Abstract
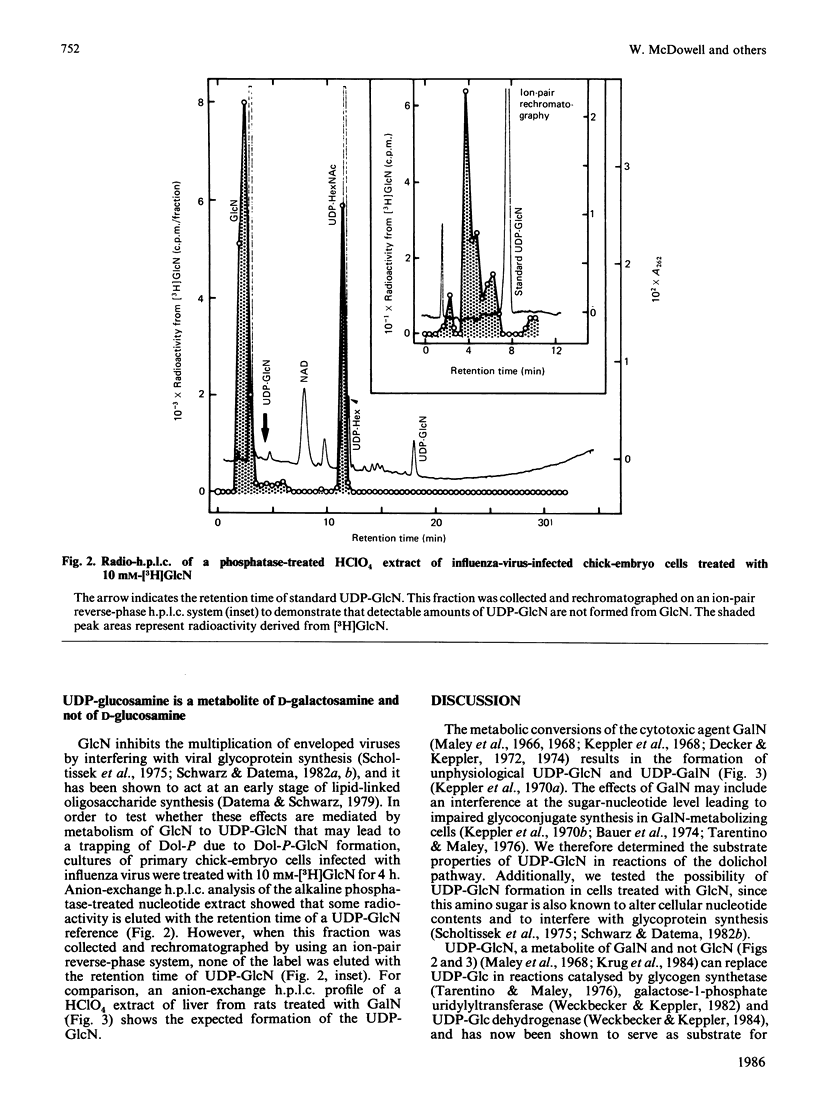
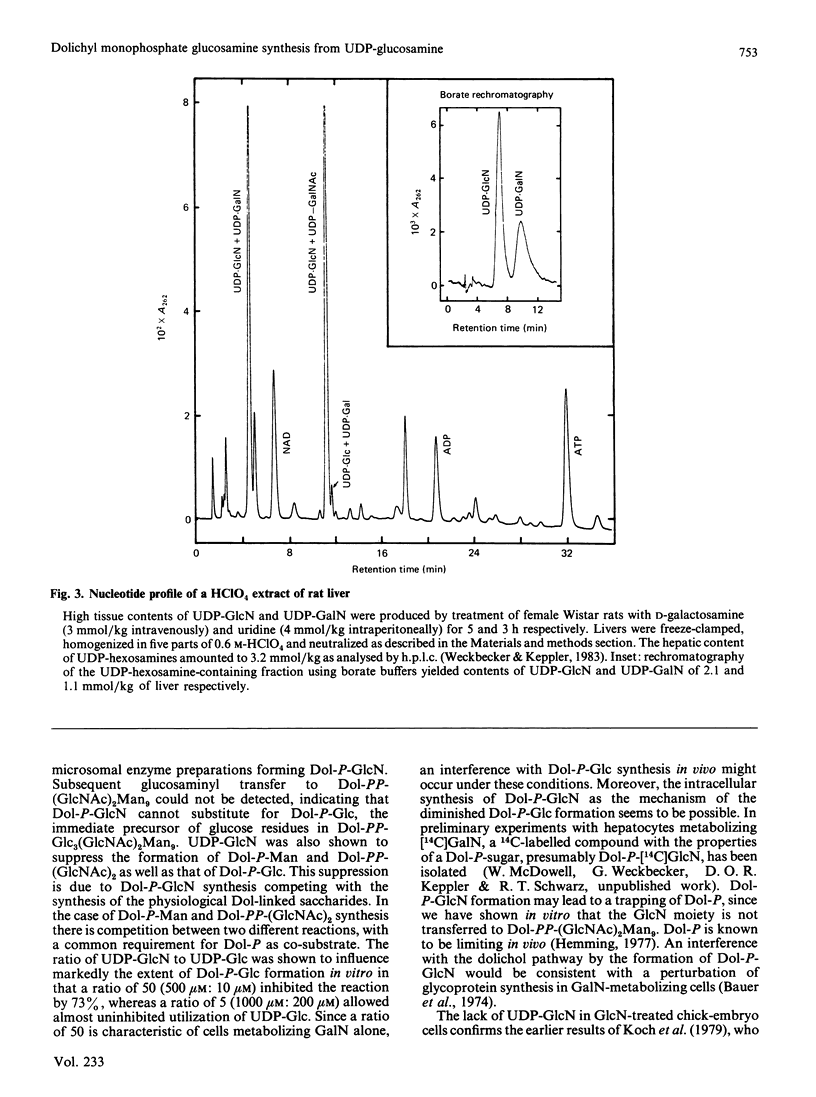
The sugar nucleotide analogue UDP-glucosamine was found to function as a sugar donor in microsomal preparations of both chick-embryo cells and rat liver, yielding dolichyl monophosphate glucosamine (Dol-P-GlcN). This was characterized by t.l.c. and retention by DEAE-cellulose. Glucosamine was the only water-soluble product released on mild acid hydrolysis. Dol-P-GlcN did not serve as substrate by transferring its glucosamine moiety to dolichol-linked oligosaccharide. Competition experiments between UDP-[3H]glucose and UDP-glucosamine showed Dol-P-[3H]glucose synthesis to be depressed by 56 or 73% in microsomes from chick-embryo cells and rat liver respectively. The concentrations of the UDP-sugars in this experiment were comparable with those occurring in galactosamine-metabolizing liver. These findings suggest that Dol-P-GlcN, formed as a metabolite of D-galactosamine, may interfere with Dol-P-dependent reactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer C. H., Lukaschek R., Reutter W. G. Studies on the golgi apparatus. Cumulative inhibition of protein and glycoprotein secretion by D-galactosamine. Biochem J. 1974 Aug;142(2):221–230. doi: 10.1042/bj1420221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Datema R., Schwarz R. T. Interference with glycosylation of glycoproteins. Inhibition of formation of lipid-linked oligosaccharides in vivo. Biochem J. 1979 Oct 15;184(1):113–123. doi: 10.1042/bj1840113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datema R., Schwarz R. T., Jankowski A. W. Fluoroglucose-inhibition of protein glycosylation in vivo. Inhibition of mannose and glucose incorporation into lipid-linked oligosaccharides. Eur J Biochem. 1980 Aug;109(2):331–341. doi: 10.1111/j.1432-1033.1980.tb04799.x. [DOI] [PubMed] [Google Scholar]
- Decker K., Keppler D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol. 1974;(71):77–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- Decker K., Keppler D. Galactosamine induced liver injury. Prog Liver Dis. 1972;4:183–199. [PubMed] [Google Scholar]
- Hemming F. W. Dolichol phosphate, a coenzyme in the glycosylation of animal membrane-bound glycoproteins. Biochem Soc Trans. 1977;5(4):1223–1231. doi: 10.1042/bst0051223. [DOI] [PubMed] [Google Scholar]
- Holstege A., Schulz-Holstege C., Henninger H., Reiffen K. A., Schneider F., Keppler D. O. Uridylate trapping induced by the C-2-modified D-glucose analogs glucosone, fluoroglucose, and glucosamine. Eur J Biochem. 1982 Jan;121(2):469–474. doi: 10.1111/j.1432-1033.1982.tb05811.x. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Keppler D. O., Rudigier J. F., Bischoff E., Decker K. F. The trapping of uridine phosphates by D-galactosamine. D-glucosamine, and 2-deoxy-D-galactose. A study on the mechanism of galactosamine hepatitis. Eur J Biochem. 1970 Dec;17(2):246–253. doi: 10.1111/j.1432-1033.1970.tb01160.x. [DOI] [PubMed] [Google Scholar]
- Keppler D., Lesch R., Reutter W., Decker K. Experimental hepatitis induced by D-galactosamine. Exp Mol Pathol. 1968 Oct;9(2):279–290. doi: 10.1016/0014-4800(68)90042-7. [DOI] [PubMed] [Google Scholar]
- Koch H. U., Schwarz R. T., Scholtissek C. Glucosamine itself mediates reversible inhibition of protein glycosylation. A study of glucosamine metabolism at inhibitory concentrations in influenza-virus-infected cells. Eur J Biochem. 1979 Mar;94(2):515–522. doi: 10.1111/j.1432-1033.1979.tb12920.x. [DOI] [PubMed] [Google Scholar]
- Krag S. S., Robbins P. W. Sindbis envelope proteins as endogenous acceptors in reactions of guanosine diphosphate-[14C]Mannose with preparations of infected chicken embryo fibroblasts. J Biol Chem. 1977 Apr 25;252(8):2621–2629. [PubMed] [Google Scholar]
- Krug E., Zweibaum A., Schulz-Holstege C., Keppler D. D-glucosamine-induced changes in nucleotide metabolism and growth of colon-carcinoma cells in culture. Biochem J. 1984 Feb 1;217(3):701–708. doi: 10.1042/bj2170701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley F., McGarrahan J. F., DelGiacco R. Galactosamine: a precursor of glycogen glucosamine. Biochem Biophys Res Commun. 1966 Apr 6;23(1):85–91. doi: 10.1016/0006-291x(66)90273-7. [DOI] [PubMed] [Google Scholar]
- Maley F., Tarentino A. L., McGarrahan J. F., Delgiacco R. The metabolism of d-galactosamine and N-acetyl-d-galactosamine in rat liver. Biochem J. 1968 May;107(5):637–644. doi: 10.1042/bj1070637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. D., Reutter W. Inhibition of induction of rat liver tyrosine aminotransferase by D-galactosamine. J Biol Chem. 1973 Mar 10;248(5):1562–1567. [PubMed] [Google Scholar]
- Scholtissek C., Rott R., Klenk H. D. Two different mechanisms of the inhibition of the multiplication of enveloped viruses by glucosamine. Virology. 1975 Jan;63(1):191–200. doi: 10.1016/0042-6822(75)90384-0. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Datema R. Inhibition of the dolichol pathway of protein glycosylation. Methods Enzymol. 1982;83:432–443. doi: 10.1016/0076-6879(82)83041-3. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Datema R. The lipid pathway of protein glycosylation and its inhibitors: the biological significance of protein-bound carbohydrates. Adv Carbohydr Chem Biochem. 1982;40:287–379. doi: 10.1016/s0065-2318(08)60111-0. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Schmidt M. F., Datema R. Inhibition of glycosylation of viral glycoproteins. Biochem Soc Trans. 1979 Apr;7(2):322–326. doi: 10.1042/bst0070322. [DOI] [PubMed] [Google Scholar]
- Sharma C. B., Babczinski P., Lehle L., Tanner W. The role of dolicholmonophosphate in glycoprotein biosynthesis in Saccharomyces cerevisiae. Eur J Biochem. 1974 Jul 1;46(1):35–41. doi: 10.1111/j.1432-1033.1974.tb03594.x. [DOI] [PubMed] [Google Scholar]
- Shidoji Y., De Luca L. M. Rat liver microsomes catalyse mannosyl transfer from GDP-D-mannose to retinyl phosphate with high efficiency in the absence of detergents. Biochem J. 1981 Dec 15;200(3):529–538. doi: 10.1042/bj2000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Direct evidence that D-galactosamine incorporation into glycogen occurs via UDP-glucosamine. FEBS Lett. 1976 Oct 15;69(1):175–178. doi: 10.1016/0014-5793(76)80680-1. [DOI] [PubMed] [Google Scholar]
- Weckbecker G., Keppler D. O. Dual role of hexose-1-phosphate uridylyltransferase in galactosamine metabolism. Eur J Biochem. 1982 Nov;128(1):163–168. doi: 10.1111/j.1432-1033.1982.tb06947.x. [DOI] [PubMed] [Google Scholar]
- Weckbecker G., Keppler D. O. Separation and analysis of 4'-epimeric UDP-sugars by borate high-performance liquid chromatography. Anal Biochem. 1983 Jul 15;132(2):405–412. doi: 10.1016/0003-2697(83)90027-1. [DOI] [PubMed] [Google Scholar]
- Weckbecker G., Keppler D. O. Substrate properties of 5-fluorouridine diphospho sugars detected in hepatoma cells. Biochem Pharmacol. 1984 Jul 15;33(14):2291–2298. doi: 10.1016/0006-2952(84)90669-5. [DOI] [PubMed] [Google Scholar]



