebellum, cortical gray matter and brain parenchymal fraction (BPF) were calculated by using FreeSurfer. We tested the association of the subcortical volumes, cortical gray matter volume and BPF with scores of the Mini-mental state examination (MMSE), cognitive domains, and the diagnosis of vascular cognitive impairment (VCI) which was defined as MMSE score <24.
Results
The thalamus and putamen were consistently associated with the MMSE (thalamus adjusted beta per SD decrement -1.41 [95 % CI -2.68–(-0.14)], p = 0.03; R²=0.25; putamen -1.93 [95 % CI -2.99–(-0.86)], p < 0.001; R²=0.36) and VCI (thalamus OR per SD-decrement 3.66 [95 % CI 1.38–9.72], p = 0.009), putamen (OR 3.06 [95 % CI 1.21–7.73], p = 0.02). A larger thalamus volume was also associated with better executive function and visuospatial perception. The cortical gray matter volume and the BPF showed associations with various cognitive outcomes in all analyses.
Conclusion
Although cortical gray matter volume and the BPF still appear to be robust markers of cognitive performance in CADASIL, the volumes of the thalamus and the putamen might also be promising regions of interest for future research.
Introduction
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) is a hereditary small vessel disease that predisposes to strokes, cognitive decline and subsequent vascular dementia [1]. In concomitance with lacunes, cerebral microbleeds and white matter alterations, brain atrophy was described as correlate of cognitive impairment in CADASIL patients [[2], [3], [4]]. Brain size is a common proxy of the brain reserve capacity. The brain reserve essentially represents a protective buffer against stressors acting on the brain [5,6]. The brain reserve capacity of CADASIL patients might also be reflected by subcortical volumes. The structural alterations of the vessels may cause shortcomings in energy and oxygen supply and eventually also lead to regional subcortical atrophy.
Research from other fields (e.g., into Alzheimer's disease) suggests that subcortical volumes are playing a substantial role in cognition [7,8]. Accordingly, the volume reduction of subcortical structures might also be related to vascular cognitive impairment (VCI) in CADASIL. Although subcortical alterations are probably causing cortical changes [9], there is a lack of studies that systematically analyzed subcortical volumes and their association with cognition in CADASIL. However, several diffusion imaging studies have focused on single subcortical structures indicating an involvement in cognition. In light of the brain reserve theory, diffusion abnormalities might go hand in hand with volumetric changes of the respective structures. Diffusion alterations in the thalamus and the putamen were shown to be present in 20 French CADASIL patients compared to 12 healthy controls [10]. This finding was affirmed by a British study group which again found abnormal diffusivity in the thalamus, putamen and pallidum of 18 CADASIL patients compared to 12 healthy controls. Thalamic diffusion metrics were also correlated with executive function [11]. A study of 242 patients with cerebral small vessel disease unrelated to CADASIL showed smaller volumes of the thalamus and caudate in patients with cognitive impairment defined by the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) [12].
The objective of this exploratory study was to analyze the impact of subcortical volumes on different aspects of cognition in patients with CADASIL. We hypothesized that smaller subcortical volumes may be associated with worse cognitive performance in patients with CADASIL.
Methods
Study design and participants
This paper has been written in accordance with the ‘Strengthening the reporting of observational studies in epidemiology’ (STROBE) checklist. The participants were recruited from the Taiwan Associated Genetic and Nongenetic Small Vessel Disease (TAG-SVD) cohort [13]. The research ethics committee's approval was obtained (NTUH-REC:201810003RIND), and informed consent was obtained from the participants or their relatives. The TAG-SVD cohort enrolled patients with clinical and neuroimaging features of CSVD. Clinical features encompassed stroke cognitive impairment, gait disturbance, parkinsonism, headache or a positive family history of hereditary CSVD. Neuroimaging features of CSVD such as lacunae, white matter hyperintensity (WMH), CMBs and enlarged perivascular space (EPVS) were defined according to the standards for reporting vascular changes on neuroimaging [14]. We also report on imaging characteristics according to the recently developed CADA-MRIT inventory tool, specifically designed for reporting imaging features in CADASIL [15]. All enrolled patients carried known pathogenic NOTCH3 variants, of those the majority harbored the R544C pathogenic variant.
Cognitive testing
Every patient underwent a Mini-mental State Examination (MMSE) to evaluate their global cognitive performance. VCI was defined as MMSE score <24 [[16], [17], [18]]. For individuals with cognitive complaints, a comprehensive neuropsychological examination was performed by experienced neuropsychologists. Executive function was evaluated using the working memory index subscale from the Wechsler Adult Intelligence Scale–Third Edition (WAIS-III), verbal fluency test, and the Wisconsin Card Sorting test. Processing speed was evaluated using the Processing Speed Index subscale from the WAIS-III. Episodic memory was evaluated using the Logical memory test from the WAIS-III, the Word Sequence Learning test, and the Benton Visual Retention Test (BVRT). 3D block construction test was used to evaluate visuospatial function. Language function was evaluated using visual naming test and the Token 44 test.
Imaging acquisition
All patients and healthy controls were scheduled for a study MRI session. We deployed 3-T MRI scanners (TIM Trio, Siemens) for imaging acquisition. For this analysis we mainly used the data of a 3D T1-magnetization- prepared rapid gradient-echo (MPRAGE) sequence (repetition time 1850–2530 ms; echo time 2.27–4.6 ms; inversion time 720–1100 ms, slice thickness 1 mm).
We further assessed CSVD markers including the number of cerebral microbleeds and lacunes. The entire scanning protocol and visual assessment of CSVD markers of CADASIL patients was published elsewhere [13].
Imaging processing
The structural T1-weighted MRI data was processed using Freesurfer (v7.2.0) for MacOS. Freesurfer is a free software which automatically delineates and calculates the volume of subcortical structures (http://surfer.nmr.mgh.harvard.edu/) [19].
We manually assessed and rated the quality of the Freesurfer output. We searched for severe anatomical defects such as large intracranial hemorrhages (by MF). A manual correction of Freesurfer's automatically assigned labels was not performed, because it was reported to not have any advantage over the automated segmentation and might hamper reproducibility [20].
We extracted the volumes of the following subcortical structures: thalamus, caudate, putamen, pallidum, cerebellar gray and white matter. We additionally created a basal ganglia variable consisting of caudate, putamen and pallidum. At first, we calculated the mean hemispherical volume for each subcortical structure [21]. Subsequently, the mean hemispherical volumes were expressed as percentage of the estimated intracranial volume [22,23].
The Brain Parenchymal Fraction (BPF) was calculated as quotient out of unnormalized brain volume and intracranial volume. To put the estimates of subcortical volumes into perspective, we also analyzed the volume of the cortical gray matter and the BPF. The cortical gray matter and the BPF were strongly correlated with cognitive function in CADASIL [2,9]. Furthermore, WMH was segmented on FLAIR imaging using the Lesion Segmentation Tool toolbox version 3.0.0 (www.statistical- modelling.de/lst.html) for Statistical Parametric Mapping [24]. The results of WMH volume were expressed in cm3.
Statistical analysis
The present study was exploratory by design. We refrained from drawing conclusions about statistical significance. However, we relied on conventional assumptions of statistical significance (e.g., p-value < 0.05, polarity of CI or CI not including 1) to identify regions of interest. Only adjusted variables were considered for interpretation. Adjustment variables were selected, when they were considered potential confounders, i.e., affect subcortical volumes and cognition alike. We intended to avoid overfitting and limited the number of covariates according to our sample size. The adjustment variables were age, sex, history of stroke, hypertension, diabetes mellitus and the number of cerebral microbleeds and lacunes per region (thalamus, cerebellum, basal ganglia, cortex, global). There was a large number of microbleeds located in subcortical structures in our cohort, potentially affecting the volume of intact gray matter. Cerebral microbleeds were also shown to be associated with cognitive decline [4,25].
To test the associations between regional brain volumes and MMSE score, linear regression was used with MMSE score as the dependent variable. There was a regression model for each subcortical volume, BPF, cortical gray matter, the number of cerebral microbleeds as well as lacunes. We report raw and adjusted estimates. Here, the regression models for the subcortical volumes were adjusted for cerebral microbleeds and lacunes in that respective region (thalamus, basal ganglia and cerebellum). The regression model for the cortical gray matter included the number of cortical microbleeds. We present the regression coefficient beta, 95 % CI and R² for each model. To further ascertain the robustness of our results, we additionally calculated a linear regression model only containing the different subcortical volumes (thalamus, cerebellar white matter, cerebellar gray matter, caudate, putamen, pallidum) as independent variables. By doing so, we could account for intercorrelations between the different regions.
We summarized cognitive test results in sum scores for each domain (executive function, memory, processing speed, language and visuospatial perception) using composite z-scores. For each domain, z-scores of cognitive tests were summarized and subsequently divided by the standard deviation of the summarized z-score. The composite z-scores reflecting different domains served as dependent variables in several linear regressions. We firstly performed simple linear regressions, in which each subcortical volume was set as a single independent variable. We then calculated a set of multiple linear regressions containing the adjustment variables. We reported the regression coefficient beta, 95 % CI and R² for each model.
To assess the impact of subcortical volumes on VCI, we calculated odds ratio using logistic regressions. The presence of VCI (binary) was set as a dependent variable. Again, we calculated raw and adjusted estimates integrating the adjustment covariates. We report odds ratio and their 95 % CI. To further ascertain the robustness of our results, we additionally calculated a logistic regression model only containing the different subcortical volumes (thalamus, cerebellar white matter, cerebellar gray matter, caudate, putamen, pallidum) as independent variables. By doing so, we could account for intercorrelations between the different regions.
All statistical analyses and data visualizations were carried out using GraphPad Prism (version 10.3.0). Other visualizations were made with BioRender.com
Results
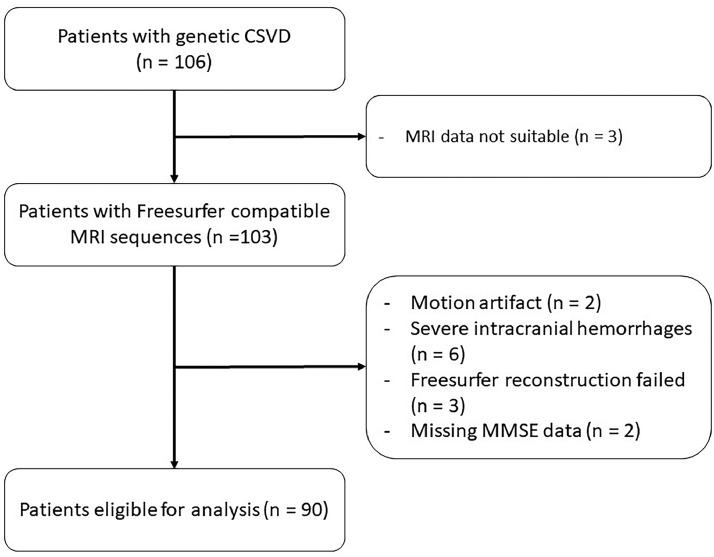
Of the initial 106 patients with NOTCH3 variants, a total of 90 were eligible for further analysis. The reasons for exclusion were MRI data not compatible with Freesurfer (n = 3), motion artefacts (n = 2), severe intracranial hemorrhages (n = 6), Freesurfer reconstruction failed (N = 3) and missing MMSE data (n = 2; Figs. 1, 2).
Fig. 1.
Flow chart.
Note: The flow chart shows the inclusion process, reasons for exclusion and the final sample size. (CSVD = cerebral small vessel disease; MRI = magnetic resonance imaging; MMSE = Mini-Mental State Examination)
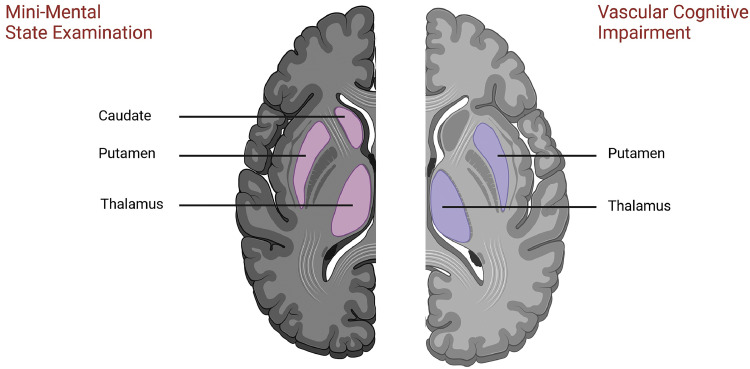
Fig. 2.
Subcortical volumes of interest.
Note: The schematic overview highlights potential regions of interest for the Mini-Mental State Examination (left) and vascular cognitive impairment (right).
Genetic sequencing revealed that 83 patients (92.2 %) were carriers of the R544C pathogenic variant. Volumetric data were missing for cerebellar gray matter (n = 1), caudate (n = 1) and the pallidum (n = 6).
Associations between brain structures and mini mental state examination
MMSE data were available for all 90 CADASIL patients. The median time between the MMSE and the MRI acquisition was 7 days (Q1 to Q3, −132 to 115).
By running adjusted linear regressions, the following structures could be identified as regions of interest: thalamus (adjusted beta per SD decrement −1.41 [95 % CI −2.68 – (−0.14)], p = 0.03; R²=0.25), caudate (adjusted beta −1.25 [95 % CI −2.26 – (−0.25)], p = 0.02; R²=0.33), putamen (adjusted beta −1.93 [95 % CI −2.99 – (−0.86)], p < 0.001; R²=0.36), cortical gray matter (adjusted beta −2.02 [95 % CI −3.35 – (−0.68)], p = 0.003; R²=0.33) and the BPF (adjusted beta −2.04 [95 % CI −3.31 – (−0.76)], p = 0.002; R²=0.33; Table 1). Aside from volumetric variables, the total number of cerebral lacunes (adjusted beta per SD decrement in the number of cerebral lacunes 1.17 [95 % CI 0.01 – 2.34], p = 0.048; R²=0.25) was inversely associated with the MMSE score. The volumes of cerebellar white matter and cerebellar gray matter and the pallidum do not appear to be associated with the MMSE scores of CADASIL patients. The model containing the subcortical volumes altogether revealed that only the thalamus was statistically significant (adjusted beta per SD decrement −1.82 [95 % CI −3.38 - (−0.27)] p = 0.02; Supplemental Table 1).
Table 1.
Subcortical volumes associated with MMSE.
| Estimate | 95 % CI | p-value | Adj. R² | ||
|---|---|---|---|---|---|
| Thalamus | Unadj. | −2.43 | −3.49 – (−1.36) | <0.001 | 0.18 |
| Adj. | −1.41 | −2.68 – (−0.14) | 0.03 | 0.25 | |
| Cerebellar White Matter | Unadj. | −1.53 | −2.66 – (−0.39) | 0.009 | 0.06 |
| Adj. | −0.24 | −1.50 – 1.02 | 0.70 | 0.20 | |
| Cerebellar Gray Matter | Unadj. | −1.23 | −2.40 – (−0.06) | 0.04 | 0.04 |
| Adj. | −0.09 | −1.29 – 1.11 | 0.88 | 0.22 | |
| Caudate | Unadj. | −1.03 | −2.20 – 0.15 | 0.09 | 0.02 |
| Adj. | −1.25 | −2.26 – (−0.25) | 0.02 | 0.33 | |
| Putamen | Unadj. | −1.96 | −3.07 – (−0.86) | <0.001 | 0.11 |
| Adj. | −1.93 | −2.99 – (−0.86) | <0.001 | 0.36 | |
| Pallidum | Unadj. | −0.91 | −2.12 – 0.30 | 0.14 | 0.01 |
| Adj. | −1.08 | −2.29 – 0.13 | 0.08 | 0.26 | |
| Basal Ganglia | Unadj. | −1.85 | −3.02 – (−0.67) | 0.002 | 0.10 |
| Adj. | −2.02 | −3.10 – (−0.94) | <0.001 | 0.36 | |
| Cortical Gray Matter | Unadj. | −2.99 | −3,98 – (−1.99) | <0.001 | 0.28 |
| Adj. | −2.02 | −3.35 – (−0.68) | 0.003 | 0.33 | |
| Brain Parenchymal Fraction | Unadj. | −3.03 | −4.02 – (−2.03 | <0.001 | 0.29 |
| Adj. | −2.04 | −3.31 – (−0.76) | 0.002 | 0.33 | |
| Cerebral Microbleeds | Unadj. | 1.79 | 0.67 – 2.91 | 0.002 | 0.09 |
| Adj. | 1.03 | −0.10 – 2.15 | 0.07 | 0.24 | |
| Lacunes | Unadj. | 1.89 | 0.77 – 3.00 | 0.001 | 0.10 |
| Adj. | 1.17 | 0.01 – 2.34 | 0.048 | 0.25 |
Note: The table shows the association of volumes with the Mini-Mental State Examination (MMSE) of n = 90 CADASIL patients. The volumes were calculated as percentage of the estimated total intracranial volume per mean volume of both hemispheres. The volumetric variables were standardized and estimates are for each decrement in standard deviation. Volumetric data were missing for cerebellar gray matter (n = 1), caudate (n = 1) and the pallidum (n = 6). Adjustment variables were age, sex, history of stroke, hypertension, diabetes mellitus and the number of cerebral microbleeds and lacunes in the respective subcortical or cortical region.
Associations between brain structures and vascular cognitive impairment
The volumes of the thalamus, cerebellar white matter, cerebellar gray matter, putamen and cortical gray matter were smaller in CADASIL patients with VCI compared to those without VCI. The BPF was also smaller in patients with VCI. Furthermore, the VCI group had a higher number of lacunes (median 10 vs 3, p = 0.008) and cerebral microbleeds (median 34 vs 6, p = 0.002) than the non-VCI group (See Table 2). Table 3 lists the patient's imaging characteristics according to the CADA-MRIT inventory.
Table 2.
Characteristics between CADASIL patients with and without vascular cognitive impairment (VCI).
| No VCI | VCI | p- value | |
|---|---|---|---|
| N = 73 | N = 17 | ||
| Age [years] – mean (SD) | 59.9 (10.5) | 70.8 (9.2) | < 0.001 |
| Female | 38 (52.11 %) | 11 (64.7 %) | 0.35 |
| Mini-Mental State Examination (MMSE) – median (25th-75th percentile) | 29 (27 – 30) | 16 (12 – 21) | < 0.001 |
| Education [years] - median (25th-75th percentile) | 12 (9 – 16), N = 66 | 12 (6 – 14) | 0.09 |
| mRS - median (25th-75th percentile) | 0 (0 – 1) | 3 (1 – 4) | < 0.001 |
| Body Mass Index- mean (SD) | 24.5 (3.4), N = 71 | 25.3 (5.6) | 0.45 |
| Diabetes Mellitus | 18 (24.6 %) | 3 (17.6 %) | 0.54 |
| Hypertension | 39 (53.4 %) | 12 (70.6 %) | 0.20 |
| Hyperlipidemia | 37 (50.6 %) | 8 (47.1 %) | 0.79 |
| Psychiatric | 17 (23.3 %) | 13 (76.5 %) | < 0.001 |
| Cognitive | 42 (57.5 %) | 13 (76.5 %) | 0.15 |
| Gait | 28 (38.4 %) | 14 (82.4 %) | < 0.001 |
| Headache | 12 (16.4 %) | 2 (11.8 %) | 0.83 |
| CAD | 1 (1.4 %) | 1 (5.9 %) | 0.26 |
| Atrial Fibrillation | 5 (6.8 %) | 2 (11.8 %) | 0.50 |
| Congestive Heart Failure | 0 | 0 | NA |
| Smoking | 18 (24.6 %) | 5 (29.4 %) | 0.69 |
| Alcohol | 7 | 1 | 0.63 |
| History of stroke | 40 | 13 | 0.17 |
| Age first stroke – mean (SD) | 57.3 (11.1) | 59.9 (11.1), N = 12 | 0.66 |
| Baseline Stroke type | |||
| None | 33 (45.2 %) | 4 (23.5 %) | 0.13 |
| Ischemic | 24 (32.9 %) | 7 (41.2 %) | |
| Hemorrhagic | 13 (17.8 %) | 3 (17.6 %) | |
| Combined | 3 (4.1 %) | 3 (17.6 %) | |
| Baseline Stroke Episode | 40 (54.8 %) | 13 (76.5 %) | 0.10 |
| Mortality | 1 (1.4 %) | 0 | 0.99 |
| Lacunes - median (25th-75th percentile) | 3 (0 - 9) | 10 (4 – 13) | 0.008 |
| Cerebral Microbleeds - median (25th-75th percentile) | 6 (1 – 26) | 34 (7 – 78) | 0.002 |
| Volume of White Matter Hyperintensities [cm³] – mean (SD) | 29.78 (22.73) | 52.26 (18.05) | <0.001 |
| MRI volumetric analysis (% of intracranial volume) – median (25th-75th percentile) | |||
| Thalamus | 0.47 (0.42 – 0.51) | 0.41 (0.38 – 0.42) | 0.0007 |
| Cerebellar White Matter | 0.93 (0.85 – 1.02) | 0.85 (0.78 – 0.98) | 0.04 |
| Cerebellar Cortex | 3.51 (3.18 – 3.61) | 3.14 (3.11 – 3.29) | 0.04 |
| Caudate | 0.26 (0.24 – 0.29) | 0.25 (0.22 – 0.28) | 0.36 |
| Putamen | 0.35 (0.32 – 0.40) | 0.29 (0.26 – 0.37) | 0.03 |
| Pallidum | 0.14 (0.12 – 0.15) | 0.14 (0.12 – 0.15) | 0.76 |
| Basal Ganglia | 0.78 (0.72 – 0.84) | 0.69 (0.63 – 0.82) | 0.06 |
| Brain Parenchymal Fraction | 73.76 (71.42 – 76.68) | 67.46 (65.98 – 71.37) | < 0.001 |
| Cortical Gray Matter | 29.21 (28.42 – 30.10) | 25.92 (25.51 – 28.69) | < 0.001 |
Note: The table shows characteristics of patients with and without vascular cognitive impairment (VCI). We present total numbers and percentages in parentheses for categorial data. Significance testing of these data was performed by using the chi-square test. Normally distributed continuous variables are listed with mean and standard deviation (SD). Statistically significant differences between groups were determined with t-tests. Median and percentiles are given for not normally distributed data. For these cases, we used the Mann-Whitney-U test to test for significant differences. If there are data missing or not applicable, N of patients with available data is shown in grey.
Table 3.
CADA-MRIT inventory across groups.
| No VCI |
VCI |
p- value | |||
|---|---|---|---|---|---|
| N = 73 | N = 17 | ||||
| Age [years] – mean (SD) | 59.9 (10.5) | 70.8 (9.2) | < 0.001 | ||
| Female | 38 (52.11 %) | 11 (64.7 %) | 0.35 | ||
| Periventricular WMH (PVH) [in contact with the ventricles] | |||||
| Absence | 3 | 4.1 % | 0 | 0.0 % | 0.02 |
| “Caps” or pencil-thin lining | 9 | 12.3 % | 0 | 0.0 % | |
| Smooth “halo” | 23 | 31.5 % | 1 | 5.9 % | |
| PVH extending into deep white matter partly merging with deep WMH | 31 | 42.5 % | 11 | 64.7 % | |
| PVH extending into deep white matter completely merging with deep WMH | 7 | 9.6 % | 5 | 29.4 % | |
| Deep WMH [between PVH and superficial WMH] | |||||
| Absence | 7 | 9.6 % | 0 | 0.0 % | 0.04 |
| Punctate foci | 10 | 13.7 % | 0 | 0.0 % | |
| Beginning confluence of foci | 24 | 32.9 % | 3 | 17.6 % | |
| Large confluent areas | 32 | 43.8 % | 14 | 82.4 % | |
| Superficial WMH | |||||
| Absence | 56 | 76.7 % | 14 | 82.4 % | 1.0 |
| Punctate foci | 12 | 16.4 % | 3 | 17.6 % | |
| Beginning confluence of foci | 2 | 2.7 % | 0 | 0.0 % | |
| Confluent WMH completely merging with deep WMH | 3 | 4.1 % | 0 | 0.0 % | |
| Lacune [number] | |||||
| 0 | 19 | 26.0 % | 2 | 11.8 % | 0.27 |
| 1–5 | 33 | 45.2 % | 7 | 41.2 % | |
| 6–10 | 11 | 15.1 % | 6 | 35.3 % | |
| >10 | 10 | 13.7 % | 2 | 11.8 % | |
| CMB [number] | |||||
| 0 | 18 | 24.7 % | 0 | 0.0 % | 0.01 |
| 1–5 (T2) / 1–10 (SWI) | 24 | 32.9 % | 6 | 35.3 % | |
| 6–10 (T2) / 11–20 (SWI) | 12 | 16.4 % | 1 | 5.9 % | |
| >10 (T2) / >20 (SWI) | 19 | 26.0 % | 10 | 58.8 % | |
| Dilated perivascular spaces – Centrum semiovale [number] | |||||
| <10 | 30 | 41.1 % | 14 | 82.4 % | 0.01 |
| 10–20 | 26 | 35.6 % | 2 | 11.8 % | |
| >20 | 17 | 23.3 % | 1 | 5.9 % | |
| Dilated perivascular spaces – Basal ganglia [number] | |||||
| <10 | 12 | 16.4 % | 1 | 5.9 % | 0.42 |
| 10–20 | 22 | 30.1 % | 4 | 23.5 % | |
| >20, innumerable or status cribrosum | 39 | 53.4 % | 12 | 70.6 % | |
| Superficial atrophy | |||||
| Absence | 17 | 23.3 % | 1 | 5.9 % | <0.001 |
| Mild | 35 | 47.9 % | 1 | 5.9 % | |
| Moderate | 15 | 20.5 % | 8 | 47.1 % | |
| Severe | 6 | 8.2 % | 7 | 41.2 % | |
| Deep atrophy | |||||
| Absence | 27 | 37.0 % | 1 | 5.9 % | 0.001 |
| Mild | 28 | 38.4 % | 6 | 35.3 % | |
| Moderate | 16 | 21.9 % | 5 | 29.4 % | |
| Severe | 2 | 2.7 % | 5 | 29.4 % | |
| Large infarct (>20 mm) [number] | |||||
| 0 | 71 | 97.3 % | 16 | 94.1 % | 0.47 |
| 1 | 2 | 2.7 % | 1 | 5.9 % | |
| Macrobleeds (>15 mm) [number] | |||||
| 0 | 58 | 79.5 % | 12 | 70.6 % | 0.60 |
| 1 | 11 | 15.1 % | 4 | 23.5 % | |
| >1 | 4 | 5.5 % | 1 | 5.9 % | |
Note: The table shows the imaging properties according to the CADA-MRIT. WMH = white matter hyperintensities; T2 = T2- weighted magnetic resonance imaging; SWI = susceptibility weighted imaging.
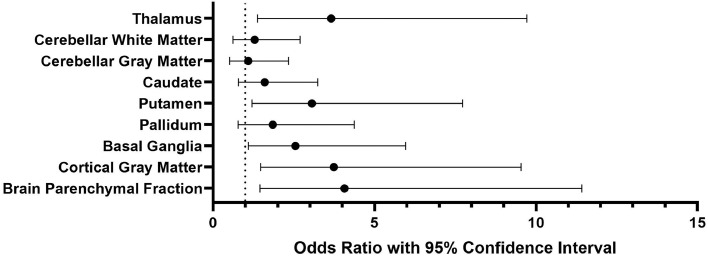
The adjusted logistic regressions revealed that the volumes of the thalamus (OR per SD-decrement 3.66 [95 % CI 1.38 – 9.72], p = 0.009), putamen (OR per SD-decrement 3.06 [95 % CI 1.21 – 7.73], p = 0.02), basal ganglia (OR per SD-decrement 2.55 [95 % CI 1.09 – 5.96], p = 0.03), cortical gray matter (OR per SD-decrement 3.74 [95 % CI 1.47 – 9.54], p = 0.006) and the BPF (OR per SD-decrement 4.07 [95 % CI 1.45 – 11.42], p = 0.008) are independently associated with VCI in patients with CADASIL (Table 4 & Fig. 3). When mutually adjusting for the different subcortical volumes, only the thalamus remained statistically significant (OR per SD-decrement 3.18 [95 % CI 1.11 – 11.3], p = 0.048; Supplemental Table 2).
Table 4.
Subcortical volumes associated with vascular cognitive impairment.
| Odds Ratio | 95 % CI | p-value | ||
|---|---|---|---|---|
| Thalamus | Unadj. | 3.34 | 1.56 – 7.17 | 0.002 |
| Adj. | 3.66 | 1.38 – 9.72 | 0.009 | |
| Cerebellar White Matter | Unadj. | 1.98 | 1.08 – 3.63 | 0.03 |
| Adj. | 1.29 | 0.62 – 2.70 | 0.50 | |
| Cerebellar Gray Matter | Unadj. | 1.99 | 1.09 – 3.65 | 0.03 |
| Adj. | 1.09 | 0.51 – 2.34 | 0.83 | |
| Caudate | Unadj. | 1.40 | 0.80 – 2.47 | 0.24 |
| Adj. | 1.60 | 0.79 – 3.24 | 0.19 | |
| Putamen | Unadj. | 2.01 | 1.06 – 3.82 | 0.03 |
| Adj. | 3.06 | 1.21 – 7.73 | 0.02 | |
| Pallidum | Unadj. | 1.19 | 0.67 – 2.13 | 0.55 |
| Adj. | 1.85 | 0.78 – 4.37 | 0.16 | |
| Basal Ganglia | Unadj. | 1.90 | 0.99 – 3.66 | 0.05 |
| Adj. | 2.55 | 1.09 – 5.96 | 0.03 | |
| Cortical Gray Matter | Unadj. | 3.92 | 1.97 – 7.80 | < 0.001 |
| Adj. | 3.74 | 1.47 – 9.54 | 0.006 | |
| Brain Parenchymal Fraction | Unadj. | 5.05 | 2.19 – 11.65 | < 0.001 |
| Adj. | 4.07 | 1.45 – 11.42 | 0.008 |
Note: The table shows the association of volumes with vascular cognitive impairment defined as Mini-Mental State Examination score of 23 or less (n = 90). The volumes were calculated as percentage of the estimated total intracranial volume per mean volume of both hemispheres. Volumetric data were missing for cerebellar gray matter (n = 1), caudate (n = 1) and the pallidum (n = 6). The volumetric variables were standardized and odds ratio are for each decrement in standard deviation. Adjustment variables were age, sex, history of stroke, hypertension, diabetes mellitus and the number of microbleeds and microbleeds within the specific region.
Fig. 3.
Subcortical volumes in vascular cognitive impairment.
Note: The forest plot displays odds ratio (dots) with 95 % confidence intervals for each region of interest in vascular cognitive impairment (see Table 3). Adjustment variables were age, sex, history of stroke, hypertension, diabetes mellitus and the number of microbleeds and microbleeds within the specific region.
Neuropsychological tests
Of the 90 patients that received a MMSE, 73 underwent an extensive neuropsychological testing. The median time between cognitive testing and MRI time span was 71 days (Q1 to Q3, −40 to 145). For executive function, 54 patients received all the tests which were needed to form a composite z-score. Memory was fully assessed in 56, processing speed in 64, language in 59 and visuospatial perception in 66 patients.
The thalamus (R² = 0.20) and pallidum (R² = 0.24) were observed to be positively associated with executive functioning. The tests reflecting processing speed were not related to any of the examined structures. Language abilities were associated with cortical gray matter volume and the BPF (R² from 0.32∼0.35). The BPF yielded suggestive results related to the cognitive domain of memory. Visuospatial perception in CADASIL patients was associated with the volumes of the thalamus, basal ganglia, cortical gray matter and the BPF (R² from 0.24∼0.35; Supplemental Table 3).
Discussion
Our study found that various subcortical volumes were associated with MMSE scores in CADASIL patients, including the thalamus, caudate, putamen and pallidum. However, the in-depth examination of cognitive domains showed that only the thalamus, putamen, pallidum and cerebellar gray matter are potentially of interested in future research endeavors. In our cohort, only the subcortical volumes of the thalamus and the putamen were associated with VCI. In contrast, established markers like the cortical gray matter volume and the BPF presented with several associations across all the analyses.
Our results indicate that the thalamus might play a role in the cognition of CADASIL patients. This observation aligns with previous diffusion MRI findings in CADASIL patients [10,11]. It further underscores that micro- and macrostructural abnormalities might be closely interrelated. The thalamic impact on cognition also receives growing attention [26,27]. The thalamus was described to be involved in executive functioning, attention, memory and visual perception [[28], [29], [30], [31]]. By changing cortical representations, thalamic nuclei probably coordinate cognitive function [32,33]. The thalamus was also discussed to be a biomarker in neurodegenerative diseases [34]. In mild cognitive impairment and Alzheimer's disease the thalamic volume potentially serves as predictor of cognitive decline [7,8]. Noteworthy, the thalamus represents one of the main sites of cerebral microbleeds in the same Taiwanese CADASIL cohort [13]. However, in the present study, the association between thalamic volume and cognition was independent of the number of microbleeds.
Alongside the thalamus, diffusion MRI in CADSIL patients has also identified the putamen as region of interest [10]. We were able to show that findings derived from diffusion imaging might be transferable to volumetric analyses. As with the thalamus, the putamen volume was reported to be strongly reduced in patients with Alzheimer's disease [7]. Furthermore, the volume of the putamen was smaller in patients with cerebral amyloid angiopathy compared to healthy controls [21]. Whereas there is a growing body of literature revolving around the thalamus in cognition, cognitive implications of putamen functioning are poorly understood. However, a study in older patients with and without cognitive impairment has suggested that the atrophy of the putamen might represent the brain structural link between cognition and gait [35].
We observed that the volume of the pallidum might be associated with executive function. So far, there is only limited evidence in obese adolescents which is also suggesting a relation between the pallidum and executive function [36]. In the present study, cerebellar gray matter was identified as potential region of interest in episodic memory. There are previous findings indicating a cerebellar involvement in working memory [37,38]. The cognitive domain of visuospatial perception was associated with cerebellar white matter, cortical gray matter and the BPF. This is in line with the established knowledge about the complex cortical pathways which process visual information [39]. The cerebellum was also previously described to contribute to visuospatial perception [38,40].
Apart from the putamen and the thalamus, these subcortical volumes were only partly associated with cognition (e.g., only the MMSE) in our cohort. This might indicate that these regions are less reliable as cognitive markers. Aside from this, we must state that conventional marker of brain reserve capacity such as the volume of cortical gray matter and the BPF were associated with cognitive outcomes in each analysis. The BPF might also be reflective of deep gray matter to a certain degree, while subcortical atrophy and global atrophy are interdependent. Other imaging markers such as the white matter hyperintensities represent established imaging correlates of cognition [41]. Previous research has revealed the association between the hippocampal volume and cognitive function in CADASIL [42], identifying the hippocampus as another imaging biomarker. Furthermore, another study found that the volume of the thalamus and caudate are significantly smaller in patients with cerebral small vessel disease compared to healthy controls [12]. Both findings suggest that volumetric imaging represents a sensible approach to detect structural abnormalities related to CADASIL. The atrophy of specific brain regions could be attributed to vascular neurodegeneration which describes the impaired structural integrity of cerebral blood vessels. As a secondary factor, neuroinflammation aggravates neurodegenerative processes [43,44]. Our results support the idea that both the thalamus and the putamen play roles in cognitive processes. In line with shared cognitive markers seen in CADASIL, it is possible that the thalamus and putamen contribute to a disease-specific signature in CADASIL.
Limitation
Most limitations are related to the nature of an exploratory pilot study. The sample size was not prespecified prior data acquisition and was of rather modest size. This work is indented to be hypothesis-generating. Therefore, we did not correct for multiple testing. Further studies with larger sample sizes are required. More than 90 % of the included CADASIL patients harbor the R544C pathogenic variant. Although this appears to be in line with observed prevalences in Taiwan [45,46], it may also affect the generalizability of the results. To corroborate our results, we advocate for further replication studies into different populations which are also harboring different pathogenic variants.
The time span between MRI acquisition and the administration of the MMSE and/or the neuropsychological test battery were relatively large for some patients. We cannot rule out that cognitive test scores may have slightly differed if obtained closer to the date of MRI acquisition. However, the large variation might bias the results toward the null. Furthermore, other cognitive test such as the Montreal Cognitive Assessment might be more suitable instruments to detect a VCI. Another limitation concerns the cognitive testing of patients with severe VCI. Due to their severely impaired cognitive abilities (e.g., MMSE <15), they were unable to take more complex cognitive tests. Different levels of complexity of the cognitive tests explain the varying sample sizes for the sum scores of different cognitive domains.
Conclusion
Apart from already established markers of cognition in CADASIL such as cortical gray matter volume or the BPF, the volumes of the thalamus and the putamen might also represent neural correlates of cognition in CADASIL patients.
CRediT authorship contribution statement
Marinus Fislage: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Chih-Hao Chen: Writing – review & editing, Supervision, Resources, Methodology, Investigation, Data curation, Conceptualization. Yu-Wen Cheng: Writing – review & editing, Supervision, Methodology, Investigation, Data curation. Ya-Fang Chen: Writing – review & editing, Methodology, Investigation, Data curation. Sung-Chun Tang: Writing – review & editing, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Sung-Chun Tang reports financial support was provided by Taiwan Ministry of Science and Technology. Marinus Fislage reports a relationship with German Academic Scholarship Foundation that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding statement
This study has received funding by Taiwan Ministry of Science and Technology (grants 105–2314-B-002–094-MY3, 106–2628-E-002–003-MY3, and 109–2314-B-002–031-MY3).
Acknowledgements
Marinus Fislage has been awarded a China-scholarship by the Studienstiftung des deutschen Volkes (German Academic Scholarship Foundation) and the Alfried Krupp von Bohlen und Halbach-Stiftung.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cccb.2024.100371.
Appendix. Supplementary materials
Data availability
Data that support the current analysis are available upon reasonable request to the corresponding author.
References
- 1.Chabriat H., Joutel A., Dichgans M., Tournier-Lasserve E., Bousser M.G. CADASIL. Lancet Neurol. 2009;8:643–653. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 2.Viswanathan A., Godin O., Jouvent E., et al. Impact of MRI markers in subcortical vascular dementia: a multi-modal analysis in CADASIL. Neurobiol. Aging. 2010;31:1629–1636. doi: 10.1016/j.neurobiolaging.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Jolly A.A., Nannoni S., Edwards H., Morris R.G., Markus H.S. Prevalence and predictors of vascular cognitive impairment in patients with CADASIL. Neurology. 2022;99:e453–e461. doi: 10.1212/WNL.0000000000200607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akoudad S., Wolters F.J., Viswanathan A., et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMa Neurol. 2016;73:934–943. doi: 10.1001/jamaneurol.2016.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reserve S.R.T. Brain changes, and decline. Neuroimaging Clin. N. Am. 2012;22:99–105. doi: 10.1016/j.nic.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Deary I.J., Penke L., Johnson W. The neuroscience of human intelligence differences. Nat. Rev. Neurosci. 2010;11:201–211. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- 7.de Jong L.W., van der Hiele K., Veer I.M., et al. Strongly reduced volumes of putamen and thalamus in Alzheimer's disease: an MRI study. Brain. 2008;131:3277–3285. doi: 10.1093/brain/awn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zidan M., Boban J., Bjelan M., et al. Thalamic volume loss as an early sign of amnestic mild cognitive impairment. J. Clin. Neurosci. 2019;68:168–173. doi: 10.1016/j.jocn.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Lambert C., Sam Narean J., Benjamin P., Zeestraten E., Barrick T.R., Markus H.S. Characterising the grey matter correlates of leukoaraiosis in cerebral small vessel disease. Neuroimage Clin. 2015;9:194–205. doi: 10.1016/j.nicl.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molko N., Pappata S., Mangin J.F., et al. Diffusion tensor imaging study of subcortical gray matter in CADASIL. Stroke. 2001;32:2049–2054. doi: 10.1161/hs0901.094255. [DOI] [PubMed] [Google Scholar]
- 11.O'Sullivan M., Singhal S., Charlton R., Markus H.S. Diffusion tensor imaging of thalamus correlates with cognition in CADASIL without dementia. Neurology. 2004;62:702–707. doi: 10.1212/01.wnl.0000113760.72706.d2. [DOI] [PubMed] [Google Scholar]
- 12.Sun W., Huang L., Cheng Y., et al. Medial temporal atrophy contributes to cognitive impairment in cerebral small vessel disease. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.858171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C.H., Chu Y.T., Chen Y.F., et al. Comparison of clinical and neuroimaging features between NOTCH3 mutations and nongenetic spontaneous intracerebral haemorrhage. Eur. J. Neurol. 2022;29:3243–3254. doi: 10.1111/ene.15485. [DOI] [PubMed] [Google Scholar]
- 14.Duering M., Biessels G.J., Brodtmann A., et al. Neuroimaging standards for research into small vessel disease—advances since 2013. Lancet Neurol. 2023 doi: 10.1016/S1474-4422(23)00131-X. 22:602-618. [DOI] [PubMed] [Google Scholar]
- 15.Zhang R., Chen C.H., Tezenas Du Montcel S., et al. The CADA-MRIT: an MRI inventory tool for evaluating cerebral lesions in CADASIL across cohorts. Neurology. 2023;101:e1665–e1677. doi: 10.1212/WNL.0000000000207713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng Y.W., Liao Y.C., Chen C.H., et al. Contribution of the APOE genotype to cognitive impairment in individuals with NOTCH3 cysteine-altering variants. J. Am. Heart Assoc. 2023;12 doi: 10.1161/JAHA.123.032689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H.C., Lin K.N., Teng E.L., et al. Prevalence and subtypes of dementia in Taiwan: a community survey of 5297 individuals. J. Am. Geriatr. Soc. 1995;43:144–149. doi: 10.1111/j.1532-5415.1995.tb06379.x. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y., Lee H.J., Yang S.C., et al. A nationwide survey of mild cognitive impairment and dementia, including very mild dementia, in Taiwan. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischl B., Salat D.H., Busa E., et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy C.S., Ramprashad A., Thompson C., Botti J.A., Coman I.L., Kates W.R. A comparison of FreeSurfer-generated data with and without manual intervention. Front. Neurosci. 2015;9:379. doi: 10.3389/fnins.2015.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C.H., Khnaijer M.K., Beaudin A.E., et al. Subcortical volumes in cerebral amyloid angiopathy compared with Alzheimer's disease and controls. Front. Neurosci. 2023;17 doi: 10.3389/fnins.2023.1139196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fotiadis P., Pasi M., Charidimou A., et al. Decreased basal ganglia volume in cerebral amyloid angiopathy. J. Stroke. 2021;23:223–233. doi: 10.5853/jos.2020.04280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell J.H., Elif G., Alex J.B., et al. Cerebellar atrophy and its implications on gait in cerebral amyloid angiopathy. J. Neurol. Neurosurg. Psychiatry. 2022;93:802. doi: 10.1136/jnnp-2021-328553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt P., Gaser C., Arsic M., et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. 2012;59:3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 25.Chung C.P., Chen J.W., Chang F.C., et al. Cerebral microbleed burdens in specific brain regions are associated with disease severity of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rikhye R.V., Wimmer R.D., Halassa M.M. Toward an integrative theory of thalamic function. Annu. Rev. Neurosci. 2018;41:163–183. doi: 10.1146/annurev-neuro-080317-062144. [DOI] [PubMed] [Google Scholar]
- 27.Shine J.M., Lewis L.D., Garrett D.D., Hwang K. The impact of the human thalamus on brain-wide information processing. Nat. Rev. Neurosci. 2023;24:416–430. doi: 10.1038/s41583-023-00701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Werf Y.D., Scheltens P., Lindeboom J., Witter M.P., Uylings H.B., Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003;41:1330–1344. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- 29.Halassa M.M., Kastner S. Thalamic functions in distributed cognitive control. Nat. Neurosci. 2017;20:1669–1679. doi: 10.1038/s41593-017-0020-1. [DOI] [PubMed] [Google Scholar]
- 30.Van der Werf Y.D., Jolles J., Witter M.P., Uylings H.B. Contributions of thalamic nuclei to declarative memory functioning. Cortex. 2003;39:1047–1062. doi: 10.1016/s0010-9452(08)70877-3. [DOI] [PubMed] [Google Scholar]
- 31.Saalmann Y.B., Kastner S. Cognitive and perceptual functions of the visual thalamus. Neuron. 2011;71:209–223. doi: 10.1016/j.neuron.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parnaudeau S., Bolkan S.S., Kellendonk C. The mediodorsal thalamus: an essential partner of the prefrontal cortex for cognition. Biol. Psychiatry. 2018;83:648–656. doi: 10.1016/j.biopsych.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rikhye R.V., Gilra A., Halassa M.M. Thalamic regulation of switching between cortical representations enables cognitive flexibility. Nat. Neurosci. 2018;21:1753–1763. doi: 10.1038/s41593-018-0269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Power B.D., Looi J.C. The thalamus as a putative biomarker in neurodegenerative disorders. Aust. N. Z. J. Psychiatry. 2015;49:502–518. doi: 10.1177/0004867415585857. [DOI] [PubMed] [Google Scholar]
- 35.Li P., Wang Y., Jiang Y., Chen X., Dong Q., Cui M. Association between cognition and gait in elderly people: the putamen as a shared substrate. Alzheimer's & Dement. 2020;16 [Google Scholar]
- 36.de Groot C.J., van den Akker E.L.T., Rings E.H.H.M., Delemarre-van de Waal H.A., van der Grond J. Brain structure, executive function and appetitive traits in adolescent obesity. Pediatr. Obes. 2017;12:e33–e36. doi: 10.1111/ijpo.12149. [DOI] [PubMed] [Google Scholar]
- 37.Won J., Callow D.D., Purcell J.J., Smith J.C. Differential associations of regional cerebellar volume with gait speed and working memory. Sci. Rep. 2022;12:2355. doi: 10.1038/s41598-022-06180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baier B., Müller N.G., Dieterich M. What part of the cerebellum contributes to a visuospatial working memory task? Ann. Neurol. 2014;76:754–757. doi: 10.1002/ana.24272. [DOI] [PubMed] [Google Scholar]
- 39.Trés E.S., Brucki S.M.D. Visuospatial processing: a review from basic to current concepts. Dement. Neuropsychol. 2014;8:175–181. doi: 10.1590/S1980-57642014DN82000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molinari M., Leggio M.G. Cerebellar information processing and visuospatial functions. Cerebellum. 2007;6:214–220. doi: 10.1080/14734220701230870. [DOI] [PubMed] [Google Scholar]
- 41.Guo W., Shi J. White matter hyperintensities volume and cognition: a meta-analysis. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.949763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Sullivan M., Ngo E., Viswanathan A., et al. Hippocampal volume is an independent predictor of cognitive performance in CADASIL. Neurobiol. Aging. 2009;30:890–897. doi: 10.1016/j.neurobiolaging.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brodtmann A., Khlif M.S., Egorova N., Veldsman M., Bird L.J., Werden E. Dynamic regional brain atrophy rates in the first year after ischemic stroke. Stroke. 2020;51:e183–e192. doi: 10.1161/STROKEAHA.120.030256. [DOI] [PubMed] [Google Scholar]
- 44.Hannawi Y. Cerebral small vessel disease: a review of the pathophysiological mechanisms. Transl. Stroke Res. 2023 doi: 10.1007/s12975-023-01195-9. [DOI] [PubMed] [Google Scholar]
- 45.Lin H.J., Chen C.H., Su M.W., et al. Modifiable vascular risk factors contribute to stroke in 1080 NOTCH3 R544C carriers in Taiwan Biobank. Int. J. Stroke. 2024;19:105–113. doi: 10.1177/17474930231191991. [DOI] [PubMed] [Google Scholar]
- 46.Liao Y.C., Hsiao C.T., Fuh J.L., et al. Characterization of CADASIL among the Han Chinese in Taiwan: distinct genotypic and phenotypic profiles. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data that support the current analysis are available upon reasonable request to the corresponding author.