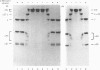
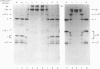
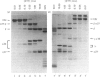
Abstract
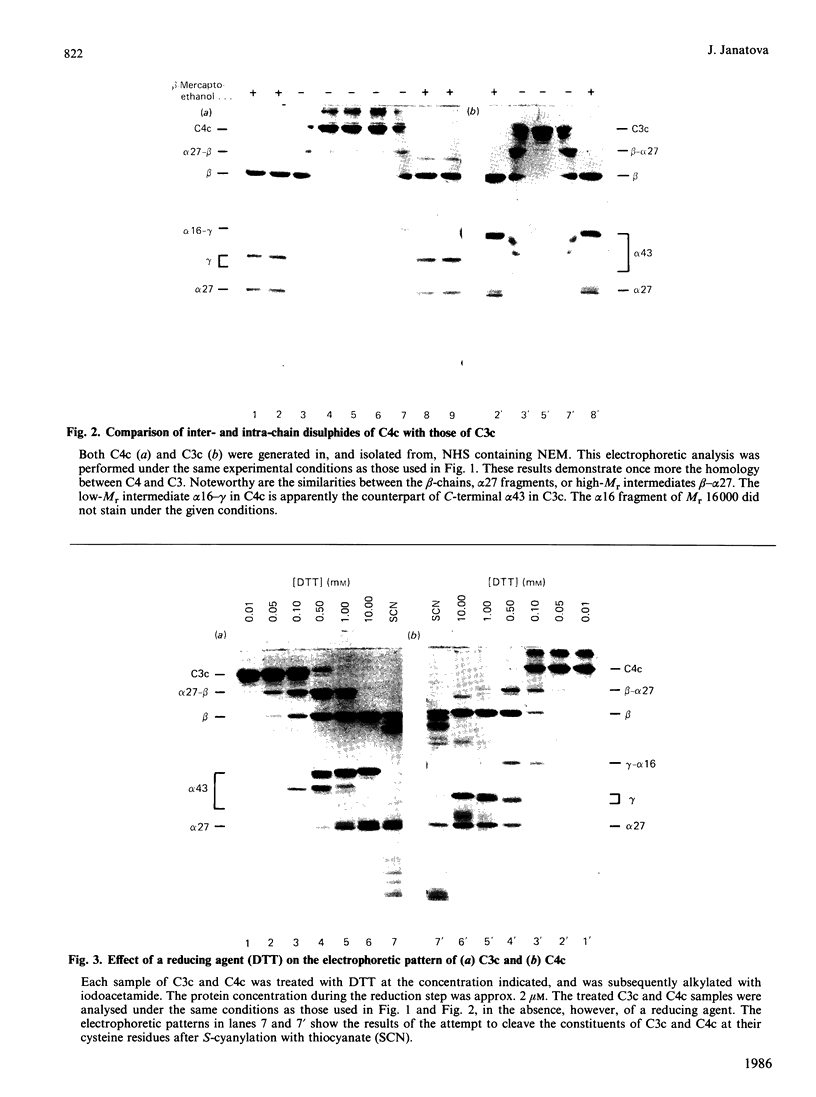
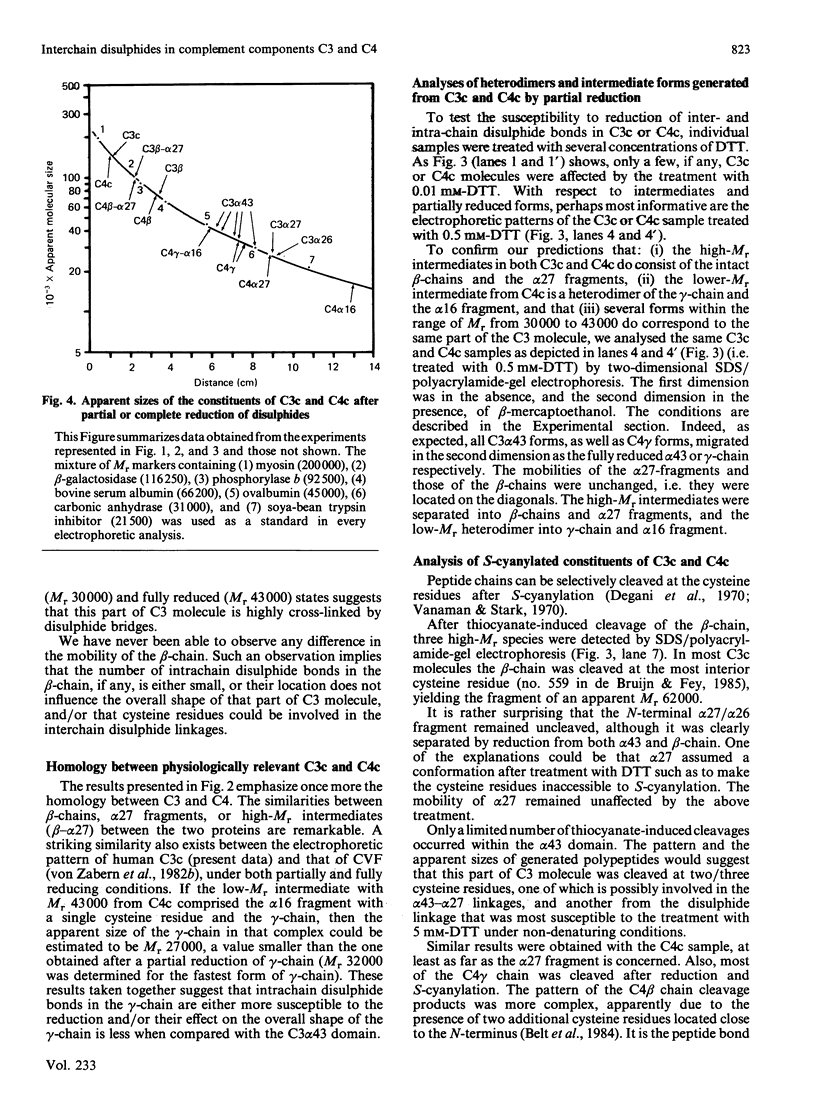
Disulphide bonds contribute significantly to the maintenance of structural/functional integrity of many proteins. Therefore it was of interest to study the distribution and the effect of disulphides on conformation of complement components C3 and C4. These proteins are precursors of several fragments with various binding sites and distinct physiological functions. The constituents of C3c (beta, alpha 27, alpha 43) and those of C4c (beta, alpha 27, alpha 16, gamma) were investigated, since other fragments of C3 or C4 do not participate in interchain linkages. Inter-and intra-chain disulphide bonds in C3c and C4c were localized by using a modification of conventional SDS (sodium dodecyl sulphate)/polyacrylamide-gel electrophoresis such that the change in mobility of disulphide-bond-containing proteins can be detected throughout the transition from a non-reduced to a fully reduced state. Several forms of the alpha 43 fragment from C3, and of the gamma-chain of C4, with different mobilities can exist, depending on the number of intra-chain disulphide bonds reduced. The intermediates (heterodimers) generated by a partial reduction of C3c or C4c were characterized by two-dimensional SDS/polyacrylamide-gel electrophoresis performed in the absence, then in the presence, of beta-mercaptoethanol. The inter-chain linkages in C3c were determined to be beta-alpha 27 and alpha 27- alpha 43, thus indicating the presence of only one interchain bond in C3. The two interchain bonds in C4c are beta-alpha 27 and alpha 16-gamma. The third interchain bond in C4 (alpha 27-gamma, tentative) remains to be determined.
Full text
PDF

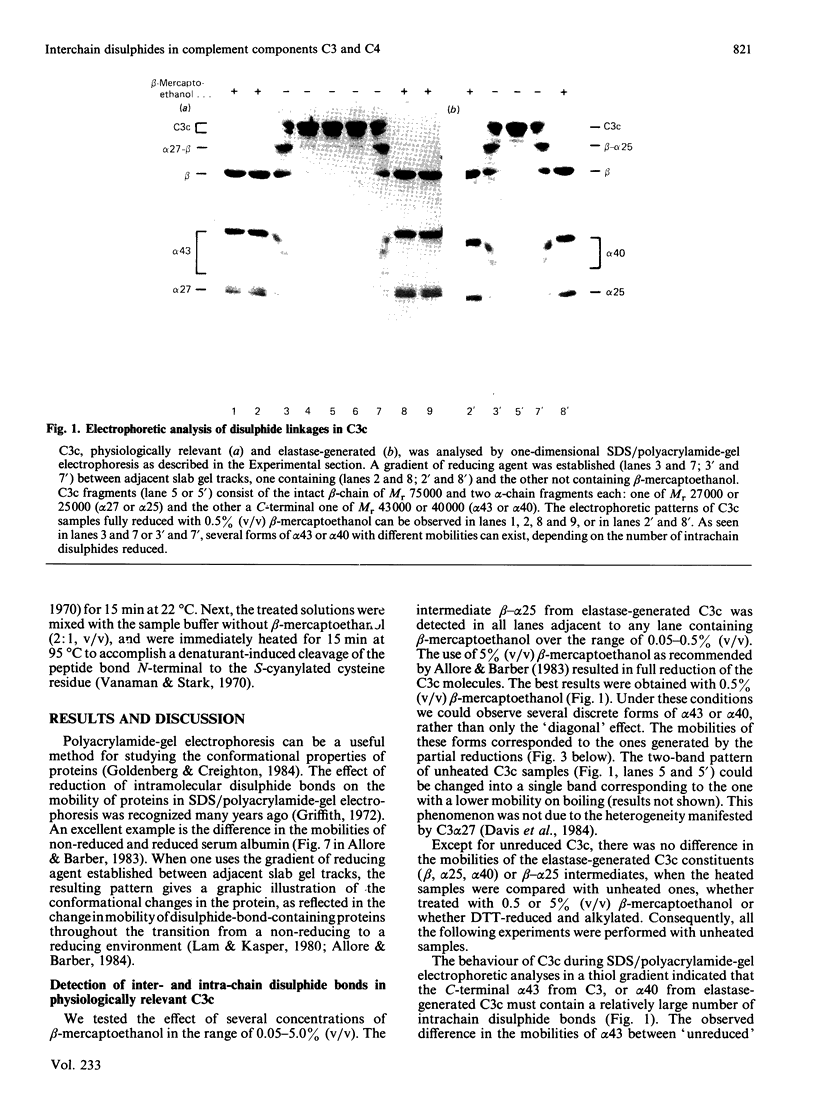


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allore R. J., Barber B. H. A recommendation for visualizing disulfide bonding by one-dimensional sodium dodecyl sulfate--polyacrylamide gel electrophoresis. Anal Biochem. 1984 Mar;137(2):523–527. doi: 10.1016/0003-2697(84)90121-0. [DOI] [PubMed] [Google Scholar]
- Allore R. J., Barber B. H. Inter- and intramolecular disulfide bonding among lymphocyte plasma membrane proteins and glycoproteins. Mol Immunol. 1983 Apr;20(4):383–395. doi: 10.1016/0161-5890(83)90020-2. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Colten H. R. Complement C3 receptors: structure and function. Mol Immunol. 1984 Dec;21(12):1191–1199. doi: 10.1016/0161-5890(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Belt K. T., Carroll M. C., Porter R. R. The structural basis of the multiple forms of human complement component C4. Cell. 1984 Apr;36(4):907–914. doi: 10.1016/0092-8674(84)90040-0. [DOI] [PubMed] [Google Scholar]
- Davis A. E., 3rd, Harrison R. A., Lachmann P. J. Physiologic inactivation of fluid phase C3b: isolation and structural analysis of C3c, C3d,g (alpha 2D), and C3g. J Immunol. 1984 Apr;132(4):1960–1966. [PubMed] [Google Scholar]
- Davis A. E., 3rd, Harrison R. A. Structural characterization of factor I mediated cleavage of the third component of complement. Biochemistry. 1982 Nov 9;21(23):5745–5749. doi: 10.1021/bi00266a003. [DOI] [PubMed] [Google Scholar]
- Degani Y., Neumann H., Patchornik A. Selective cyanylation of sulfhydryl groups. J Am Chem Soc. 1970 Nov 18;92(23):6969–6971. doi: 10.1021/ja00726a043. [DOI] [PubMed] [Google Scholar]
- Fujita T., Gigli I., Nussenzweig V. Human C4-binding protein. II. Role in proteolysis of C4b by C3b-inactivator. J Exp Med. 1978 Oct 1;148(4):1044–1051. doi: 10.1084/jem.148.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger G., Abraham G. N., Williams J., Colten H. R. NH2-terminal sequence analysis of pro-C4, the precursor of the fourth component of guinea pig complement. J Biol Chem. 1980 Aug 10;255(15):7071–7074. [PubMed] [Google Scholar]
- Goldenberg D. P., Creighton T. E. Gel electrophoresis in studies of protein conformation and folding. Anal Biochem. 1984 Apr;138(1):1–18. doi: 10.1016/0003-2697(84)90761-9. [DOI] [PubMed] [Google Scholar]
- Griffith I. P. The effect of cross-links on the mobility of proteins in dodecyl sulphate-polyacrylamide gels. Biochem J. 1972 Feb;126(3):553–560. doi: 10.1042/bj1260553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenman D. E., Sundsmo J. S., Cooper N. R. The effects of mild reduction on the structure and function of C3. J Immunol. 1980 Oct;125(4):1798–1805. [PubMed] [Google Scholar]
- Janatova J. The third (C3) and the fourth (C4) components of complement: labile binding site and covalent bond formation. Ann N Y Acad Sci. 1983;421:218–234. doi: 10.1111/j.1749-6632.1983.tb18111.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam K. S., Kasper C. B. Electrophoretic analysis of three major nuclear envelope polypeptides. Topological relationship and sequence homology. J Biol Chem. 1979 Nov 25;254(22):11713–11720. [PubMed] [Google Scholar]
- Lam K. S., Kasper C. B. Selective endogenous phosphorylation of two liver microsomal polypeptides in the presence of micromolar levels of Mg2+ ion. J Biol Chem. 1980 Jan 10;255(1):259–266. [PubMed] [Google Scholar]
- Matsuda T., Seya T., Nagasawa S. Location of the inter-chain disulfide bonds of the third component of human complement. Biochem Biophys Res Commun. 1985 Feb 28;127(1):264–269. doi: 10.1016/s0006-291x(85)80153-4. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Iida K., Mold C., Nussenzweig V. Unique role of the complement receptor CR1 in the degradation of C3b associated with immune complexes. J Exp Med. 1982 Dec 1;156(6):1739–1754. doi: 10.1084/jem.156.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris K. M., Goldberger G., Colten H. R., Aden D. P., Knowles B. B. Biosynthesis and processing of a human precursor complement protein, pro-C3, in a hepatoma-derived cell line. Science. 1982 Jan 22;215(4531):399–400. doi: 10.1126/science.7199205. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Schreiber R. D. Molecular biology and chemistry of the alternative pathway of complement. Adv Immunol. 1980;29:1–53. doi: 10.1016/s0065-2776(08)60042-5. [DOI] [PubMed] [Google Scholar]
- Press E. M., Gagnon J. Human complement component C4. Structural studies on the fragments derived from C4b by cleavage with C3b inactivator. Biochem J. 1981 Nov 1;199(2):351–357. doi: 10.1042/bj1990351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Lambris J. D., Cain J. A., Newman S. L. Generation of three different fragments of bound C3 with purified factor I or serum. I. Requirements for factor H vs CR1 cofactor activity. J Immunol. 1982 Nov;129(5):2051–2060. [PubMed] [Google Scholar]
- Sim E., Wood A. B., Hsiung L. M., Sim R. B. Pattern of degradation of human complement fragment, C3b. FEBS Lett. 1981 Sep 14;132(1):55–60. doi: 10.1016/0014-5793(81)80426-7. [DOI] [PubMed] [Google Scholar]
- Thornton J. M. Disulphide bridges in globular proteins. J Mol Biol. 1981 Sep 15;151(2):261–287. doi: 10.1016/0022-2836(81)90515-5. [DOI] [PubMed] [Google Scholar]
- Vanaman T. C., Stark G. R. A study of the sulfhydryl groups of the catalytic subunit of Escherichia coli aspartate transcarbamylase. The use of enzyme--5-thio-2-nitrobenzoate mixed disulfides as intermediates in modifying enzyme sulfhydryl groups. J Biol Chem. 1970 Jul 25;245(14):3565–3573. [PubMed] [Google Scholar]
- de Bruijn M. H., Fey G. H. Human complement component C3: cDNA coding sequence and derived primary structure. Proc Natl Acad Sci U S A. 1985 Feb;82(3):708–712. doi: 10.1073/pnas.82.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zabern I., Bloom E. L., Chu V., Gigli I. The fourth component of human complement treated with amines or chaotropes or frozen-thawed (C4b-like C4): interaction with C4 binding protein and cleavage by C3b/C4b inactivator. J Immunol. 1982 Mar;128(3):1433–1438. [PubMed] [Google Scholar]
- von Zabern I., Przyklenk H., Vogt W. Chain structure of cobra venom factor from Naja naja and Naja haje venom. Scand J Immunol. 1982 Apr;15(4):357–362. doi: 10.1111/j.1365-3083.1982.tb00659.x. [DOI] [PubMed] [Google Scholar]