Abstract
Background
The 2023 “International Working Group on the Diabetic Foot/Infectious Disease Society of America Guidelines on the Diagnosis and Treatment of Diabetes-Related Foot Infections” (DFIs) provides recommendations for Pseudomonas coverage based on the climate region.
Methods
This was a retrospective national study of veterans between 1/1/2010 and 3/23/2024 with diabetes mellitus and a culture below the malleolus wound. Prevalence of Pseudomonas was categorized based on climate zones according to the International Energy Conservation Code. Multivariable logistic regression was used to determine odds ratios and 97.5% CIs.
Results
The prevalence of Pseudomonas significantly varied between US climates. Pseudomonas was most prevalent within the Hot Humid climate, where it was isolated in 11.6% of DFI cultures. Pseudomonas was least prevalent within the Very Cold climate, where it was isolated in 6.2% of cultures. In the multivariable logistic regression model, hot and humid climates were associated with an odds of P. aeruginosa of 1.92 (97.5% CI, 1.69–2.20), a hot, dry climate was associated with an odds of 1.65 (97.5% CI, 1.44–1.90), and a humid climate was associated with an odds of 1.65 (97.5% CI, 1.45–1.89). A lower Charlson Comorbidity Index, inpatient admission, recent antipseudomonal antibiotic use, and swabs were less likely to have Pseudomonas. Recent admission increased the odds of P. aeruginosa (odds ratio [OR], 1.34; 97.5% CI, 1.27–1.41). History of P. aeruginosa was associated with an increase in P. aeruginosa (OR, 8.90; 97.5% CI, 8.29–9.56).
Conclusions
The prevalence of DFI organisms varies within different US climates. Utilization of local climate information may allow for more accurate and targeted empiric antibiotic selection when treating DFIs.
Keywords: diabetic foot infection, Pseudomonas, climate, Pseudomonas aeruginosa, osteomyelitis
The prevalence of Pseudomonas aeruginosa varies within different United States climate designations. Further studies are needed to determine a threshold for which empirical Pseudomonas treatment should be considered when treating diabetes-related foot infections.
Diabetes is a highly prevalent chronic disease. It is estimated that 38.4 million Americans, or 11.6% of the US population, currently have diabetes. This number is expected to rise over the coming years [1]. Diabetes-related foot infections (DFIs) are a common complication of uncontrolled diabetes. The American Diabetes Association estimates that the lifetime risk of a foot ulcer is 19%–34% in patients with diabetes, and this number is climbing with increased longevity and complexity of people with diabetes. DFIs are often difficult to treat and can result in amputation of a limb or death if left untreated or improperly managed [2].
Pseudomonas aeruginosa is one of the most common causes of DFI globally and is a leading cause of diabetes-associated sepsis and amputation [3]. P. aeruginosa's high inherent resistance to many commonly used antibiotics and its ability to release proteins that delay and/or prevent wound healing makes treating DFI associated with P. aeruginosa more difficult [4]. Prolonged water and heat exposure are known natural risk factors for P. aeruginosa infection. In general, Pseudomonas species primarily live in soil, seawater, and fresh water [5]. This correlates to greater water exposure, increasing a patient's risk of acquiring Pseudomonas. The genes that encode for P. aeruginosa's virulence mechanisms are upregulated at 37°C, increasing P. aeruginosa's pathogenicity in warmer environments [6]. P. aeruginosa is also a common cause of nosocomial infections due to its intrinsic antimicrobial resistance and ability to grow in various environments.
The 2023 “International Working Group on the Diabetic Foot (IWGDF)/Infectious Disease Society of America (IDSA) Guidelines on the Diagnosis and Treatment of Diabetes-Related Foot Infections” provides recommendations on how to properly treat DFIs to reduce the risk of morbidity and mortality. The guidelines recommend avoidance of empirical P. aeruginosa treatment in climates that are temperate unless they meet certain criteria. These criteria include P. aeruginosa isolated from cultures of the affected site within the previous few weeks or moderate/severe infection in a patient living in Asia or North Africa. This recommendation stems from previous findings indicating that P. aeruginosa is not as prevalent in North America or Europe but is more common in the tropical or subtropical climates [7]. To support this recommendation, the guidelines cite a study that prospectively examined the bacteriology profile of DFI specimens from 522 patients in Turkey, where P. aeruginosa was isolated in 12.4% of patients [8]. The relatively small sample sizes of previous studies examining P. aeruginosa prevalence in different climates call into question the generalizability of recommendations.
According to the IWGDF/IDSA recommendations, because the United States falls within the temperate solar climate zone, P. aeruginosa should not be empirically targeted for DFIs within the United States unless the patient has had a previous DFI culture identifying P. aeruginosa. There are varied climates within the United States. Our study seeks to discover how climate zones impact the prevalence of P. aeruginosa within the United States. The numerous different climate regions within the United States were classified using the International Energy Conservation Code (IECC), which utilizes annual temperature and precipitation data to assign a climate designation to each county within the United States. While this classification system is intended to be used for climate-optimized building practices, we have extrapolated to evaluate for differences between climates within the United States.
The objective of this study was to evaluate the rates of P. aeruginosa within different climate designations within the United States. Though guidelines do not indicate microbiological thresholds for when P. aeruginosa should be empirically covered, this information will be helpful for clinicians deciding empirical regimens for DFI.
METHODS
This was a retrospective cohort study using data obtained from the Corporate Data Warehouse (CDW) and analyzed in the Veterans Affairs (VA) Informatics and Computing Infrastructure (VINCI). CDW data were obtained through Structure Query Language (SQL) via the SQL Server Management System (SSMS). Data analysis was performed using R Studio in the VINCI platform to protect confidential patient health information. This study was approved by the Research and Development Committee at the VA Western New York Healthcare System in Buffalo, New York.
The data query included patients who received care within the VA Healthcare System across the United States, were diagnosed with diabetes, and had a lower extremity culture taken from January 1, 2010, to April 3, 2024. The diagnosis of diabetes was defined using International Classification of Diseases, Ninth or Tenth Revision (ICD-9 or ICD-10), coding indicating a diagnosis of diabetes, a patient receiving a diabetes-specific medication, or if a patient had a documented glycated hemoglobin (A1c) value >6.5%. Diabetes-specific medications were defined as those that are primarily used only for the treatment of diabetes; sodium-glucose cotransporter-2 inhibitors (SGLT2i) and glucagon-like peptide (GLP-1) agents were not included in this definition due to their propensity to be used for treatment of other disease states, such as heart failure and obesity. Metformin was also not included due to the potential for non-diabetes-related indications when used as monotherapy. The date of diabetes diagnosis was defined as the first time any of the diagnoses or events occurred. Patients were excluded if they had ICD coding indicating a temporary diabetes diagnosis, such as gestational diabetes, or if they did not meet inclusion criteria. The climate in which a patient was located at the time of sampling was determined using the IECC for the location of the VA site where the sample was obtained. The definitions for the different climate zones used within this study can be found in the “Building America Best Practice Series Volume 7.3 Guide to Determining Climate Regions by County” [9]. Charlson scores were calculated using ICD codes. Patients were included in this study if they were 18 years of age or older and had a lower extremity sample taken after the date of diabetes diagnosis that yielded microbiology culture results within the study time frame. Samples were further divided by type of culture including bone, tissue, abscess/fluid, or swab. Cultures were only included if they were taken from below the malleolus. Site of culture and culture comments were reviewed to ensure that it was a foot culture. Cultures were only included if they were associated with an appropriate ICD code (for diabetic foot infection, foot ulceration, or osteomyelitis) within 24 hours before to 7 days after culture was obtained, if a patient was receiving antibiotics within 24 hours of a culture being obtained, or if a patient had a lower extremity amputation within 30 days of the culture.
Each P. aeruginosa culture was included only once every 30 days to prevent duplication. The first culture in this time frame was used, prioritizing bone, then tissue, then abscess/fluid, and finally swab. Percentages of P. aeruginosa for each climate zone were calculated using R Studio based on amount of P. aeruginosa in cultures taken from diabetic foot wounds. A heat map was also generated based on these percentages. Percentages of P. aeruginosa for each climate zone were calculated based on amount of P. aeruginosa in cultures taken from diabetic foot wounds. A heat map was generated based on these percentages. To show distribution of P. aeruginosa rates throughout each state, a second map was constructed by taking an inverse distance weighted average of percentages over VA locations for each county within the mainland United States. Python's Matplotlib library was used for rendering, and Geopandas for parsing and manipulating the geographical data.
In the contingency table, statistics were conducted using analysis of variance for continuous variables. For categorical data, a chi-square test was used. To ensure that climate was a contributor to rates of P. aeruginosa, other risk factors were included in a multivariable logistic regression analysis. The factors included a dichotomized Charlson score (0–5 vs >5), age, history of osteomyelitis in the past 90 days, admission in the past 90 days, if the culture was taken as an inpatient, if the patient had a Pseudomonas history in the prior 90 days, use of antibiotics specifically active against P. aeruginosa in the prior 90 days, and, finally, culture sample type. A patient was deemed to have a Pseudomonas history in the prior 90 days if they had any cultures, from any sites, that were positive for Pseudomonas in the 90 days preceding their foot culture. Adjusted odds ratios and 97.5% CIs were reported.
Patient Consent
The design of this study was approved by the Research and Development Committee (RDC). The patients’ written consent was not obtained as this study was deemed exempt by the RDC.
RESULTS
A total of 100 070 below-the-malleolus cultures from diabetic patients were identified in this study. The different climate designation groups were well matched in terms of cumulative comorbidities (Supplementary Table 1). The mean Charlson score, a marker of comorbidity burden, was similar across groups at ∼5.5 (Table 1).
Table 1.
Patient Characteristics and Comorbidities
| Cool Dry | Cool Humid | Dry | Hot Dry | Hot Humid | Humid | Marine | Very Cold | Warm Marine | P Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | 4200 (4.2) | 26 035 (26.0) | 1882 (1.9) | 12 629 (12.6) | 26 427 (26.4) | 23 795 (23.8) | 3292 (3.3) | 48 (0.05) | 1762 (1.8) | |
| Age, mean (SD), y | 67.4 (9.7) | 67.6 (10.0) | 68.3 (10.3) | 66.1 (9.7) | 65.9 (9.9) | 66.8 (9.9) | 66.3 (9.3) | 67.0 (9.8) | 68.2 (9.2) | <.001 |
| Male | 4121 (98.1) | 25 576 (98.2) | 1847 (98.1) | 12 455 (98.6) | 25 887 (98.0) | 23 449 (98.5) | 3231 (98.1) | 45 (93.8) | 1736 (98.5) | <.001 |
| Black Other White |
142 (3.4) 353 (8.4) 3705 (88.2) |
3128 (12.0) 1502 (5.8) 21 405 (82.2) |
63 (3.3) 426 (22.6) 1393 (74.0) |
1857 (14.7) 1609 (12.7) 9163 (72.6) |
7000 (26.5) 1780 (6.7) 17 647 (66.8) |
5179 (21.8) 1124 (4.7) 17 492 (73.5) |
201 (6.1) 427 (13.0) 2664 (80.9) |
8 (16.7) 4 (8.3) 36 (75.0) |
254 (14.4) 268 (15.2) 1240 (70.4) |
<.001 |
| Charlson score, mean (SD) | 5.5 (2.7) | 5.9 (2.8) | 5.6 (2.8) | 5.4 (2.8) | 5.8 (2.9) | 6.0 (2.9) | 5.6 (2.8) | 4.3 (2.1) | 5.8 (2.9) | <.001 |
| Charlson score >5 | 1974 (47.0) | 13 344 (51.3) | 870 (46.2) | 5513 (43.7) | 13 000 (49.2) | 12 372 (52.0) | 1502 (45.6) | 13 (27.1) | 853 (48.4) | <.001 |
| Charlson score 0–5 (%) | 2226 (53.0) | 12 691 (48.7) | 1012 (53.8) | 7116 (56.3) | 13 427 (50.8) | 11 423 (48.0) | 1790 (54.4) | 35 (72.9) | 909 (51.6) | <.001 |
| Admit within 1 y before specimen | 2100 (50.0) | 14 054 (54.0) | 875 (46.5) | 7139 (56.5) | 14 598 (55.2) | 13 449 (56.5) | 1911 (58.0) | 1 (2.1) | 1046 (59.4) | <.001 |
| Osteomyelitis in past 90 d | 684 (16.3) | 4849 (18.6) | 252 (13.4) | 2367 (18.7) | 6188 (23.4) | 4716 (19.8) | 765 (23.2) | 5 (10.4) | 442 (25.1) | <.001 |
| Admitted in the past 90 d | 661 (15.7) | 4726 (18.2) | 268 (14.2) | 2314 (18.3) | 5027 (19.0) | 4343 (18.3) | 642 (19.5) | 0 (0) | 389 (22.1) | <.001 |
| Culture obtained while inpatient | 1814 (43.2) | 12 765 (49.0) | 837 (44.5) | 6365 (50.4) | 14 363 (54.3) | 10 901 (45.8) | 1642 (49.9) | 1 (2.1) | 870 (49.4) | <.001 |
| Pseudomonas history in prior 90 d | 72 (1.7) | 807 (3.1) | 50 (2.7) | 606 (4.8) | 1038 (3.9) | 1166 (4.9) | 90 (2.7) | 0 (0) | 98 (5.6) | <.001 |
| Antipseudomonal antibiotic received in prior 90 d | 833 (19.8) | 8402 (32.3) | 559 (29.7) | 4313 (34.2) | 10 336 (39.1) | 8478 (35.6) | 1386 (42.1) | 7 (14.6) | 583 (33.1) | <.001 |
| Culture type: Abscess Bone Swab Tissue |
285 (6.8) 161 (3.8) 2938 (70.0) 816 (19.4) |
1930 (7.4) 3927 (15.1) 17 402 (66.8) 2776 (10.7) |
77 (4.1) 105 (5.6) 1500 (79.7) 200 (10.6) |
996 (7.9) 1293 (10.2) 8971 (71.0) 1369 (10.8) |
2786 (10.5) 3877 (14.7) 16 600 (62.8) 3164 (12.0) |
1327 (5.6) 2714 (11.4) 16 159 (67.9) 3595 (15.1) |
214 (6.5) 722 (21.9) 1470 (44.7) 886 (26.9) |
1 (2.1) 2 (4.2) 45 (93.8) 0 (0) |
49 (2.8) 385 (21.9) 719 (40.8) 609 (34.6) |
<.001 |
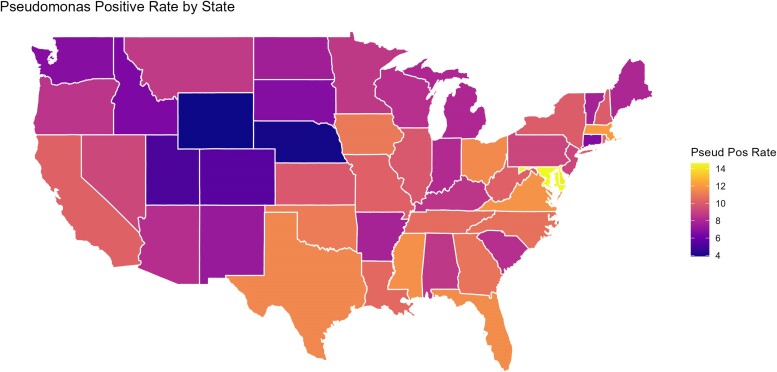
The prevalence of P. aeruginosa ranged from 6.2% to 11.6% within the different US climate designations. The average prevalence of P. aeruginosa in the United States was 10.1% in this study. P. aeruginosa was most common in the Hot Humid climate designation, where it was identified in 11.6% of DFI cultures. P. aeruginosa was least prevalent in the Very Cold climate designation, where it was identified in only 6.2% of DFI cultures. P. aeruginosa was also seen in 6.4%, 8.9%, 6.8%, 10.7%, 11.0%, 7.4%, and 8.2% of DFI cultures in the Cool Dry, Cool Humid, Dry, Hot Dry, Humid, Marine, and Warm Marine climate zones, respectively (Table 2). Locations of the various zones/percentages are located in Figure 1.
Table 2.
Pseudomonas aeruginosa Prevalence
| Cool Dry | Cool Humid | Dry | Hot Dry | Hot Humid | Humid | Marine | Very Cold | Warm Marine | P Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa present, No. (%) | 269 (6.4) | 2312 (8.9) | 128 (6.8) | 1347 (10.7) | 3056 (11.6) | 2621 (11.0) | 243 (7.4) | 3 (6.2) | 145 (8.2) | <.001 |
Figure 1.
Heat map of Pseudomonas aeruginosa prevalence.
In the multivariable logistic regression model, hot and humid climates were associated with an odds of P. aeruginosa of 1.92 (95% CI, 1.69–2.20). A hot, dry climate was associated with an odds of P. aeruginosa of 1.65 (97.5% CI, 1.44–1.90). A humid climate zone was associated with an odds of P. aeruginosa of 1.65 (97.5% CI, 1.45–1.89). Those patients with a lower Charlson Comorbidity Index were less likely to have P. aeruginosa (odds ratio [OR], 0.83; 97.5% CI, 0.79–0.87). Admission in the prior 90 days increased the odds of P. aeruginosa (OR, 1.34; 97.5% CI, 1.27–1.41). A culture taken as an inpatient was associated with a lower odds of P. aeruginosa (OR, 0.90; 97.5% CI, 0.86–0.94). History of P. aeruginosa in the 90 days preceding the culture was associated with an increase in P. aeruginosa (OR, 8.90; 97.5% CI, 8.29–9.56). History of treatment with an antipseudomonal antibiotic in the prior 90 days was associated with a decreased odds of P. aeruginosa (OR, 0.82; 97.5% CI, 0.78–0.86). Finally, swabs were more likely to have P. aeruginosa compared with tissue and bone (Table 3)
Table 3.
Odds of P. aeruginosa
| Odds Ratio | Confidence Interval | |
|---|---|---|
| Climate zones | ||
| Cool Humid | 1.37 | 1.20–1.57 |
| Dry | 1.01 | 0.81–1.26 |
| Hot Dry | 1.65 | 1.44–1.90 |
| Hot Humid | 1.92 | 1.69–2.20 |
| Humid | 1.65 | 1.45–1.89 |
| Marine | 1.20 | 1.0–1.44 |
| Very Cold | 1.10 | 0.26–3.04 |
| Warm Marine | 1.11 | 0.89–1.38 |
| Patient factors | ||
| Age | 1.02 | 1.01–1.02 |
| Male | 1.49 | 1.23–1.83 |
| Charlson score 0–5 | 0.83 | 0.79–0.87 |
| Admitted within the past 90 d | 1.34 | 1.27–1.41 |
| Osteomyelitis in the past 90 d | 1.05 | 0.99–1.11 |
| Inpatient specimen | 0.90 | 0.86–0.94 |
| History of Pseudomonas in the past 90 d | 8.90 | 8.29–9.56 |
| Received antipseudomonal antibiotic within the past 90 d | 0.82 | 0.78–0.86 |
| Type of culture | ||
| Bone | 1.40 | 1.25–1.56 |
| Swab | 1.53 | 1.39–1.68 |
| Tissue | 1.42 | 1.28–1.59 |
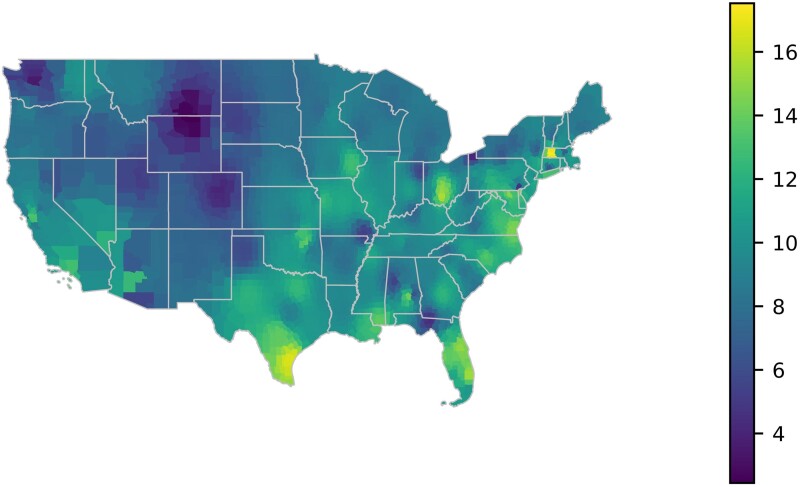
Percentages of P. aeruginosa for each climate zone were calculated based on amount of P. aeruginosa in cultures taken from diabetic foot wounds. A heat map was generated based on these percentages (Figure 1). To show the distribution of P. aeruginosa rates throughout each state, a second map (Figure 2) was constructed by taking an inverse distance weighted average of percentages over VA locations for each county within the mainland United States.
Figure 2.
Distribution of P. aeruginosa rates throughout each state.
DISCUSSION
The average prevalence of P. aeruginosa seen in this study is similar to results of prior studies. In a global epidemiologic study conducted by Garousi et al., the prevalence of P. aeruginosa in diabetic foot ulcer infection in Asia, Africa, and Western countries was 18.5%, 16.3%, and 11.1%, respectively [3]. In a meta-analysis conducted by Coyle et al., the pooled estimation of prevalence of P. aeruginosa in DFI in the United States specifically was 8.06% based on the 10 studies examined, which included a total of 9513 patients, but varied from 4.46% to 14.71% between studies [10].
Findings of this study reinforce the correlation between climate and prevalence of P. aeruginosa seen in previous studies. The IWGDF/IDSA guidelines indicate that P. aeruginosa is not as prevalent in North America or Europe but is more common in the tropical or subtropical climates [7]. This recommendation is based on a study that prospectively examined the bacteriology profile of DFI specimens from 522 patients in Turkey. Interestingly, despite Turkey falling mostly within the temperate climate designation, the authors of this study concluded that P. aeruginosa should be empirically targeted by clinicians in Turkey as it was identified in 12.4% of DFIs [8]. In our study, we found that P. aeruginosa was more prevalent in hot and humid climates. These findings are consistent with a previous study by Spichler et al., which found that P. aeruginosa is more likely to be the causative DFI pathogen in hot, humid climates [11].
The difference in P. aeruginosa prevalence within different US climate designations seen in this study highlights the importance of considering local climate trends when deciding if empiric P. aeruginosa coverage is warranted when treating DFIs. While the 2023 IWGDF/IDSA guidelines recommend against empiric P. aeruginosa coverage for DFIs in the United States based on general global climate trends, they do not clarify what P. aeruginosa prevalence is significant enough to warrant empiric coverage [7]. While a local P. aeruginosa prevalence of 6.2% may not warrant empiric coverage, a local prevalence of 11.6% may be more clinically significant, necessitating empiric coverage depending on the severity of infection. Even accounting for various risk factors for P. aeruginosa, warmer and more humid environments had a higher odds of P. aeruginosa. In the multivariable logistic regression model, hot and humid climates were associated with an odds of P. aeruginosa of 1.92 (97.5% CI, 1.69–2.20). A hot, dry climate was associated with an odds of P. aeruginosa of 1.65 (97.5% CI, 1.44–1.90). A humid climate zone was associated with an odds of P. aeruginosa of 1.65 (97.5% CI, 1.45–1.89). Future recommendations based on climate and local prevalence patterns may be more applicable to clinicians vs those rooted in general climate designations. In the meantime, it is likely beneficial for clinicians to consider the guideline recommendations based on climate in conjunction with local prevalence patterns when deciding whether empiric P. aeruginosa coverage is warranted when treating DFIs.
It is also pertinent to consider the impact of changing global weather patterns on the prevalence of P. aeruginosa. The National Oceanic and Atmospheric Administration (NOAA) reports that 2023 was the warmest year since global records began in 1850 by a wide margin [12]. Mean global temperatures are expected to continue to rise in the upcoming years, even with attempts to slow or limit yearly emissions [13, 14]. Based on the findings of this study and previous studies that P. aeruginosa is more prevalent in warmer climate conditions, it is likely that the prevalence of P. aeruginosa will increase in upcoming years as global temperatures increase. This further places importance on regularly examining P. aeruginosa prevalence and climate patterns to guide empiric antibiotic selection when treating DFIs.
The strengths of this study include its large sample size and representation of P. aeruginosa prevalence from across the United States. A total of 100 070 lower extremity cultures from diabetic patients were identified in this study, which is a much larger sample size than prior studies examining the prevalence of P. aeruginosa within the United States [6]. Individuals within the different climate designation groups in this study were relatively well matched in comorbidities, allowing for better comparison between groups. Utilization of IECC climate designations allowed us to examine the prevalence of microbes within various local climates within the United States. To our knowledge, this is the first study to examine the correlation between local US climate designations and the prevalence of P. aeruginosa.
The limitations of this study include its retrospective nature and limitations in the data we were able to obtain via SQL coding. In this study, a patient was assumed to have a DFI if they had documented lower extremity microbiology culture results following a diagnosis of diabetes. Other signs/symptoms of infection were not assessed, which may have led to us including microbiological data from colonized, below-the-malleolus cultures. An additional limitation was the variation in number of cultures obtained from patients within different US climate designations. For example, there were 26 427 cultures obtained within the Hot Humid climate designation compared with only 48 cultures from the Very Cold climate designation. The difference in representation of different climate designations is likely related to differences in population density within areas of the United States and makes it more challenging to accurately compare microbiology between local climates. Additionally, the results of this study may not be generalizable beyond our study population of predominantly Caucasian, male veterans. Older patients tend to be at higher risk of infection and more health care exposure, which can increase the risk of acquisition of nosocomial pathogens, such as P. aeruginosa.
CONCLUSIONS
The prevalence of P. aeruginosa varies within different US climate designations. Utilization of local climate information and P. aeruginosa prevalence may allow for more accurate and targeted empiric antibiotic selection when treating DFIs. Further studies are needed to determine a threshold for which empirical P. aeruginosa treatment should be considered.
Supplementary Material
Acknowledgments
Author contributions. Rebecca Winski, PharmD—primary investigator, primary author; Jiachen Xu, PharmD—data collection; Jonathon Townsend—manuscript preparation; Arthur Chan, PharmD—data collection, original manuscript preparation; Bethany Wattengel, BCPS, BCIDP—manuscript preparation; Mathew Davis, PharmD—manuscript preparation; Andrew Puckett, MD—manuscript preparation; Kyle Huntsman, MD—manuscript preparation; Ashley O’Leary, PharmD, AAHIVP—manuscript preparation; Kari Mergenhagen, PharmD, BCPS, BCIDP—senior investigator.
Disclaimer. The contents of this article do not reflect or represent the views of the Department of Veterans Affairs or the United States Government.
Financial support. No funding was provided for this research study.
Contributor Information
Rebecca Winski, Department of Pharmacy, Veterans Affairs Western New York Healthcare System, Buffalo, New York, USA.
Jiachen Xu, Department of Pharmacy, Veterans Affairs Western New York Healthcare System, Buffalo, New York, USA.
Jonathan Townsend, Department of Pharmacy, Veterans Affairs Western New York Healthcare System, Buffalo, New York, USA.
Arthur Chan, Department of Pharmacy, Veterans Affairs Western New York Healthcare System, Buffalo, New York, USA.
Bethany A Wattengel, Department of Pharmacy, Veterans Affairs Western New York Healthcare System, Buffalo, New York, USA.
Matthew Davis, Department of Pharmacy, Veterans Affairs Western New York Healthcare System, Buffalo, New York, USA.
Andrew Puckett, Department of Pharmacy, Veterans Affairs Western New York Healthcare System, Buffalo, New York, USA.
Kyle Huntsman, Department of Pharmacy, Veterans Affairs Western New York Healthcare System, Buffalo, New York, USA.
Ashley L O’Leary, D’Youville University School of Pharmacy, Buffalo, New York, USA.
Kari A Mergenhagen, Department of Pharmacy, Veterans Affairs Western New York Healthcare System, Buffalo, New York, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Centers for Disease Control and Prevention . National diabetes statistics report: estimates of diabetes and its burden in the United States. 2023. Available at: https://www.cdc.gov/diabetes/php/data-research/index.html. Accessed November 29, 2023. [Google Scholar]
- 2. McDermott K, Fang M, Boulton AJM, Selvin E, Hicks CW. Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care 2023; 46:209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garousi M, MonazamiTabar S, Mirazi H, Farrokhi Z, Khaledi A, Shakerimoghaddam A. Epidemiology of Pseudomonas aeruginosa in diabetic foot infections: a global systematic review and meta-analysis. Germs 2023; 13:362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prasad ASB, Shruptha P, Prabhu V, et al. Pseudomonas aeruginosa virulence proteins pseudolysin and protease IV impede cutaneous wound healing. Lab Invest 2020; 100:1532–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mena KD, Gerba CP. Risk assessment of Pseudomonas aeruginosa in water. Rev Environ Contam Toxicol 2009; 201:71–115. [DOI] [PubMed] [Google Scholar]
- 6. Tribelli PM, Lopez NI. Insights into the temperature responses of Pseudomonas species in beneficial and pathogenic host interactions. Appl Microbiol Biotechnol 2022; 106:7699–709. [DOI] [PubMed] [Google Scholar]
- 7. Senneville E, Albalawi Z, van Asten SA, et al. IWGDF/IDSA guidelines on the diagnosis and treatment of diabetes-related foot infections (IWGDF/IDSA 2023). Diabetes Metab Res Rev 2024; 40:e3687. [DOI] [PubMed] [Google Scholar]
- 8. Hatipoglu M, Mutluoglu M, Turhan V, et al. Causative pathogens and antibiotic resistance in diabetic foot infections: a prospective multi-center study. J Diabetes Complications 2016; 30:910–6. [DOI] [PubMed] [Google Scholar]
- 9. Michael Beachler TG, Cole P, Hefty M, Ruiz K. Building America Best Practice Series Volume 7.3 Guide to Determining Climate Regions by County. US Department of Energy; 2015. [Google Scholar]
- 10. Coye T, Foote C, Stasko PA. Prevalence of Pseudomonas aeruginosa in diabetic foot infections in the United States: a systematic review and meta-analysis. Foot Ankle Surg Techniques Reports Cases 2022; 2:100189. [Google Scholar]
- 11. Spichler A, Hurwitz BL, Armstrong DG, Lipsky BA. Microbiology of diabetic foot infections: from Louis Pasteur to ‘crime scene investigation.’ BMC Med 2015; 13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Oceanic and Atmospheric Administration . Annual 2023 Global Climate Report. National Oceanic and Atmospheric Administration; 2024.
- 13. United States Global Change Research Program . Climate Science Special Report (CSSR) Fourth National Assessment (NCA4). United States Global Change Research Program; 2017. [Google Scholar]
- 14. Bell DA, Kovach RP, Muhlfeld CC, et al. Climate change and expanding invasive species drive widespread declines of native trout in the northern Rocky Mountains, USA. Sci Adv 2021; 7:eabj5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.