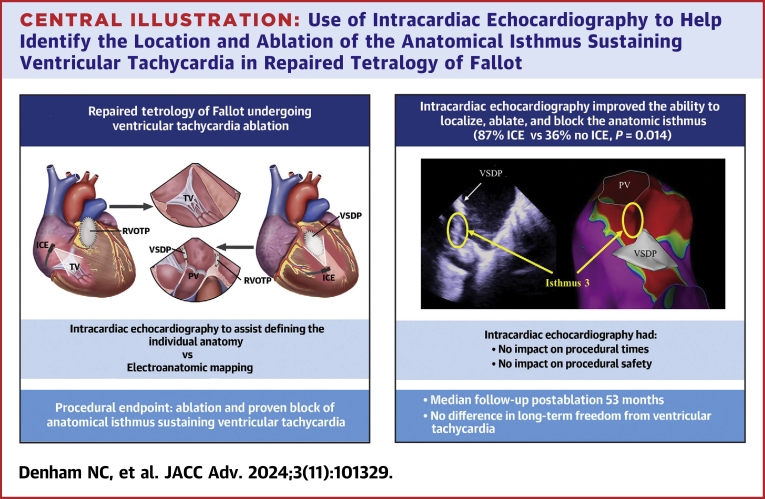
Abstract
Background
Successful catheter ablation of ventricular tachycardia (VT) in repaired tetralogy of Fallot (TOF) can be achieved by targeting 1 or more anatomical isthmuses. However, significant interindividual variability in the size and location of surgical patches means careful mapping is required to design ablation lines to block the isthmus. Intracardiac echocardiography (ICE) may assist ablation by accurate identification of individual TOF anatomy.
Objectives
The authors hypothesized ICE-guided VT ablation improved isthmus recognition, ablation, and procedural outcomes.
Methods
Retrospective study of adults with repaired TOF undergoing VT ablation between January 1, 2017 and December 31, 2022. ICE integration was compared to a strategy using electroanatomical mapping only to identify anatomic boundaries. All cases underwent ablation and had proven isthmus block as the procedural endpoint.
Results
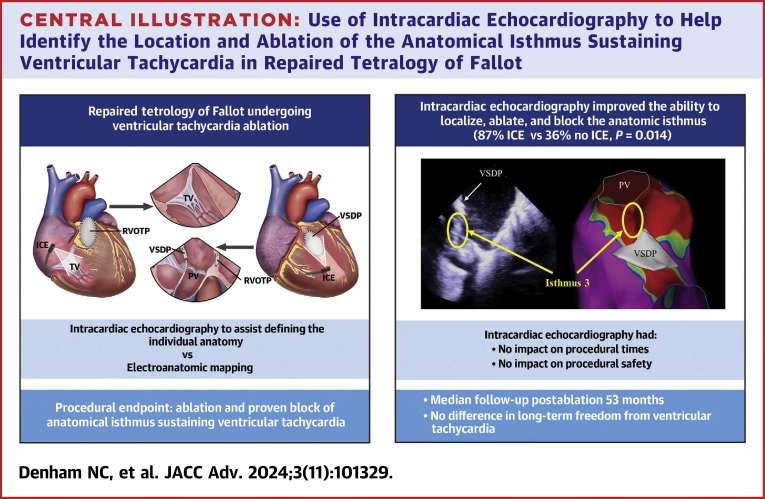
Twenty-three patients (age 47 ± 14 years; 61% male) underwent 27 VT ablations (ICE: 16/27 [59%]; no ICE: 11/27 [41%]). ICE improved the ability to localize and ablate the anatomical isthmus (ICE: 13/15 [87%] vs no ICE: 4/11 [36%]; P = 0.014); however, there was no difference in long-term freedom from VT (ICE: 9/12 [75%] vs no ICE: 8/11 [73%]; P = 0.901). ICE had no impact on procedural times (ICE: 173 ± 48 minutes vs no ICE: 157 ± 47 minutes; P = 0.399), fluoroscopy time (ICE: 30 ± 16 minutes vs no ICE: 29 ± 10 minutes; P = 0.864), or major complications (ICE: 1/16 [6%] vs no ICE 0/11; P = 1.000).
Conclusions
ICE improves ablation of the anatomical isthmus for sustaining VT in patients with repaired TOF by demonstrating the individual anatomy but does not improve long-term outcomes.
Key words: adult congenital heart disease, image integration, intracardiac echocardiography, tetralogy of Fallot, ventricular tachycardia
Central Illustration
Ventricular arrhythmias are a frequent source of late morbidity and mortality in patients who have undergone surgical repair of tetralogy of Fallot (TOF).1,2 The majority manifest as monomorphic ventricular tachycardia (VT),3 which utilizes a macro re-entrant circuit primarily dependent on distinct anatomical isthmuses between:
-
1.
Tricuspid annulus and the right ventricular outflow tract (RVOT) patch/incision
-
2.
Pulmonary annulus and the RVOT incision
-
3.
Pulmonary annulus and the ventricular septal defect (VSD) patch
-
4.
Tricuspid annulus and the VSD patch4
Catheter ablation to block 1 or more isthmuses has been shown to reduce the recurrence of VT and implantable cardioverter-defibrillator (ICD) shocks.5, 6, 7 Given many VTs are frequently rapid with poor hemodynamic tolerance,8 detailed activation and entrainment mapping can be challenging, leading to a reliance on substrate mapping to identify areas of low voltage corresponding to the isthmus.4,9 However, the repaired RVOT and septum frequently have extensive areas of low voltage, making accurate differentiation between true surgical barriers and scarred myocardium difficult.10 Noncapture with high-output pacing is a useful adjunctive strategy of tissue differentiation,11 but this can be time-consuming and may also underestimate the extent of inaccessible tissue behind prosthetic material.
There is considerable anatomical variation in patients with repaired TOF, both in terms of the original anatomy and the type of surgical repair.12,13 As a result, not every patient will have each anatomical isthmus and not every isthmus will be capable of sustaining monomorphic VT.9 In addition, there are anatomical features that can prevent block across the isthmus contributing to acute procedural failure, including right ventricular hypertrophy, overlying surgical patches, and percutaneous pulmonary valve apparatus.9,12,14
Intracardiac echocardiography (ICE) has the potential to overcome some of these challenges by allowing true isthmus identification with real-time visualization and localization of surgical patches, incisions, and valve annuli. This, alongside conventional electroanatomical mapping (EAM), can be utilized to design optimal lesion sets for more effective conduction block,15 particularly for isthmus 3, which is the commonest ablation site.4 We hypothesized image-integrated EAM using ICE improved isthmus recognition, ablation, and outcomes in patients with VT and repaired TOF.
Methods
Patient selection
A retrospective study was performed of all patients with repaired TOF undergoing VT ablation as an adult (>18 years of age) at our institution from January 1, 2017, to December 31, 2022, inclusive. Approval for the study was provided by the research and ethics review board at University Health Network. Data were collected from clinical notes, procedure reports, device interrogations, and ambulatory rhythm monitors, including baseline characteristics, procedural outcomes, and follow-up. Cases utilizing ICE to create image-integrated EAM were considered the interventional group. Patients who did not undergo ablation (electrophysiology study only) or received ablation for frequent focal premature ventricular contractions with no VT were excluded.
Electrophysiology studies and ICE imaging
Electrophysiology studies and ablation were performed either under conscious sedation or with general anesthesia using the Carto-3 system (Biosense Webster). After obtaining femoral venous access, anatomical mapping with ICE was performed at the discretion of the operating electrophysiologist. ICE imaging was performed using a 9Fr phased-array ultrasound catheter (Soundstar, Biosense Webster) from the right atrium (RA), right ventricle (RV), and the RVOT. Image-integration was performed during the procedure using the CartoSound module (Biosense Webster) by manually contouring selected ICE images defining (where present): the tricuspid annulus, the pulmonary annulus, the geometry of the RV, the RVOT patch, and the VSD patch.
We start by obtaining views from the home (neutral) position in the RA to mark the position of the tricuspid annulus (Figure 1A) and create geometry of the RV. From this position, we rotate clockwise in order to view the long axis of the RVOT and add to the RV geometry. Dependent upon the type of TOF repair the patient has had, we define the presence of any RVOT or transannular patches that come into view. Further clockwise rotation brings the left ventricular outflow tract (LVOT) into view, from which we try to outline the VSD patch and the pulmonary valve annulus. We perform small amounts of posterior/anterior tilt to obtain better visualization, particularly when the ventricle is dilated or there is rotation of the RVOT and pulmonary annulus with respect to the LVOT and aortic annulus.
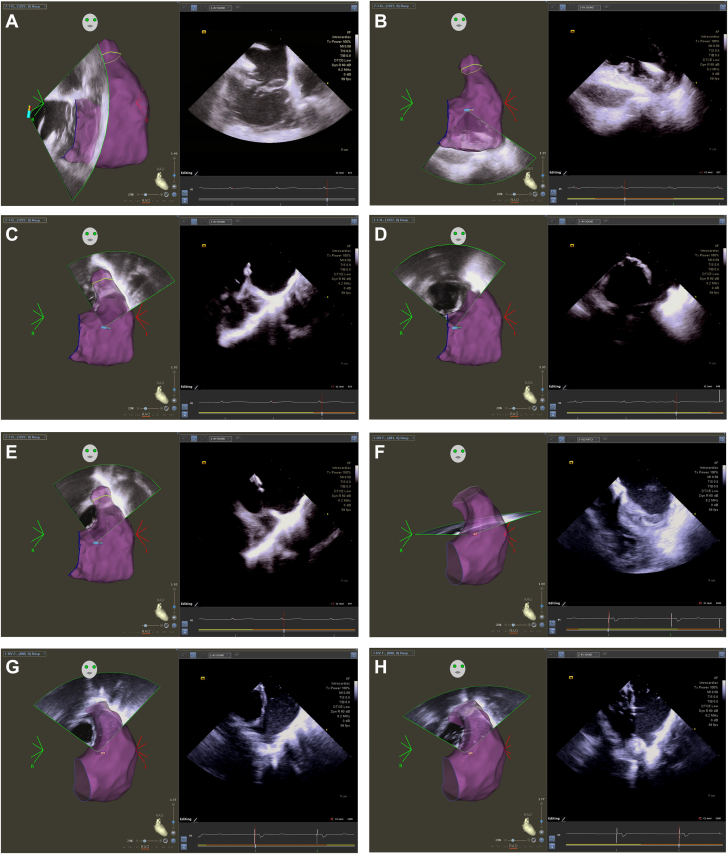
Figure 1.
Workflow for Intracardiac Echocardiography in Repaired Tetralogy of Fallot
(A) Views from the right atrium are used to create right ventricular geometry and mark the location of the tricuspid annulus. (B) The intracardiac echocardiography (ICE) catheter is advanced into the right ventricle (RV) with anterior tilt to create additional RV geometry and mark the defibrillator lead position. (C) The best view in our experience is with posterior tilt in the RV looking through the right ventricular outflow tract (RVOT) into the pulmonary artery. The location of the pulmonary annulus is marked. (D to F) The location and extent of the ventricular septal defect patch are defined by ICE, using small degrees of rotation and tilt to optimize the image. (G and H) Finally, the RVOT/transannular patch location is defined. Enlarged and annotated views are provided in Supplemental Figures 1 to 8 and Supplemental Video 1, Supplemental Video 2, Supplemental Video 3, Supplemental Video 4, Supplemental Video 5.
After obtaining views from the RA, we return to the home view and cross into the RV through the tricuspid valve with anterior tilt. We add RV geometry and annotate the position of any defibrillator or pacemaker leads (Figure 1B). We then withdraw back into the RA and then re-enter the RV, this time with posterior tilt. This allows us to look directly up through the RVOT to visualize the VSD patch, the RVOT patch, and the pulmonary annulus (Figure 1C). Once we are in this position, the extent of the surgical patches are defined with minor clockwise/counter-clockwise rotation and anterior/posterior tilt to optimize the views. Any additional RVOT abnormalities are noted and contoured, including pouches, prominent septo-parietal bands, and additional prosthetic material. The VSD patch (Figures 1D to 1F) and RVOT patch (Figure 1G and 1H) can be recognized by an abrupt change in tissue thickness and a greater echodensity compared to the surrounding myocardium, in addition to their location on the RV anatomic shell.
From the RV, we pass up into the RVOT by gently releasing the tilt with clockwise rotation. Typically, we do not collect left ventricular geometry and continue to rotate until we open up our final view, the short axis of the RVOT and the pulmonary valve annulus. Once the anatomy has been collected, the ICE catheter is withdrawn to the home position in the RA. Enlarged, annotated still images and videos are available as supplementary materials.
Electroanatomic mapping and ablation
Thereafter, the study was continued using our routine workflow for a VT ablation in repaired TOF as previously described.6 In brief, a VT stimulation protocol was performed from 2 different RV sites (apex and RVOT) at 2 different drive trains (8 beats at 600/400 ms) with a minimum of 3 extrastimuli down to 200 ms, or the effective refractory period. If hemodynamically tolerated monomorphic VT was induced, activation mapping was performed using a Pentaray catheter (Biosense Webster), and the isthmus was confirmed with entrainment mapping. If VT was not inducible or resulted in hemodynamic instability requiring cardioversion, substrate-based mapping was performed in sinus rhythm. A bipolar voltage map of the RV was created using a Pentaray taking <1.5 mV as a cut-off for diseased tissue. The presence of late potentials and fractionated electrograms (EGM) were tagged as sites of interest for potential substrate modification. Where a 12-lead electrocardiogram (ECG) of the clinical VT was available, pace mapping was used to assist in localizing the VT exit site. Where the clinical VT could be induced during the study, the VT exit site was defined as a >95% pace map match (PASO module, Biosense Webster). However, when the VT could not be induced, a ≥11/12 QRS match to the historical ECG of VT was taken as the putative exit site.
The choice of ablation site was determined in a hierarchical manner dependent upon the mapping strategy. The primary target was an anatomical isthmus proven to be critical to the VT based on entrainment or activation mapping. Where this was not possible, the second target was an anatomical isthmus based on pace mapping. If neither were possible, a substrate-based approach was performed by ablating the isthmus with the highest frequency of late potentials and fractionated EGMs. Additional ablation lesions for substrate modification outside of the isthmus could be performed at the operator’s discretion as part of any ablation strategy.
Ablation was performed using power of 25-40W and temperature limited to 45 °C for up to 60 seconds, aiming for a 10 to 15 Ω impedance drop with a 3.5 mm irrigated ablation catheter (ThermoCool SmartTouch or ThermoCool SmartTouch SF, Biosense Webster), guided by a steerable sheath (Agilis NxT, Abbott, or Vizigo, Biosense Webster). Contact force was monitored with the aim of achieving a minimum contact force of 10 g during ablation. In cases where VT was induced, the stimulation protocol was repeated 15 minutes after ablation with the aim of achieving noninducibility.
Procedural outcomes
The main aim of the study was to determine whether ICE improved anatomical recognition and subsequent ablation of the anatomical isthmus sustaining VT. The isthmus was first chosen using the aforementioned strategy. The design line in cases without ICE was made by linking 2 regions of electrically unexcitable scar, with 1 end defined by the valve annulus and 1 other the expected location of the surgical patch. Scar was defined as the presence of low-amplitude bipolar EGMs <0.5 mV and failure to capture local tissue at a pacing output of 10 mA. When using ICE, the exact border of the patch was identified on ICE and confirmed by pacing to determine where the first anchoring ablation lesion should be placed. Conduction block was tested across the isthmus using differential pacing or activation mapping across the line during pacing.
Additional procedural outcomes included VT noninducibility, fluoroscopic, and procedural times, as well as complications defined as cardiac tamponade, vascular injury, thromboembolism, and major bleeding requiring transfusion. Any access site complication was recorded given that the use of ICE required an additional venous puncture.
Finally, data on VT in long-term follow-up postablation was collected up to December 31, 2023 (ie, all patients had a 1-year minimum follow-up). Recurrence of any VT, as well as only the clinical VT that was identified and targeted at the time of ablation, were collected.
Statistics
Continuous variables are expressed as the mean ± SD when normally distributed, and comparisons between groups were performed using a 2-tailed, unpaired Student’s t test. Non-normalized continuous data are expressed as the median (IQR) and compared with the Mann-Whitney U test. Categorical variables are expressed as numbers (%) and compared using the 2-tailed Fisher’s exact test. Statistics were performed with GraphPad Prism (GraphPad Software), where P values of <0.05 were considered statistically significant.
Results
Patient characteristics
Twenty-three patients (age 47 ± 14 years, 61% male) were identified to have undergone 1 or more VT ablations during the time period. Four patients had a repeat VT ablation resulting in 27 procedures (ICE: 16/27 [59%]; no ICE: 11/27 [41%]). The patient characteristics are summarized in Table 1. The mean age at index surgical repair was 9 ± 6 years (range: 3 months to 23 years), with patients undergoing a median of 2 sternotomies prior to ablation (IQR: 1). Three patients had surgical repair within the first year of life (all in the no ICE cohort). Transthoracic echocardiography showed the left and right ventricular ejection fractions were 49% ± 12% and 32% ± 11%, respectively. The RV basal diameter in diastole was 49 ± 7 mm, and 4 of 23 patients (17%) had severe pulmonary regurgitation. An insufficient number of patients had cross-sectional imaging for RV volumetric data. An ICD was present in 11 of 23 (48%) patients, of which 4 (36%) were originally implanted for a primary prevention indication and 7 (64%) for secondary prevention. In terms of drug management, 21 of 23 (91%) were managed with class 2 beta-blockers, while 1 of 23 (4%) was taking sotalol and 10 of 23 (43%) were taking amiodarone.
Table 1.
Baseline Characteristics, Arrhythmia History, and Procedural Details
| All Patients (N = 23) | Cases With ICE (n = 16) | Cases Without ICE (n = 11) | P Value (With vs Without ICE) | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (y) | 47 ± 14 | 51 ± 15 | 39 ± 12 | 0.037 |
| Male | 14 (61%) | 10 (63%) | 7 (64%) | 1.000 |
| Age at index surgical repair (y) | 9 ± 6 | 10 ± 7 | 6 ± 4 | 0.100 |
| Number of previous sternotomiesa | 2 ± 1 | 2 ± 1 | 3 ± 1 | 0.062 |
| Palliative shunt | 13 (57%) | 9 (56%) | 7 (64%) | 1.000 |
| Baseline QRS duration (ms) | 176 ± 36 | 177 ± 44 | 167 ± 34 | 0.532 |
| Left ventricular ejection fraction (%) | 49 ± 12 | 45 ± 15 | 52 ± 7 | 0.163 |
| Right ventricular ejection fraction (%) | 32 ± 11 | 32 ± 11 | 29 ± 10 | 0.477 |
| Right ventricular dimension in diastole (mm) | 49 ± 7 | 47 ± 6 | 51 ± 8 | 0.150 |
| Severe pulmonary stenosis | 1 (4%) | 1 (6%) | 1 (9%) | 1.000 |
| Severe pulmonary regurgitation | 4 (17%) | 4 (25%) | 2 (18%) | 1.000 |
| Arrhythmia history | ||||
| Documented VT | 22 (96%) | 16 (100%) | 10 (91%) | 0.407 |
| 12-lead ECG available | 12 (52%) | 10 (63%) | 5 (50%) | 0.689 |
| Sustained VT | 18 (69%) | 14 (88%) | 8 (80%) | 0.625 |
| Nonsustained VT | 11 (42%) | 9 (56%) | 3 (30%) | 0.248 |
| VT cycle length (ms) | 316 ± 75 | 332 ± 112 | 317 ± 59 | 0.701 |
| Previous VT storm | 1 (4%) | 1 (6%) | 0 | 1.000 |
| Previous DC cardioversion | 12 (52%) | 10 (63%) | 4 (36%) | 0.252 |
| Previous syncope | 6 (26%) | 6 (38%) | 1 (9%) | 0.183 |
| Implantable cardiac defibrillator | 11 (48%) | 8 (50%) | 5 (45%) | 1.000 |
| Primary prevention | 4 (36%) | 4 (50%) | 0 | 0.123 |
| Secondary prevention | 7 (64%) | 4 (50%) | 5 (100%) | 0.411 |
| Previous appropriate ICD shock | 3 (27%) | 5 (63%) | 0 | 0.060 |
| Previous appropriate ATP | 6 (55%) | 6 (75%) | 2 (40%) | 0.405 |
| Antiarrhythmic use | ||||
| Beta blockers | 21 (91%) | 13 (81%) | 10 (91%) | 0.624 |
| Amiodarone | 10 (43%) | 9 (56%) | 4 (36%) | 0.440 |
| Sotalol | 1 (4%) | 0 | 1 (9%) | 0.407 |
| Procedural details | ||||
| Procedure type | ||||
| Index | - | 14 (87%) | 8 (73%) | 0.371 |
| Redo | - | 2 (13%) | 3 (27%) | 0.371 |
| VT stimulation study | 16 (100%) | 11 (100%) | 1.000 | |
| Monomorphic VT inducible | - | 9 (56%) | 5 (45%) | 0.701 |
| Hemodynamically tolerated VT | - | 4 (44%) | 4 (80%) | 0.301 |
| Ventricular fibrillation induced | - | 2 (13%) | 1 (9%) | 1.000 |
| Noninducible | - | 5 (31%) | 5 (45%) | 0.687 |
| Electroanatomic mapping | ||||
| Substrate in sinus/RV pacing | - | 15 (94%) | 9 (82%) | 0.549 |
| Pace mapping | - | 3 (19%) | 1 (9%) | 0.624 |
| Activation in VT | - | 2 (13%) | 4 (36%) | 0.187 |
| Entrainment | - | 1 (6%) | 1 (9%) | 1.000 |
Values are mean ± SD or n (%).
The dataset of all patients (n = 23) included in the study, as well as data for VT ablation cases performed using ICE (n = 16) and cases without ICE (n = 11). 4 patients underwent 2 VT ablations during the study period. Statistics are presented for comparisons between groups. Bold means statistically significant (P < 0.05).
ATP = antitachycardia pacing; DC = direct current; ECG = electrocardiogram; ICD = implantable cardioverter-defibrillator; ICE = intracardiac echocardiography; RV = right ventricle; VT = ventricular tachycardia.
Median ± SD.
Arrhythmia history
The presence of VT had been documented in 26 of 27 (96%) cases either on ambulatory rhythm monitoring, 12-lead ECG, or device interrogation. The remaining case had an episode of syncope highly suspicious for a tachyarrhythmic etiology. Sustained VT was documented in 22 of 26 (85%) cases with a mean tachycardia cycle length of 326 ± 92 ms, and 4 of 26 (15%) cases had recurrent, highly symptomatic nonsustained VT. Three patients out of the 11 with a defibrillator (27%) had previously received an appropriate shock for monomorphic VT (2 primary and 1 secondary prevention), and 6 (55%) had received appropriate antitachycardia pacing (2 primary and 4 secondary prevention). Twelve patients (52%) had required external direct current cardioversion. Only 1 patient (4%) had a history of VT storm.
Procedural details
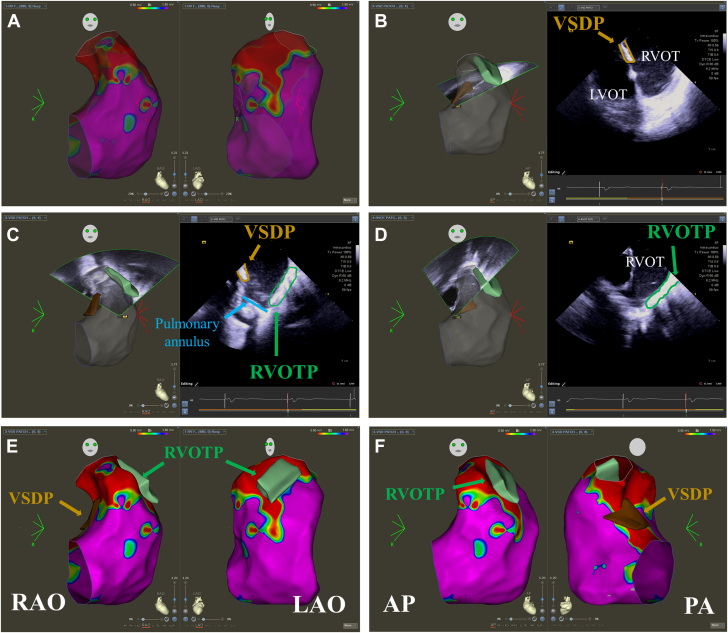
ICE was performed in 16 of 27 (59%) VT ablations. Between 2017 and 2019 inclusive, the use of ICE was solely at the discretion of the operator during the ablation procedure (ICE: 8/18 [44%]; no ICE: 10/18 [56%]). From 2020 onward, the use of ICE became routine practice for the majority of cases (ICE: 8/9 [89%]; no ICE: 1/9 [11%]). An example of integrating echo images into EAM to define the individual repaired TOF anatomy, particularly surgical patches, and the location of valve annuli is shown in Figure 2.
Figure 2.
Integration of Structures Defined on Intracardiac Echocardiography Into Electroanatomic Maps
(A) A bipolar voltage map in RAO and LAO projections using cut-offs of 0.5 to 1.5 mV. There is extensive low voltage in the right ventricular outflow tract without any clear definition of where the surgical patches sit. (B to D) Intracardiac echocardiography (ICE) is used to collect anatomy of the ventricular septal defect patch (VSDP) (brown) and the right ventricular outflow tract patch (RVOTP) (green). (E, F) The anatomy of surgical patches can be superimposed on the voltage map of the ventricle using the CartoSound module. This can then be used to plan ablation of 1 or more anatomical isthmuses. AP = anteroposterior; LAO = left anterior oblique; PA = posteroanterior; RAO = right anterior oblique.
The VT ablation was the index procedure in 21 of 27 (78%) cases and a redo procedure in 6 of 27 (22%) cases. Each redo procedure was performed on a unique patient. Four patients underwent both their index and redo procedure during the study timeframe, and therefore both procedures were included (2 cases used ICE during both procedures, 2 used ICE during the redo procedure only). Two patients only had their redo procedure during the study period; therefore, they are included once (both without ICE).
Monomorphic VT was inducible with programmed electrical stimulation in 14 of 27 cases (52%), of which 8 (57%) were hemodynamically tolerated. Activation mapping was completed in 6 cases (75%), of which 2 (33%) underwent entrainment mapping to identify the location of the isthmus. A complete activation map could not be completed in the remaining 2 cases (25%) due to repeated termination while mapping. Twenty-four (89%) cases underwent substrate mapping in sinus rhythm either due to noninducible VT (10/24, 42%), hemodynamically unstable VT (9/24, 37%), or to supplement activation mapping (5/24, 21%).
Anatomical isthmus recognition and ablation
A single anatomical isthmus deemed critical to VT maintenance was identified in 25 of 27 (92%) cases and 2 isthmuses in 1 of 27 (4%) cases. In the remaining case, an isthmus not based on the classical 4 isthmuses was identified on the RV free wall, determined by activation mapping. The commonest anatomical isthmus was isthmus 3 (85%), followed by isthmus 2 (11%) and isthmus 1 (4%). Ablation was performed in all cases with the aim of blocking the identified isthmus, and the success rate (determined by block across the anatomical isthmus) was 13/15 (87%) when using ICE to help design the ablation set vs 4/11 (36%; P = 0.014). There was no difference in the number of patients undergoing additional nonisthmus, substrate-based ablation (ICE: 14/16 [88%] vs no ICE: 10/11 [91%]; P = 1.000). A detailed overview of all cases is shown in Table 2.
Table 2.
Individual Case Overview
| Case # | ICE | Redo Procedure | Monomorphic VT Inducible Preablation | Anatomical Isthmus Number Identified as Critical | Mapping Strategy | Proven Block Across Isthmus | Monomorphic VT Inducible Postablation | Details |
|---|---|---|---|---|---|---|---|---|
| 1 | No | - | No | 2 | Sinus | No | - | Unable to block isthmus. Underwent inpatient PVR and cryoablation. |
| 2 | No | Yes (index ablation pre-2017) | Yes | 3 | VT | Yes | No | |
| 3 | No | - | Yes | 3 | VT | No | Yes | Failure to block isthmus and remained inducible. Required redo ablation 15 months later (case 17) |
| 4 | No | - | No | 3 | Sinus | No | - | Unable to induce during case. Failure to block isthmus. No VT in follow-up. |
| 5 | No | Yes (index ablation pre-2017) | Yes | 3 | VT | No | No | Procedure terminated as postablation testing was noninducible. No VT in follow-up |
| 6 | No | - | No | 3 | Sinus | Yes | - | |
| 7 | No | - | No | 3 | Sinus | No | - | Unable to induce during case. Failure to block isthmus. No VT in follow-up. |
| 8 | No | - | Yes | 3 | VT | No | Yes | Failure to block isthmus and remained inducible. Required redo ablation months later (case 13) |
| 9 | No | - | Yes | 3 | Sinus | Yes | No | |
| 10 | No | - | No | 3 | Sinus | No | - | Unable to induce during case. Failure to block isthmus. No VT in follow-up. |
| 11 | No | - | No | Sinus | Yes | - | ||
| Total | 11 | 2/11 (18%) | 5/11 (45%) | 4/11 (36%) | 2/5 (20%) |
| Case # | ICE | Redo Procedure | Monomorphic VT Inducible Preablation | Anatomical Isthmus Number Identified as Critical | Mapping Strategy | Proven Block Across Isthmus | Monomorphic VT Inducible Postablation | Details |
|---|---|---|---|---|---|---|---|---|
| 12 | Yes | - | No | 3 | Sinus | Yes | - | |
| 13 | Yes | Yes (case 8) | Yes | 3 | Sinus | Yes | No | |
| 14 | Yes | - | Yes | 3 | Sinus | Yes | No | |
| 15 | Yes | - | Yes | 3 | Sinus | No | No | Procedure terminated as postablation testing was noninducible. Required redo ablation 12 months later (case 18). |
| 16 | Yes | - | Yes | 2 | Sinus | Yes | No | |
| 17 | Yes | Yes (case 3) | Yes | NA | Sinus | NA | No | Nonclassical isthmus on RV free wall. ICE identified pouch in RVOT that contributed to failure of previous procedure. |
| 18 | Yes | Yes (case 15) | No | 3 | Sinus | Yes | - | |
| 19 | Yes | - | No | 3 | Sinus | Yes | - | |
| 20 | Yes | - | Yes | 3 | Sinus | Yes | No | |
| 21 | Yes | - | No | 1 | Sinus | Yes | - | |
| 22 | Yes | - | Yes | 3 | Sinus | Yes | No | |
| 23 | Yes | - | No | 3 | VT | Yes | - | |
| 24 | Yes | - | No | 3 | Sinus | No | - | Unable to induce during case. Required repeat procedure 1 week later (case 25) |
| 25 | Yes | Yes (case 24) | Yes | 2 and 3 | Sinus | Yes | No | |
| 26 | Yes | - | Yes | 3 | Sinus | Yes | No | |
| 27 | Yes | - | No | 3 | Sinus | Yes | - | |
| Total | 16 | 4/16 (25%) | 9/16 (56%) | 13/15 (87%) | 0/9 | |||
| P = 0.014 | P = 0.110 |
Case-level data of ventricular tachycardia ablations performed. Mapping strategy refers to how the isthmus was identified (sinus: combination of substrate and/or pace mapping; VT: combination of activation mapping and/or entrainment). Statistics are presented for comparisons between groups (ICE vs without ICE).
ICE = intracardiac echocardiography; PVR = pulmonary valve replacement; RV = right ventricle; RVOT = right ventricular outflow tract; VT = ventricular tachycardia.
In cases where there was inducible monomorphic VT at the start of the procedure, VT stimulation testing was repeated. Of the 2 patients with failed isthmus block in the ICE cohort, 1 had inducible VT and 1 had noninducible postablation. In the cohort without ICE, 3 patients with failed isthmus block were inducible at the start of the case, of which 2 remained inducible after ablation.
With regard to the redo cases, 4 of 6 (67%) underwent a successful ablation of the isthmus with proven block (3 cases isthmus 3; 1 case isthmuses 2 and 3). All 4 had failure to block the anatomical isthmus during the index procedure (2 ICE, 2 without ICE). One redo procedure failed to achieve block of isthmus 3 (no ICE); however, the procedure was terminated as the patient became noninducible. The remaining case had a nonclassical isthmus and success was demonstrated by noninducibility postablation.
Secondary procedural outcomes
The use of ICE had no impact on procedural times (ICE: 173 ± 48 minutes vs no ICE: 157 ± 47 minutes, P = 0.399), number of radiofrequency lesions (ICE: 26 ± 15 vs no ICE: 22 ± 15, P = 0.508), or duration of ablation (ICE: 1,022 ± 672s vs no ICE: 1,083 ± 735s, P = 0.828). ICE did not reduce overall fluoroscopy times during procedures for all cases (ICE: 30 ± 16 minutes vs no ICE: 29 ± 10 minutes, P = 0.864); however, it did help reduce fluoroscopy times when limited to patients with ICDs (ICE: 20 ± 2 minutes vs no ICE: 37 ± 4 minutes, P = 0.011). There was no difference in procedural complications observed between the groups (ICE: 1/16 [6%] vs no ICE 0/11, P = 1.000), with the single complication being a groin hematoma, which was managed conservatively.
Postablation follow-up
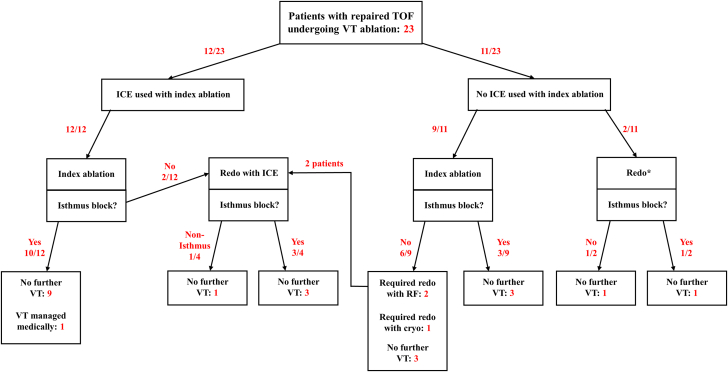
The median follow-up postablation was 53 months (IQR: 36 months). Long-term success (defined by absence of any VT postablation irrespective of isthmus block) was achieved in 8 of 11 (73%) patients whose first procedure was without ICE and in 9 of 12 (75%) patients whose first procedure was with ICE (P = 0.901). No difference was seen when considering a recurrence of the clinical VT at the time of ablation only (ICE: 10/12 [83%] vs no ICE: 8/11 [73%], P = 0.604). A diagram detailing the outcomes of both cohorts is shown in Figure 3.
Figure 3.
Patient Outcomes
A flow diagram showing the outcomes of each individual patient undergoing VT ablation in the study. The number of patients at each step is in red. ∗The index procedure for these 2 patients preceded the study inclusion period. Cryo = surgical cryoablation; ICE = intracardiac echocardiography; RF = radiofrequency ablation; TOF = tetralogy of Fallot; VT = ventricular tachycardia.
Of the cases who had proven isthmus block postablation, 4 of 4 remained free of VT in the cohort without ICE and 12 of 13 in the ICE cohort (P = 0.568). One patient in the ICE cohort underwent an empiric cryoablation at the time of pulmonary valve replacement, and another had a nonarrhythmic death as determined by ICD interrogation.
Discussion
Importance of ablating the isthmus in repaired TOF
In this retrospective study, we describe the technique and outcomes of ICE in the ablation of VT in patients with repaired TOF. Our key finding was that ICE improved the ability to define the extent of the anatomical isthmus between surgical patches and valve annuli above EAM alone, leading to a 51% increase in acute conduction block across the isthmus with linear ablation (Central Illustration). The ability to localize and perform linear ablation of anatomical isthmuses has become the cornerstone of VT ablation in repaired TOF,4 and we showed ICE was of greatest benefit in targeting isthmus 3 (from pulmonary annulus and the VSD patch), which was the commonest isthmus ablated in our study (85% of cases). This is of particular value given isthmus 3 is the commonest reported ablation site,4 and we anticipate it will only increase in proportional significance as the use of a ventriculotomy and RVOT incision reduces with contemporary surgical techniques.16
Central illustration.
Use of Intracardiac Echocardiography to Help Identify the Location and Ablation of the Anatomical Isthmus Sustaining Ventricular Tachycardia in Repaired Tetralogy of Fallot
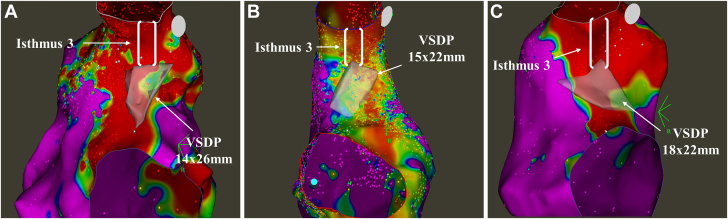
We found ICE helped to demonstrate the significant variability in the location and anatomical course of isthmus 3 (Figure 4), particularly in redo cases. In comparison, cases without ICE relied on conventional approaches to identify these anatomical barriers purely with mapping of low voltage areas and high output pacing. We believe the reduced success rate attributable to this EAM-only approach is the wide area of low voltage (but viable) tissue in the RVOT either overestimating the size of or misjudging the location of the patches, as well as failure to identify the true location of the pulmonary annulus, particularly in cases postvalve replacement.
Figure 4.
Variation in Ventricular Septal Defect Patch Location and Isthmus 3
(A to C) The variation in the location and extent of the ventricular septal defect patch (VSDP) is demonstrated in 3 patients. The boundaries of the patch as defined by intracardiac echocardiography are shown in transparent white for ease of differentiation. The anatomy of the patch cannot be determined by voltage mapping alone (bipolar voltage cut-offs; red: <0.5 mV, purple: >1.5 mV), as demonstrated by extensive low voltage in the right ventricular outflow tract. VSDP = ventricular septal defect patch.
The long-term freedom from VT recurrence is reported to be 73% to 100% over a follow-up of 30 to 53 months.4,5,9,14 Our cohort had comparable excellent long-term freedom from VT (75% and 73% at a median of 53 months for ICE and no ICE, respectively). One of the main risk factors for long-term recurrence is failure to block the anatomical isthmus, where recurrence rates may be as high as 50%.5,9 We acknowledge there was no statistical difference in long-term success from the use of ICE irrespective of isthmus block, which we attribute to experienced operators with a high number of patients undergoing additional substrate ablation in both groups.
Advantages from the routine use of ICE
Patients with repaired TOF who undergo VT ablation are recognized to display a range of anatomical variation, both in terms of the original anatomy as well as the type and extent of surgical repair.13,17 A strength of ICE is the real-time localization of valve annuli and surgical patches, which can then be integrated into EAM. Using 1 patient as an example from our cohort, the initial VT ablation without ICE was unsuccessful as we were unable to transect the anatomical isthmus given a large area of low-voltage tissue and ongoing inducibility. A subsequent procedure using ICE identified a small pouch in the RVOT, which may have contributed to the failure to achieve conduction block in the index procedure. Acute procedural success was subsequently possible with an ICE-guided line of ablation avoiding the pouch in addition to a nonclassical isthmus on the RV-free wall.
ICE may also allow for better-quality ablation lesions. We have found ICE has occasionally been useful in the event of catheter instability, manipulation close to ICD leads, or poor contact force. ICE also has the potential to guide titrated higher power ablation to hypertrophied myocardium or the identification of clearly inaccessible sites where muscle is interposed between surgical material where alternative strategies such as left-sided ablation could be employed.12,15
A final potential benefit is a reduction in fluoroscopy times.18 We did observe a reduction in fluoroscopy time when limited to patients with ICD leads but not in overall fluoroscopy time between groups. This may be due to the learning curve with ICE, as the mean duration of fluoroscopy in the first 5 cases was 42 minutes, which halved to a mean of 21 minutes for the final 5 cases. This is lower than the mean duration of fluoroscopy for non-ICE cases (29 minutes), and we expect the routine introduction of ICE to lead to a long-term reduction in fluoroscopy time in line with the larger study of congenital ablation by Headrick et al.19
Integration of ICE into VT ablation workflow
We have found the workflow described in the methods extremely helpful in understanding each patient’s anatomy and planning for linear ablation. The use of ICE did not significantly increase either procedure times or complications and therefore can be safely integrated into the workflow. Our experience using ICE has taught us the best and most reproducible view is where we enter the RV with posterior tilt (retroflexion), allowing direct visualization through the long axis of the RVOT and surgical patches. We have found imaging surgical patches can be challenging directly from the RVOT due to proximity of the patch to the probe, as well as imaging from the RA, which often does not fully define the extent of the patches in transverse plane.
Comparison with integrated cross-sectional imaging
Integration of cardiac computerized tomography (CT) has been widely described to assist in the ablation of VT in the context of structural heart disease20,21 and has been reported to be particularly useful in defining the surgical anatomy where part of the isthmus is inaccessible from the RVOT22 or there is limited venous access from the inferior vena cava.23 A recent study by Moore et al.24 reported the use of CT to define the anatomical location of patches as well as the myocardial wall thickness, which correlated with conduction properties across the isthmus and the number of radiofrequency lesions required to block the isthmus. In our practice, we do not routinely use image integration at the time of the ablation procedure, and to our knowledge, there is no study comparing the use of ICE vs cross-sectional imaging in TOF VT. We propose ICE has additional benefits over CT integration alone given that it allows for real-time imaging and is not prone to errors in registration, particularly in cases with high anatomic complexity, additional radiation, and contrast exposure, as well as the need for scheduling preprocedure.
Study limitations
This was a retrospective rather than prospective study, with the use of ICE and subsequent ablation being performed at the discretion of the operator rather than following a predefined protocol. It is therefore difficult to isolate the benefit gained from the use of ICE alone. ICE cases were also performed more recently and may have been more successful because of advances in EAM with a trend toward higher density substrate maps. While every attempt was made to review all clinical data, it is possible some outcome data including VT recurrence may have been underreported. In particular, the degree of follow-up including schedule for clinic attendance and outpatient ambulatory monitoring was variable. None of the patients in the ICE cohort underwent surgical repair as a neonate; therefore, it is uncertain whether ICE performs equally well at identifying smaller patches. Finally, we assumed that the structures annotated on ICE represented the true boundaries of the patches to mark the isthmus without either direct visualization or correlative detailed operative notes.
Conclusions
ICE improves targeted linear ablation of the anatomical isthmus for sustaining VT in patients with repaired TOF by demonstrating the individual anatomy. The introduction of ICE was neither associated with a significant increase in procedure time nor periprocedural complications and was easily integrated into ablation workflow. There was a trend toward shorter fluoroscopy times after an initial learning curve; however, ICE utilization did not affect the recurrence of VT, which was low in this contemporary cohort.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: Success of ventricular tachycardia ablation in repaired tetralogy of Fallot is dependent upon the ability to localize and block the anatomical isthmus sustaining ventricular tachycardia. Intracardiac echocardiography can be safely integrated into ventricular tachycardia ablation and improves the ability to block the anatomical isthmus however it did not improve long-term outcomes.
TRANSLATIONAL OUTLOOK: Intracardiac echocardiography allows for real-time recognition of the interindividual variation in the anatomy following repair of tetralogy of Fallot, and may provide a framework for reducing radiation during procedures. Future research should focus on how outcomes can be improved and a comparison with the use of cross-sectional imaging.
Funding support and author disclosures
Dr Nair is a consultant for Biosense Webster, dealing only with teaching of complex mapping. Biosense Webster had no role in any part of the study, including its planning or execution or the creation of the manuscript, and has not seen the contents of this manuscript. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental figures and videos, please see the online version of this paper.
Supplementary Materials
References
- 1.Gatzoulis M.A., Balaji S., Webber S.A., et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356(9234):975–981. doi: 10.1016/s0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 2.Koyak Z., Harris L., de Groot J.R., et al. Sudden cardiac death in adult congenital heart disease. Circulation. 2012;126(16):1944–1954. doi: 10.1161/circulationaha.112.104786. [DOI] [PubMed] [Google Scholar]
- 3.Khairy P., Harris L., Landzberg M.J., et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation. 2008;117(3):363–370. doi: 10.1161/circulationaha.107.726372. [DOI] [PubMed] [Google Scholar]
- 4.Zeppenfeld K., Schalij M.J., Bartelings M.M., et al. Catheter ablation of ventricular tachycardia after repair of congenital heart disease: electroanatomic identification of the critical right ventricular isthmus. Circulation. 2007;116(20):2241–2252. doi: 10.1161/circulationaha.107.723551. [DOI] [PubMed] [Google Scholar]
- 5.Kapel G.F., Reichlin T., Wijnmaalen A.P., et al. Re-entry using anatomically determined isthmuses: a curable ventricular tachycardia in repaired congenital heart disease. Circ Arrhythm Electrophysiol. 2015;8(1):102–109. doi: 10.1161/circep.114.001929. [DOI] [PubMed] [Google Scholar]
- 6.Kawada S., Chakraborty P., Downar E., et al. The role of ablation in prevention of recurrent implantable cardioverter defibrillator shocks in patients with tetralogy of Fallot. CJC Open. 2021;3(5):619–626. doi: 10.1016/j.cjco.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laredo M., Frank R., Waintraub X., et al. Ten-year outcomes of monomorphic ventricular tachycardia catheter ablation in repaired tetralogy of Fallot. Arch Cardiovasc Dis. 2017;110(5):292–302. doi: 10.1016/j.acvd.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Waldmann V., Bouzeman A., Duthoit G., et al. Long-term follow-up of patients with tetralogy of Fallot and implantable cardioverter defibrillator: the DAI-T4F nationwide registry. Circulation. 2020;142(17):1612–1622. doi: 10.1161/circulationaha.120.046745. [DOI] [PubMed] [Google Scholar]
- 9.Kapel G.F., Sacher F., Dekkers O.M., et al. Arrhythmogenic anatomical isthmuses identified by electroanatomical mapping are the substrate for ventricular tachycardia in repaired Tetralogy of Fallot. Eur Heart J. 2017;38(4):268–276. doi: 10.1093/eurheartj/ehw202. [DOI] [PubMed] [Google Scholar]
- 10.Drago F., Pazzano V., Di Mambro C., et al. Role of right ventricular three-dimensional electroanatomic voltage mapping for arrhythmic risk stratification of patients with corrected tetralogy of Fallot or other congenital heart disease involving the right ventricular outflow tract. Int J Cardiol. 2016;222:422–429. doi: 10.1016/j.ijcard.2016.07.231. [DOI] [PubMed] [Google Scholar]
- 11.Soejima K., Stevenson W.G., Maisel W.H., Sapp J.L., Epstein L.M. Electrically unexcitable scar mapping based on pacing threshold for identification of the reentry circuit isthmus: feasibility for guiding ventricular tachycardia ablation. Circulation. 2002;106(13):1678–1683. doi: 10.1161/01.cir.0000030187.39852.a7. [DOI] [PubMed] [Google Scholar]
- 12.Kapel G.F., Reichlin T., Wijnmaalen A.P., et al. Left-sided ablation of ventricular tachycardia in adults with repaired tetralogy of Fallot: a case series. Circ Arrhythm Electrophysiol. 2014;7(5):889–897. doi: 10.1161/circep.114.001661. [DOI] [PubMed] [Google Scholar]
- 13.Moore J.P., Seki A., Shannon K.M., et al. Characterization of anatomic ventricular tachycardia isthmus pathology after surgical repair of tetralogy of Fallot. Circ Arrhythm Electrophysiol. 2013;6(5):905–911. doi: 10.1161/circep.113.000450. [DOI] [PubMed] [Google Scholar]
- 14.Yang J., Brunnquell M., Liang J.J., et al. Long term follow-up after ventricular tachycardia ablation in patients with congenital heart disease. J Cardiovasc Electrophysiol. 2019;30(9):1560–1568. doi: 10.1111/jce.13996. [DOI] [PubMed] [Google Scholar]
- 15.Campbell T., Haqqani H., Kumar S. Intracardiac echocardiography to guide mapping and ablation of arrhythmias in patients with congenital heart disease. Card Electrophysiol Clin. 2021;13(2):345–356. doi: 10.1016/j.ccep.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Stellin G., Guariento A., Vida V.L. Evolving techniques for the achievement of optimal long-term results after tetralogy of Fallot repair. World J Pediatr Congenit Heart Surg. 2021;12(1):116–123. doi: 10.1177/2150135120968103. [DOI] [PubMed] [Google Scholar]
- 17.Kapel G.F.L., Laranjo S., Blom N.A., et al. Impact of surgery on presence and dimensions of anatomical isthmuses in tetralogy of Fallot. Heart. 2018;104(14):1200–1207. doi: 10.1136/heartjnl-2017-312452. [DOI] [PubMed] [Google Scholar]
- 18.Goya M., Frame D., Gache L., et al. The use of intracardiac echocardiography catheters in endocardial ablation of cardiac arrhythmia: meta-analysis of efficiency, effectiveness, and safety outcomes. J Cardiovasc Electrophysiol. 2020;31(3):664–673. doi: 10.1111/jce.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Headrick A., Ou Z., Asaki S.Y., et al. Intracardiac echocardiography in paediatric and congenital cardiac ablation shortens procedure duration and improves success without complications. Europace. 2024;26(2) doi: 10.1093/europace/euae047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esposito A., Palmisano A., Antunes S., et al. Cardiac CT with delayed enhancement in the characterization of ventricular tachycardia structural substrate: relationship between CT-segmented scar and electro-anatomic mapping. JACC Cardiovasc Imaging. 2016;9(7):822–832. doi: 10.1016/j.jcmg.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita S., Sacher F., Mahida S., et al. Image integration to guide catheter ablation in scar-related ventricular tachycardia. J Cardiovasc Electrophysiol. 2016;27(6):699–708. doi: 10.1111/jce.12963. [DOI] [PubMed] [Google Scholar]
- 22.Madaffari A., Rivetti L., Kühne M., et al. Ventricular tachycardia catheter ablation after repaired tetralogy of Fallot: how to overcome an electrical short circuit. Europace. 2020;22(11):1687. doi: 10.1093/europace/euaa167. [DOI] [PubMed] [Google Scholar]
- 23.Chuang C.M., Chung F.P., Lee P.C., Chen S.A. Successful ablation of ventricular tachycardia in repaired tetralogy of Fallot via transjugular and subclavian approach. Acta Cardiol Sin. 2019;35(4):433–436. doi: 10.6515/acs.201907_35(4).20190320a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore J.P., Su J., Shannon K.M., et al. Multidetector computed tomography assessment of anatomical ventricular tachycardia isthmuses in repaired tetralogy of Fallot. JACC Clin Electrophysiol. 2024;10(5):857–866. doi: 10.1016/j.jacep.2024.102333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.