Abstract
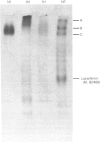
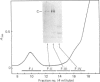
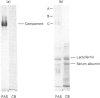
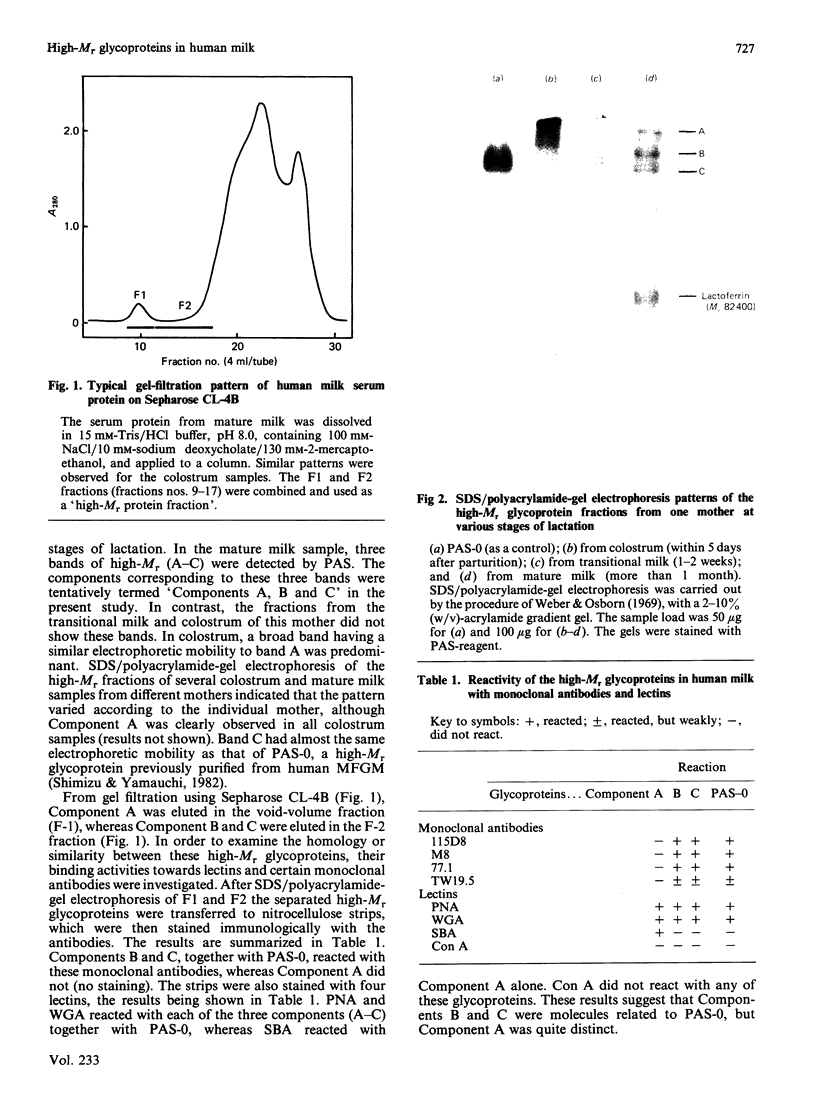
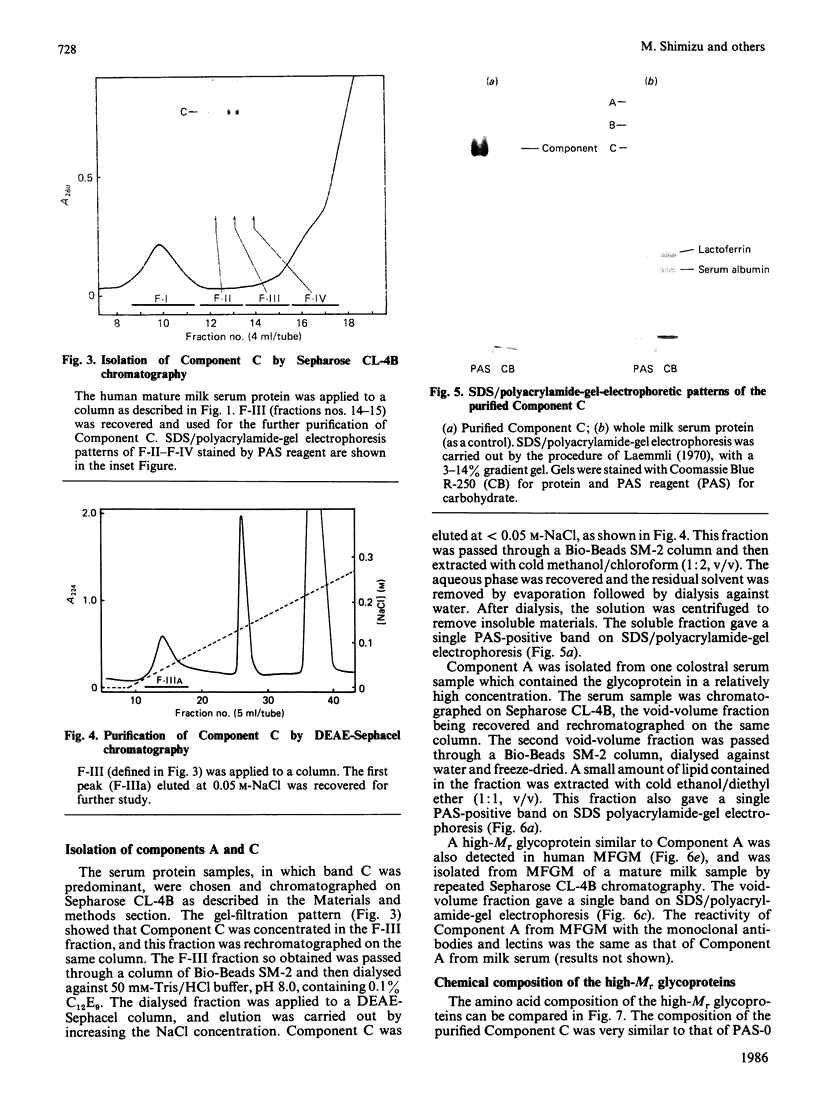
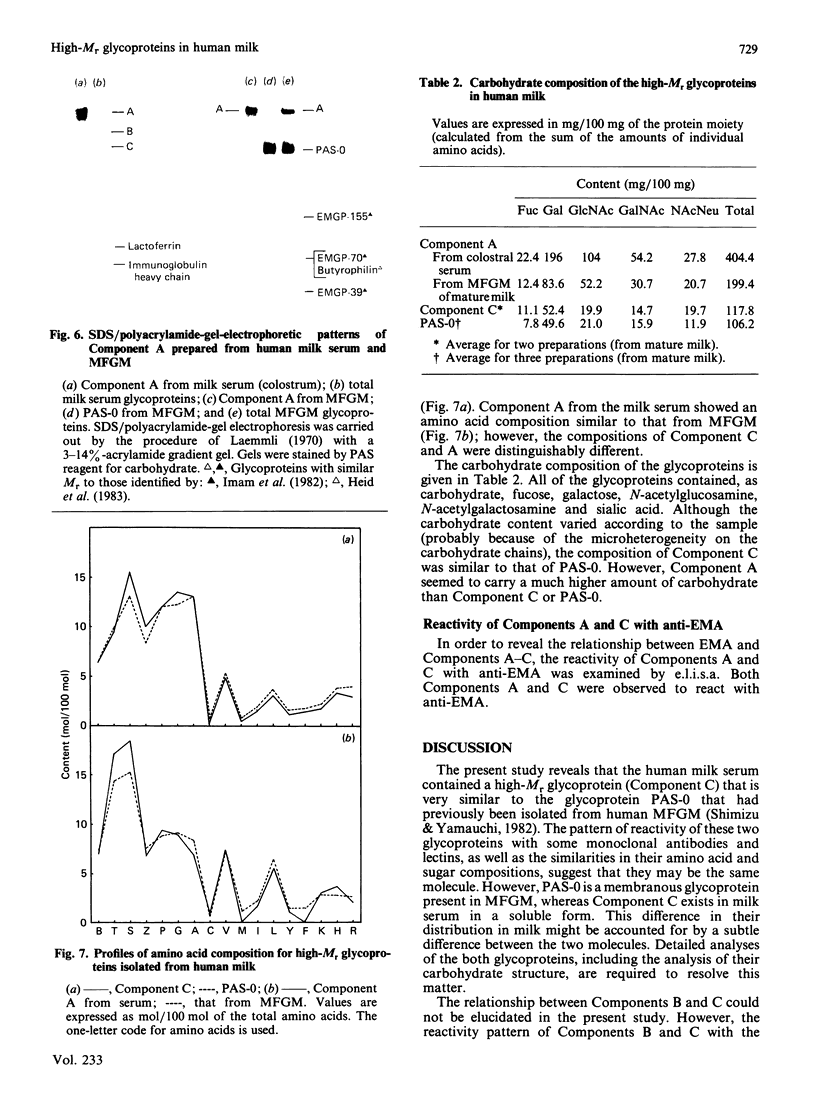
Gradient-polyacrylamide-gel electrophoresis of human milk serum separated three high-Mr glycoprotein bands. The properties of the components corresponding to the three bands (tentatively termed 'Components C, B and A' in their order of migration) were compared by staining with four monoclonal antibodies and lectins. Components B and C both reacted with the four antibodies, but Component A did not. Components B and C were stained with peanut (Arachis hypogaea) agglutinin (PNA) and wheat (Triticum)-germ agglutinin (WGA), Component A being stained with soya-bean (Glycine max) agglutinin as well as PNA and WGA. These results suggest that Components B and C were related molecules, whereas Component A was markedly different from them. The reactivities of Components B and C were the same as those of PAS-0, a high-Mr periodate/Schiff (PAS)-positive glycoprotein previously isolated from human milk fat-globule membrane (MFGM). Component C, whose electrophoretic mobility was the same as PAS-0, could have been a soluble form of PAS-0. A high-Mr glycoprotein having the same properties as Component A was also observed in MFGM. The amino acid composition of the isolated Component A was significantly different from that of Component C and PAS-0, high threonine and serine contents being characteristic of Component A. The carbohydrate content of Component A was 65-80%, and was much higher than that of Component C and PAS-0. Fucose, galactose, N-acetylglucosamine, N-acetylgalactosamine and sialic acid were each detected as constituent sugars of Component A. Component A represents, therefore, a new high-Mr glycoprotein species in human milk serum and MFGM. Since these glycoproteins were high-Mr mucin-like glycoproteins, the names 'HM glycoprotein-A' and 'HM glycoprotein-C' were proposed for Component A and Component C (PAS-O) respectively.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma N., Kadoya H., Yamauchi K. A 20,000-dalton casein fragment in human milk. J Dairy Sci. 1985 Sep;68(9):2176–2183. doi: 10.3168/jds.S0022-0302(85)81088-2. [DOI] [PubMed] [Google Scholar]
- Burchell J., Durbin H., Taylor-Papadimitriou J. Complexity of expression of antigenic determinants, recognized by monoclonal antibodies HMFG-1 and HMFG-2, in normal and malignant human mammary epithelial cells. J Immunol. 1983 Jul;131(1):508–513. [PubMed] [Google Scholar]
- Ceriani R. L., Peterson J. A., Blank E. W. Variability in surface antigen expression of human breast epithelial cells cultured from normal breast, normal tissue peripheral to breast carcinomas, and breast carcinomas. Cancer Res. 1984 Jul;44(7):3033–3039. [PubMed] [Google Scholar]
- Ceriani R. L., Peterson J. A., Lee J. Y., Moncada R., Blank E. W. Characterization of cell surface antigens of human mammary epithelial cells with monoclonal antibodies prepared against human milk fat globule. Somatic Cell Genet. 1983 Jul;9(4):415–427. doi: 10.1007/BF01543043. [DOI] [PubMed] [Google Scholar]
- Fischer J., Klein P. J., Farrar G. H., Hanisch F. G., Uhlenbruck G. Isolation and chemical and immunochemical characterization of the peanut-lectin-binding glycoprotein from human milk-fat-globule membranes. Biochem J. 1984 Dec 1;224(2):581–589. doi: 10.1042/bj2240581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster C. S., Edwards P. A., Dinsdale E. A., Neville A. M. Monoclonal antibodies to the human mammary gland. I. Distribution of determinants in non-neoplastic mammary and extra mammary tissues. Virchows Arch A Pathol Anat Histol. 1982;394(3):279–293. doi: 10.1007/BF00430671. [DOI] [PubMed] [Google Scholar]
- Freudenstein C., Keenan T. W., Eigel W. N., Sasaki M., Stadler J., Franke W. W. Preparation and characterization of the inner coat material associated with fat globule membranes from bovine and human milk. Exp Cell Res. 1979 Feb;118(2):277–294. doi: 10.1016/0014-4827(79)90153-8. [DOI] [PubMed] [Google Scholar]
- Heid H. W., Winter S., Bruder G., Keenan T. W., Jarasch E. D. Butyrophilin, an apical plasma membrane-associated glycoprotein characteristic of lactating mammary glands of diverse species. Biochim Biophys Acta. 1983 Feb;728(2):228–238. doi: 10.1016/0005-2736(83)90476-5. [DOI] [PubMed] [Google Scholar]
- Heyderman E., Steele K., Ormerod M. G. A new antigen on the epithelial membrane: its immunoperoxidase localisation in normal and neoplastic tissue. J Clin Pathol. 1979 Jan;32(1):35–39. doi: 10.1136/jcp.32.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilkens J., Buijs F., Hilgers J., Hageman P., Calafat J., Sonnenberg A., van der Valk M. Monoclonal antibodies against human milk-fat globule membranes detecting differentiation antigens of the mammary gland and its tumors. Int J Cancer. 1984 Aug 15;34(2):197–206. doi: 10.1002/ijc.2910340210. [DOI] [PubMed] [Google Scholar]
- Imam A., Laurence D. J., Neville A. M. Isolation and characterization of a major glycoprotein from milk-fat-globule membrane of human breast milk. Biochem J. 1981 Jan 1;193(1):47–54. doi: 10.1042/bj1930047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam A., Laurence D. J., Neville A. M. Isolation and characterization of two individual glycoprotein components from human milk-fat-globule membranes. Biochem J. 1982 Oct 1;207(1):37–41. doi: 10.1042/bj2070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McIlhinney R. A., Patel S., Gore M. E. Monoclonal antibodies recognizing epitopes carried on both glycolipids and glycoproteins of the human milk fat globule membrane. Biochem J. 1985 Apr 1;227(1):155–162. doi: 10.1042/bj2270155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama R., Nakashima H., Makino S., Koga S. A study on the separation of reconstituted proteoliposomes and unincorporated membrane proteins by use of hydrophobic affinity gels, with special reference to band 3 from bovine erythrocyte membranes. Anal Biochem. 1984 Jun;139(2):292–297. doi: 10.1016/0003-2697(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Moroi M., Jung S. M. Selective staining of human platelet glycoproteins using nitrocellulose transfer of electrophoresed proteins and peroxidase-conjugated lectins. Biochim Biophys Acta. 1984 Apr 24;798(3):295–301. doi: 10.1016/0304-4165(84)90101-6. [DOI] [PubMed] [Google Scholar]
- Murray L. R., Powell K. M., Sasaki M., Eigel W. N., Keenan T. W. Comparison of lectin receptor and membrane coat-associated glycoproteins of milk lipid globule membranes. Comp Biochem Physiol B. 1979;63(1):137–145. doi: 10.1016/0305-0491(79)90246-3. [DOI] [PubMed] [Google Scholar]
- Ormerod M. G., McIlhinney J., Steele K., Shimizu M. Glycoprotein PAS-0 from the milk fat globule membrane carries antigenic determinants for epithelial membrane antigen. Mol Immunol. 1985 Mar;22(3):265–269. doi: 10.1016/0161-5890(85)90160-9. [DOI] [PubMed] [Google Scholar]
- Ormerod M. G., Steele K., Westwood J. H., Mazzini M. N. Epithelial membrane antigen: partial purification, assay and properties. Br J Cancer. 1983 Oct;48(4):533–541. doi: 10.1038/bjc.1983.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton S., Keenan T. W. The milk fat globule membrane. Biochim Biophys Acta. 1975 Oct 31;415(3):273–309. doi: 10.1016/0304-4157(75)90011-8. [DOI] [PubMed] [Google Scholar]
- Peterson J. A., Ceriani R. L., Blank E. W., Osvaldo L. Comparison of rates of phenotypic variability in surface antigen expression in normal and cancerous human breast epithelial cells. Cancer Res. 1983 Sep;43(9):4291–4296. [PubMed] [Google Scholar]
- Shimizu M., Yamauchi K. Isolation and characterization of mucin-like glycoprotein in human milk fat globule membrane. J Biochem. 1982 Feb;91(2):515–524. doi: 10.1093/oxfordjournals.jbchem.a133724. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Peterson J. A., Arklie J., Burchell J., Ceriani R. L., Bodmer W. F. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int J Cancer. 1981 Jul 15;28(1):17–21. doi: 10.1002/ijc.2910280104. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]