Abstract
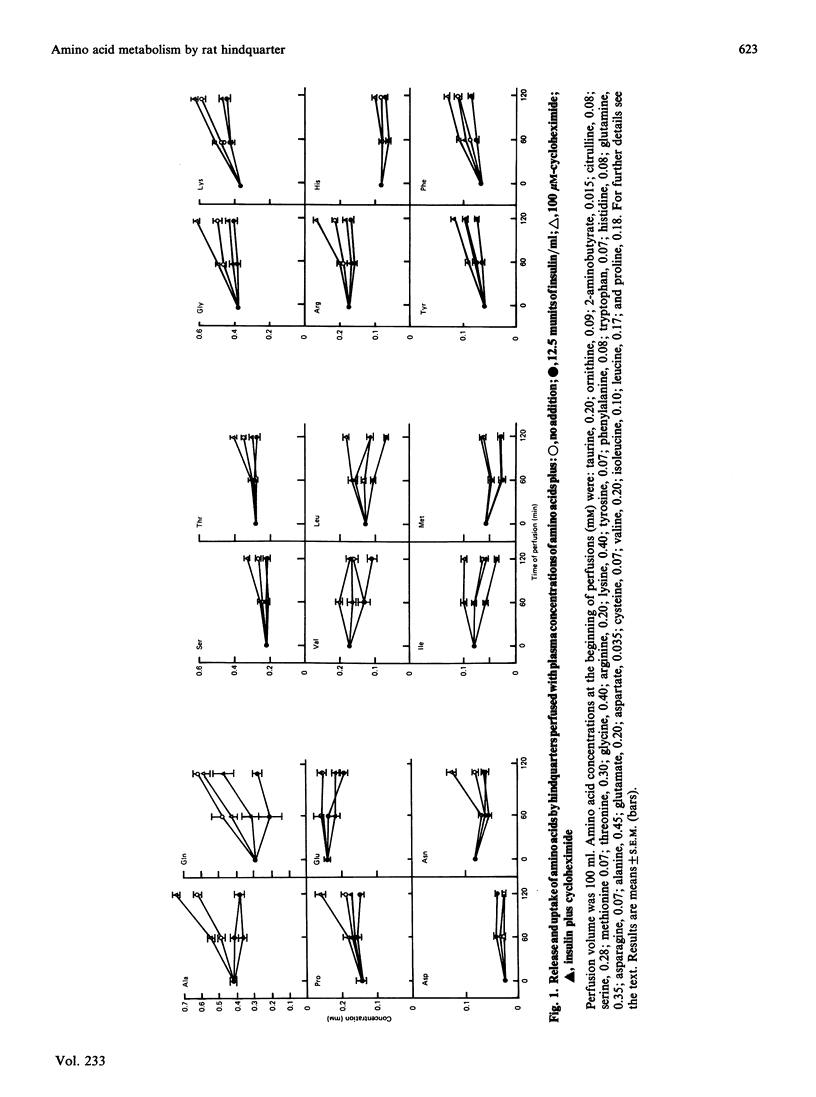
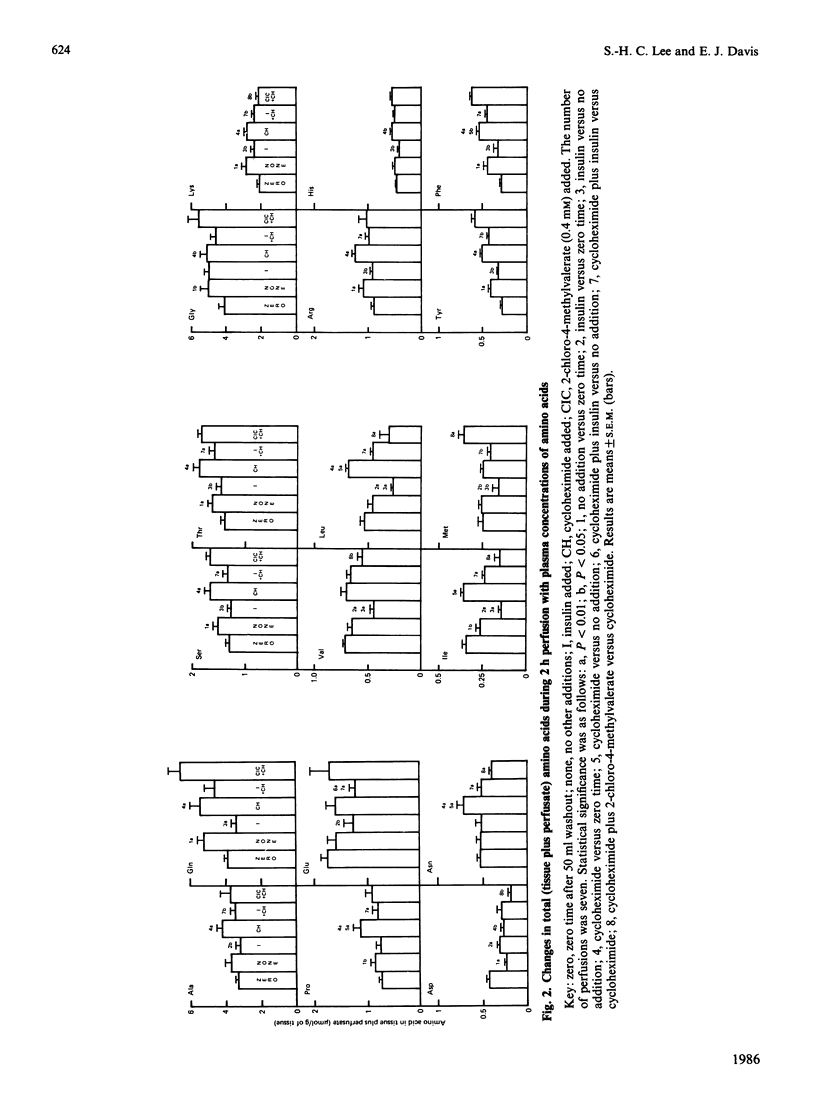
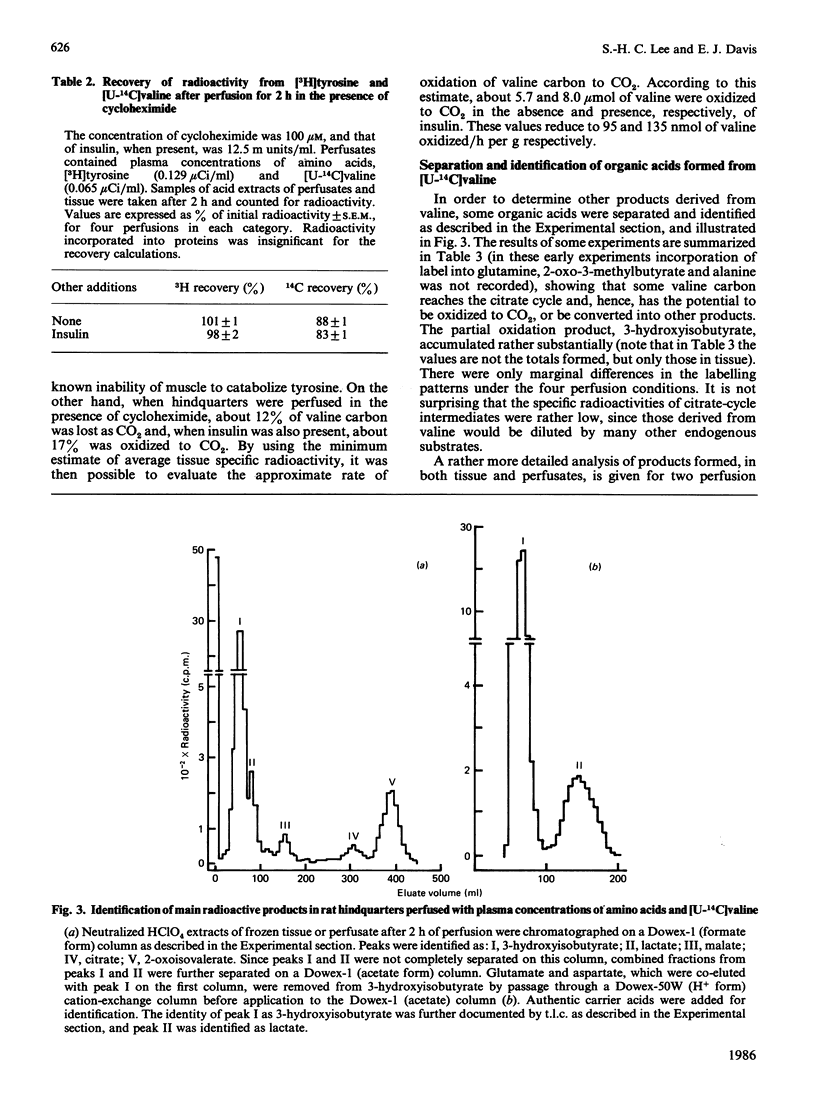
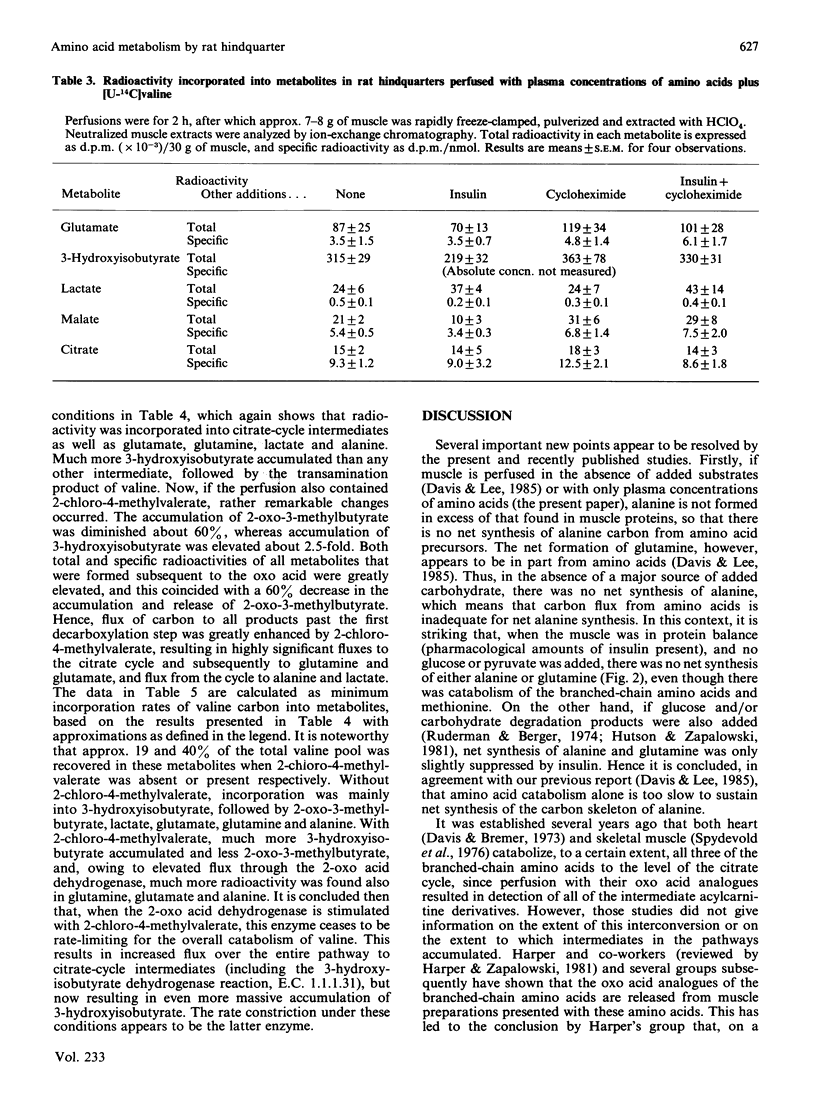
Hindquarters from starved rats were perfused with plasma concentrations of amino acids, but without other added substrates. Release of amino acids was similar to that previously reported, but, if total amino acid changes were recorded, alanine and glutamine were not formed in excess of their occurrence in muscle proteins. In protein balance (excess insulin) there was no net formation of either alanine or glutamine, even though the branched-chain amino acids and methionine were consumed. If [U-14C]valine was present, radiolabelled 3-hydroxyisobutyrate and, to a lesser extent, 2-oxo-3-methylbutyrate accumulated and radiolabel was incorporated into citrate-cycle intermediates and metabolites closely associated with the citrate cycle (glutamine and glutamate, and, to a smaller extent, lactate and alanine). If a 2-chloro-4-methylvalerate was present to stimulate the branched-chain oxo acid dehydrogenase, flux through this step was accelerated, resulting in increased accumulation of 3-hydroxyisobutyrate, decreased accumulation of 2-oxo-3-methylbutyrate, and markedly increased incorporation of radiolabel (specific and total) into all measured metabolites formed after 3-hydroxyisobutyrate. It is concluded that: amino acid catabolism by skeletal muscle is confined to degradation of the branched-chain amino acids, methionine and those that are interconvertible with the citrate cycle; amino acid catabolism is relatively minor in supplying carbon for net synthesis of alanine and glutamine; and partial degradation products of the branched-chain amino acids are quantitatively significant substrates released from muscle for hepatic gluconeogenesis. For valine, 3-hydroxyisobutyrate appears to be quantitatively the most important intermediate released from muscle. A side path for inter-organ disposition of the branched-chain amino acids is proposed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND K. W., SLATER E. C. Respiratory granules of heart muscle. Biochem J. 1953 Mar;53(4):547–556. doi: 10.1042/bj0530547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. W., Goldberg A. L. The metabolic fates of amino acids and the formation of glutamine in skeletal muscle. J Biol Chem. 1978 May 25;253(10):3685–3693. [PubMed] [Google Scholar]
- Chang T. W., Goldberg A. L. The origin of alanine produced in skeletal muscle. J Biol Chem. 1978 May 25;253(10):3677–3684. [PubMed] [Google Scholar]
- Davis E. J., Bremer J. Studies with isolated surviving rat hearts. Interdependence of free amino acids and citric-acid-cycle intermediates. Eur J Biochem. 1973 Sep 21;38(1):86–97. doi: 10.1111/j.1432-1033.1973.tb03037.x. [DOI] [PubMed] [Google Scholar]
- Davis E. J., Lee S. H. Amino acid metabolism by perfused rat hindquarter. Effects of insulin, leucine and 2-chloro-4-methylvalerate. Biochem J. 1985 Jul 1;229(1):19–29. doi: 10.1042/bj2290019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. J., Spydevold O., Bremer J. Pyruvate carboxylase and propionyl-CoA carboxylase as anaplerotic enzymes in skeletal muscle mitochondria. Eur J Biochem. 1980 Sep;110(1):255–262. doi: 10.1111/j.1432-1033.1980.tb04863.x. [DOI] [PubMed] [Google Scholar]
- Felig P., Owen O. E., Wahren J., Cahill G. F., Jr Amino acid metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):584–594. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Pozefsky T., Marliss E., Cahill G. F., Jr Alanine: key role in gluconeogenesis. Science. 1970 Feb 13;167(3920):1003–1004. doi: 10.1126/science.167.3920.1003. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. I. Glycolysis and amino acid release. J Biol Chem. 1976 Feb 10;251(3):826–835. [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. II. The precursor role of amino acids in alanine and glutamine synthesis. J Biol Chem. 1976 Feb 10;251(3):836–843. [PubMed] [Google Scholar]
- Goldberg A. L., Odessey R. Oxidation of amino acids by diaphragms from fed and fasted rats. Am J Physiol. 1972 Dec;223(6):1384–1391. doi: 10.1152/ajplegacy.1972.223.6.1384. [DOI] [PubMed] [Google Scholar]
- Harris R. A., Paxton R., DePaoli-Roach A. A. Inhibition of branched chain alpha-ketoacid dehydrogenase kinase activity by alpha-chloroisocaproate. J Biol Chem. 1982 Dec 10;257(23):13915–13918. [PubMed] [Google Scholar]
- Hutson S. M., Zapalowski C., Cree T. C., Harper A. E. Regulation of leucine and alpha-ketoisocaproic acid metabolism in skeletal muscle. Effects of starvation and insulin. J Biol Chem. 1980 Mar 25;255(6):2418–2426. [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- LEHNINGER A. L., GREVILLE G. D. The enzymic oxidation of alpha- and 2-beta-hydroxybutyrate. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):188–202. doi: 10.1016/0006-3002(53)90138-3. [DOI] [PubMed] [Google Scholar]
- Landaas S. Accumulation of 3-hydroxyisobutyric acid, 2-methyl-3-hydroxybutyric acid and 3-hydroxyisovaleric acid in ketoacidosis. Clin Chim Acta. 1975 Oct 15;64(2):143–154. doi: 10.1016/0009-8981(75)90196-5. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Davis E. J. Carboxylation and decarboxylation reactions. Anaplerotic flux and removal of citrate cycle intermediates in skeletal muscle. J Biol Chem. 1979 Jan 25;254(2):420–430. [PubMed] [Google Scholar]
- MAHLER H. R., WAKIL S. J., BOCK R. M. Studies on fatty acid oxidation. I. Enzymatic activation of fatty acids. J Biol Chem. 1953 Sep;204(1):453–468. [PubMed] [Google Scholar]
- Mallet L. E., Exton J. H., Park C. R. Control of gluconeogenesis from amino acids in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5713–5723. [PubMed] [Google Scholar]
- Palmer T. N., Caldecourt M. A., Sugden M. C. Amino acid oxidation and alanine production in rat hemidiaphragm in vitro. Effects of dichloroacetate. Biochem J. 1984 Oct 1;223(1):113–117. doi: 10.1042/bj2230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Berger M. The formation of glutamine and alanine in skeletal muscle. J Biol Chem. 1974 Sep 10;249(17):5500–5506. [PubMed] [Google Scholar]
- Ruderman N. B., Houghton C. R., Hems R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochem J. 1971 Sep;124(3):639–651. doi: 10.1042/bj1240639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Duff D. A. Branched-chain amino acid metabolism and alanine formation in rat diaphragm muscle in vitro. Effects of dichloroacetate. Biochem J. 1984 Nov 1;223(3):831–835. doi: 10.1042/bj2230831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K. Muscle alanine synthesis and hepatic gluconeogenesis. Biochem Soc Trans. 1980 Apr;8(2):205–213. doi: 10.1042/bst0080205. [DOI] [PubMed] [Google Scholar]
- Spydevold O., Hokland B. Oxidation of branched-chain amino acids in skeletal muscle and liver of rat. Effects of octanoate and energy state. Biochim Biophys Acta. 1981 Sep 4;676(3):279–288. doi: 10.1016/0304-4165(81)90161-6. [DOI] [PubMed] [Google Scholar]
- Spydevold O., Hokland B. Release of leucine and isoleucine metabolites by perfused skeletal muscle and liver of rat. Int J Biochem. 1983;15(8):985–990. doi: 10.1016/0020-711x(83)90033-2. [DOI] [PubMed] [Google Scholar]
- Spydevold O. The effect of octanoate and palmitate on the metabolism of valine in perfused hindquarter of rat. Eur J Biochem. 1979 Jul;97(2):389–394. doi: 10.1111/j.1432-1033.1979.tb13125.x. [DOI] [PubMed] [Google Scholar]
- Spydevold S., Davis E. J., Bremer J. Replenishment and depletion of citric acid cycle intermediates in skeletal muscle. Indication of pyruvate carboxylation. Eur J Biochem. 1976 Dec;71(1):155–165. doi: 10.1111/j.1432-1033.1976.tb11101.x. [DOI] [PubMed] [Google Scholar]



