Abstract
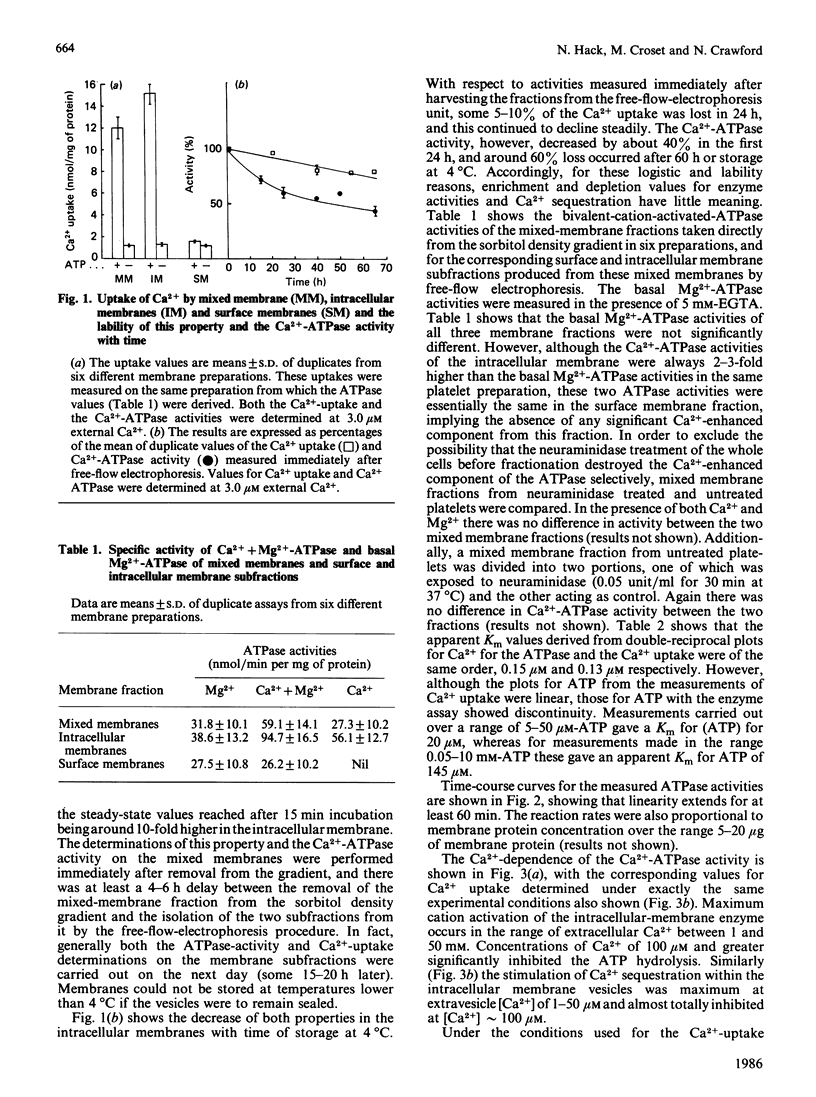
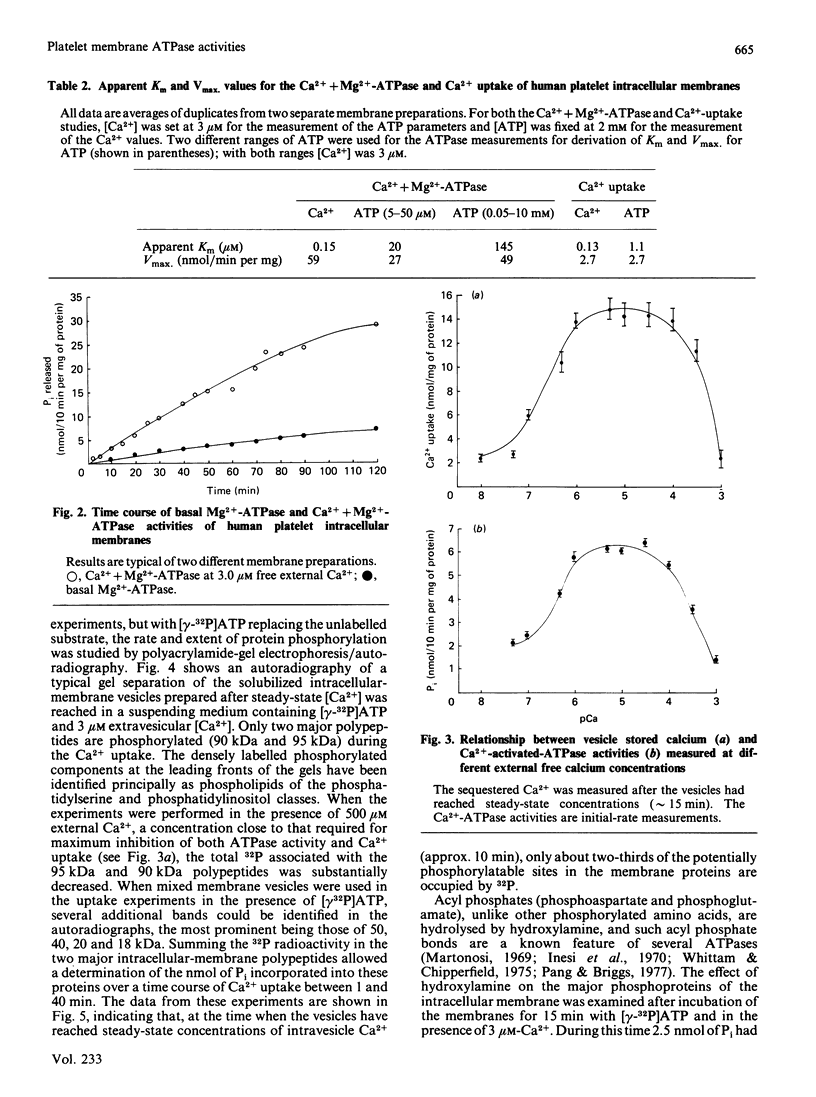
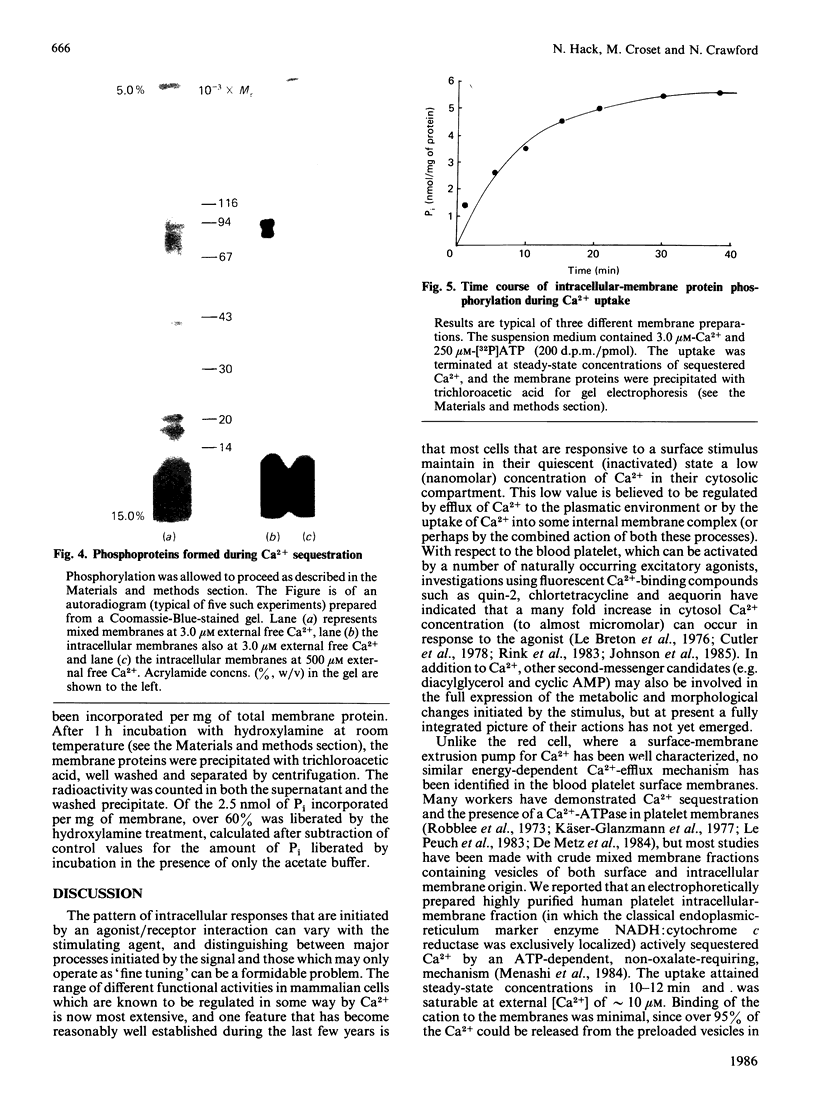
Membrane-bound Ca2+-ATPases are responsible for the energy-dependent transport of Ca2+ across membrane barriers against concentration gradients. Such enzymes have been identified in sarcoplasmic reticulum of muscle tissues and in non-muscle cells in both surface membranes and endoplasmic-reticulum-like intracellular membrane complexes. In a previous study using membrane fractionation by density-gradient and free-flow electrophoresis, we reported that the intracellular membranes of human blood platelets were a major storage site for Ca2+ and involved in maintaining low cytosol [Ca2+] in the unactivated cell. In the present report we demonstrated that the intracellular membranes also exhibit a high-affinity Ca2+-ATPase which appears to be kinetically associated with the Ca2+-sequestering process. We found that both the surface membrane and the intracellular membrane exhibited a basal Mg2+-ATPase activity, but Ca2+ activation of this enzyme was confined only to the intracellular membrane. Use of Ca2+-EGTA buffers to control the extravesicle [Ca2+] allowed a direct comparison of the Ca2+-ATPase and the Ca2+-uptake process over a Ca2+ range of 0.01 microM to 1.0 mM, and it was found that both properties were maximally expressed in the range of external [Ca2+] 1-50 microM, with concentrations greater than 100 microM showing substantial inhibition. Double-reciprocal plots for the Ca2+-ATPase activity and Ca2+ uptake gave apparent Km values for Ca2+ of 0.15 and 0.13 microM respectively. However, similar plots for ATP with the enzyme revealed a discontinuity (two affinity sites, with Km 20 and 145 microM), whereas plots for the Ca2+ uptake gave a single Km value for Ca2+, 1.1 microM. Phosphorylation studies during Ca2+ uptake using [gamma-32P]ATP revealed two components of 90 and 95 kDa phosphorylated at extravesicle [Ca2+] of 3 microM. The Ca2+-ATPase activity, Ca2+ uptake and phosphorylation were all almost completely inhibited in the presence of 500 microM-Ca2+. Similar studies using mixed membranes revealed four other phosphoproteins (50, 40, 20 and 18 kDa) formed in addition to the 90 and 95 kDa components. The findings are discussed in the context of platelet Ca2+ mobilization for function and the mechanisms whereby Ca2+ homoeostasis is controlled in the unactivated cell.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Authi K. S., Crawford N. Inositol 1,4,5-trisphosphate-induced release of sequestered Ca2+ from highly purified human platelet intracellular membranes. Biochem J. 1985 Aug 15;230(1):247–253. doi: 10.1042/bj2300247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros F., Kaczorowski G. J. Mechanisms of Ca2+ transport in plasma membrane vesicles prepared from cultured pituitary cells. II. (Ca2+ + Mg2+)-ATPase-dependent Ca2+ transport activity. J Biol Chem. 1984 Aug 10;259(15):9404–9410. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brass L. F. Ca2+ homeostasis in unstimulated platelets. J Biol Chem. 1984 Oct 25;259(20):12563–12570. [PubMed] [Google Scholar]
- Chamberlain B. K., Volpe P., Fleischer S. Inhibition of calcium-induced calcium release from purified cardiac sarcoplasmic reticulum vesicles. J Biol Chem. 1984 Jun 25;259(12):7547–7553. [PubMed] [Google Scholar]
- Cutler L., Rodan G., Feinstein M. B. Cytochemical localization of adenylate cyclase and of calcium ion, magnesium ion-activated ATPases in the dense tubular system of human blood platelets. Biochim Biophys Acta. 1978 Sep 6;542(3):357–371. doi: 10.1016/0304-4165(78)90367-7. [DOI] [PubMed] [Google Scholar]
- De Metz M., Lebret M., Enouf J., Lévy-Tolédano S. The phospholipid requirement of the (Ca2+ + Mg2+)-ATPase from human platelets. Biochim Biophys Acta. 1984 Mar 14;770(2):159–165. doi: 10.1016/0005-2736(84)90125-1. [DOI] [PubMed] [Google Scholar]
- Dean W. L. Purification and reconstitution of a Ca2+ pump from human platelets. J Biol Chem. 1984 Jun 10;259(11):7343–7348. [PubMed] [Google Scholar]
- Dean W. L., Sullivan D. M. Structural and functional properties of a Ca2+-ATPase from human platelets. J Biol Chem. 1982 Dec 10;257(23):14390–14394. [PubMed] [Google Scholar]
- Enouf J., Bredoux R., Boizard B., Wautier J. L., Chap H., Thomas J., de Metz M., Levy-Toledano S. Simultaneous isolation of two platelet membrane fractions: biochemical, immunological and functional characterization. Biochem Biophys Res Commun. 1984 Aug 30;123(1):50–58. doi: 10.1016/0006-291x(84)90378-4. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Say A. K., Haslam R. J. Subcellular distribution of the different platelet proteins phosphorylated on exposure of intact platelets to ionophore A23187 or to prostaglandin E1. Possible role of a membrane phosphopolypeptide in the regulation of calcium-ion transport. Biochem J. 1979 Dec 15;184(3):651–661. doi: 10.1042/bj1840651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghijsen W. E., De Jong M. D., Van Os C. H. ATP-dependent calcium transport and its correlation with Ca2+ -ATPase activity in basolateral plasma membranes of rat duodenum. Biochim Biophys Acta. 1982 Jul 28;689(2):327–336. doi: 10.1016/0005-2736(82)90266-8. [DOI] [PubMed] [Google Scholar]
- Hack N., Carey F., Crawford N. The inhibition of platelet cyclo-oxygenase by aspirin is associated with the acetylation of a 72kDa polypeptide in the intracellular membranes. Biochem J. 1984 Oct 1;223(1):105–111. doi: 10.1042/bj2230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack N., Crawford N. Two-dimensional polyacrylamide-gel electrophoresis of the proteins and glycoproteins of purified human platelet surface and intracellular membranes. Biochem J. 1984 Aug 15;222(1):235–246. doi: 10.1042/bj2220235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto N. Structure and function of the calcium pump protein of sarcoplasmic reticulum. Annu Rev Physiol. 1982;44:297–317. doi: 10.1146/annurev.ph.44.030182.001501. [DOI] [PubMed] [Google Scholar]
- Inesi G., Maring E., Murphy A. J., McFarland B. H. A study of the phosphorylated intermediate of sarcoplasmic reticulum ATPase. Arch Biochem Biophys. 1970 May;138(1):285–294. doi: 10.1016/0003-9861(70)90309-7. [DOI] [PubMed] [Google Scholar]
- Johnson P. C., Ware J. A., Cliveden P. B., Smith M., Dvorak A. M., Salzman E. W. Measurement of ionized calcium in blood platelets with the photoprotein aequorin. Comparison with Quin 2. J Biol Chem. 1985 Feb 25;260(4):2069–2076. [PubMed] [Google Scholar]
- Käser-Glanzmann R., Jakäbovä M., George J. N., Lüscher E. F. Stimulation of calcium uptake in platelet membrane vesicles by adenosine 3',5'-cyclic monophosphate and protein kinase. Biochim Biophys Acta. 1977 May 2;466(3):429–440. doi: 10.1016/0005-2736(77)90336-4. [DOI] [PubMed] [Google Scholar]
- Lagarde M., Bryon P. A., Guichardant M., Dechavanne M. A simple and efficient method for platelet isolation from their plasma. Thromb Res. 1980 Feb 1;17(3-4):581–588. doi: 10.1016/0049-3848(80)90098-5. [DOI] [PubMed] [Google Scholar]
- Lagarde M., Guichardant M., Menashi S., Crawford N. The phospholipid and fatty acid composition of human platelet surface and intracellular membranes isolated by high voltage free flow electrophoresis. J Biol Chem. 1982 Mar 25;257(6):3100–3104. [PubMed] [Google Scholar]
- Le Peuch C. J., Le Peuch D. A., Katz S., Demaille J. G., Hincke M. T., Bredoux R., Enouf J., Levy-Toledano S., Caen J. Regulation of calcium accumulation and efflux from platelet vesicles. Possible role for cyclic-AMP-dependent phosphorylation and calmodulin. Biochim Biophys Acta. 1983 Jun 23;731(3):456–464. doi: 10.1016/0005-2736(83)90041-x. [DOI] [PubMed] [Google Scholar]
- Martonosi A. Sarcoplasmic reticulum. VII. Properties of a phosphoprotein intermediate implicated in calcium transport. J Biol Chem. 1969 Feb 25;244(4):613–620. [PubMed] [Google Scholar]
- Menashi S., Authi K. S., Carey F., Crawford N. Characterization of the calcium-sequestering process associated with human platelet intracellular membranes isolated by free-flow electrophoresis. Biochem J. 1984 Sep 1;222(2):413–417. doi: 10.1042/bj2220413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menashi S., Davis C., Crawford N. Calcium uptake associated with an intracellular membrane fraction prepared from human blood platelets by high-voltage, free-flow electrophoresis. FEBS Lett. 1982 Apr 19;140(2):298–302. doi: 10.1016/0014-5793(82)80918-6. [DOI] [PubMed] [Google Scholar]
- Menashi S., Weintroub H., Crawford N. Characterization of human platelet surface and intracellular membranes isolated by free flow electrophoresis. J Biol Chem. 1981 Apr 25;256(8):4095–4101. [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Pang D. C., Briggs F. N. Effect of calcium and magnesium on binding of beta, gamma-methylene ATP to sarcoplasmic reticulum. J Biol Chem. 1977 May 25;252(10):3262–3266. [PubMed] [Google Scholar]
- Pershadsingh H. A., Landt M., McDonald J. M. Calmodulin-sensitive ATP-dependent Ca2+ transport across adipocyte plasma membranes. J Biol Chem. 1980 Oct 10;255(19):8983–8986. [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Robblee L. S., Shepro D., Belamarich F. A. Calcium uptake and associated adenosine triphosphatase activity of isolated platelet membranes. J Gen Physiol. 1973 Apr;61(4):462–481. doi: 10.1085/jgp.61.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals J. R., McDonald J. M., Bruns D., Jarett L. A sensitive and precise isotopic assay of ATPase activity. Anal Biochem. 1978 Oct 15;90(2):785–795. doi: 10.1016/0003-2697(78)90169-0. [DOI] [PubMed] [Google Scholar]
- Statland B. E., Heagan B. M., White J. G. Uptake of calcium by platelet relaxing factor. Nature. 1969 Aug 2;223(5205):521–522. doi: 10.1038/223521a0. [DOI] [PubMed] [Google Scholar]
- Trumble W. R., Sutko J. L., Reeves J. P. Cardiac sarcolemmal and sarcoplasmic reticulum membrane vesicles exhibit distinctive (Ca-Mg)-ATPase substrate specificities. J Biol Chem. 1981 Jul 25;256(14):7101–7104. [PubMed] [Google Scholar]
- Whittam R., Chipperfield A. R. The reaction mechanism of the sodium pump. Biochim Biophys Acta. 1975 Jun 30;415(2):149–171. doi: 10.1016/0304-4157(75)90001-5. [DOI] [PubMed] [Google Scholar]




