Abstract
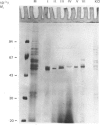
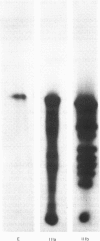
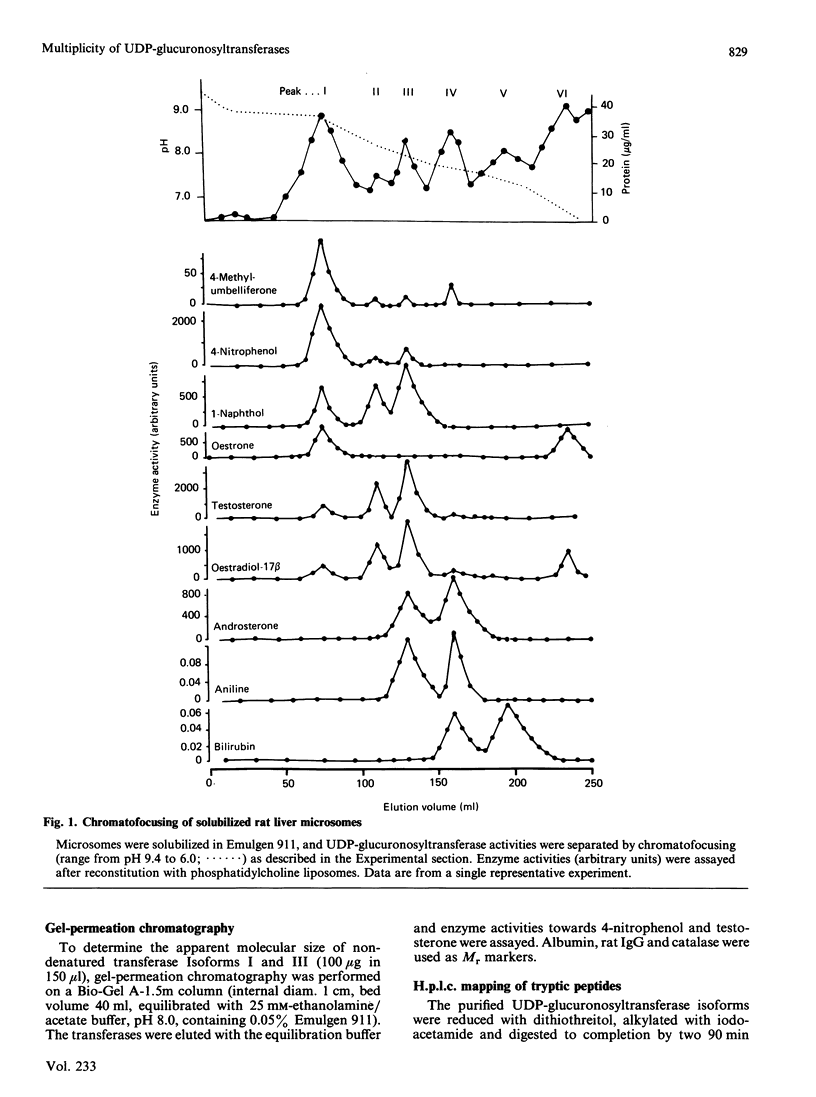
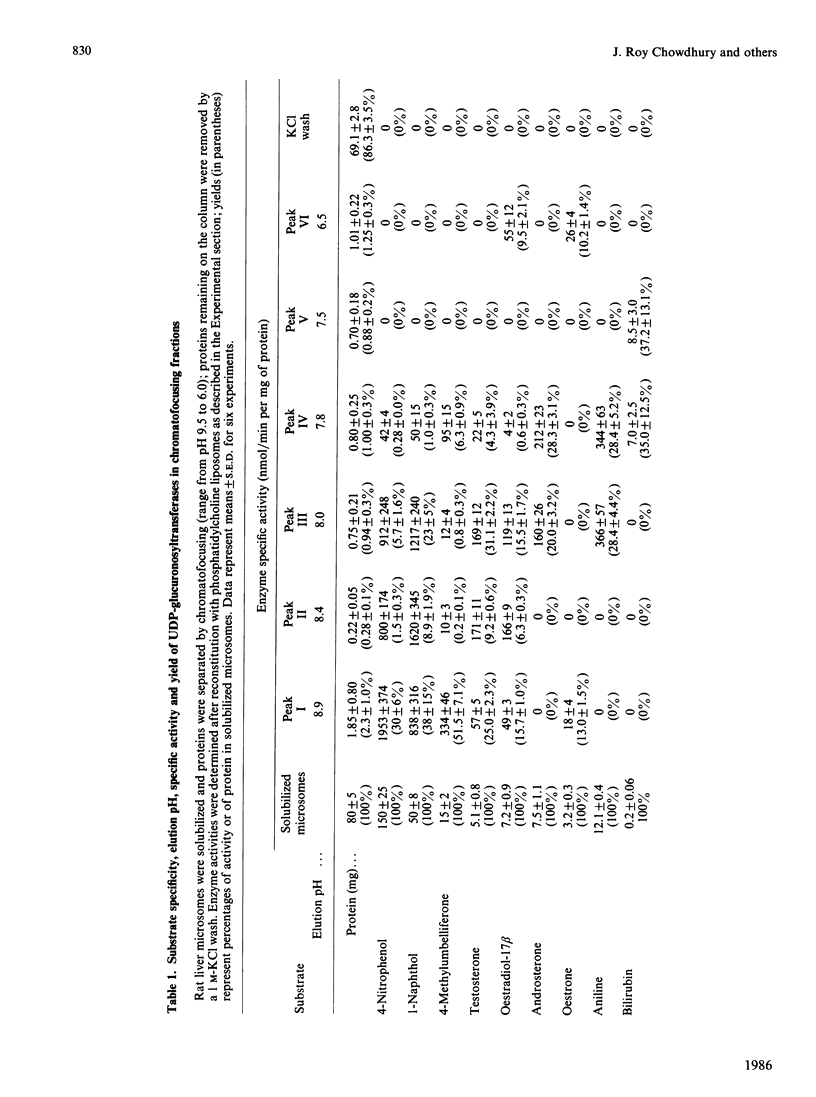
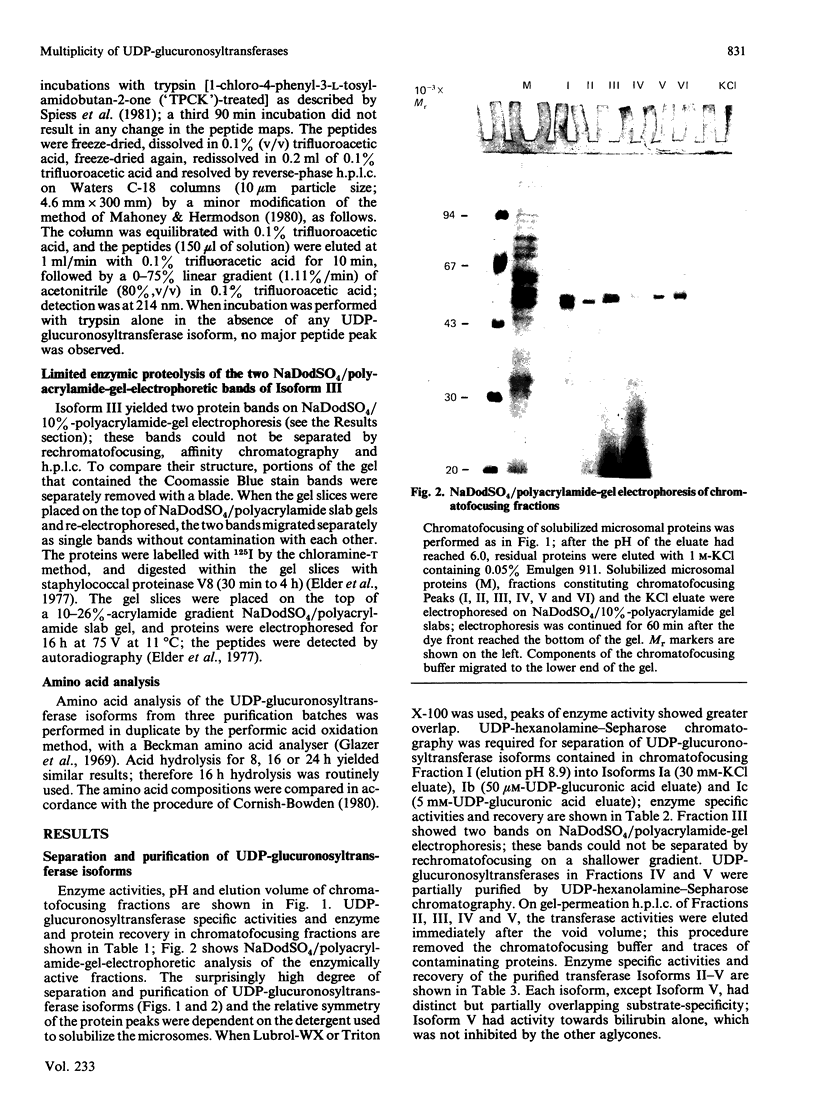
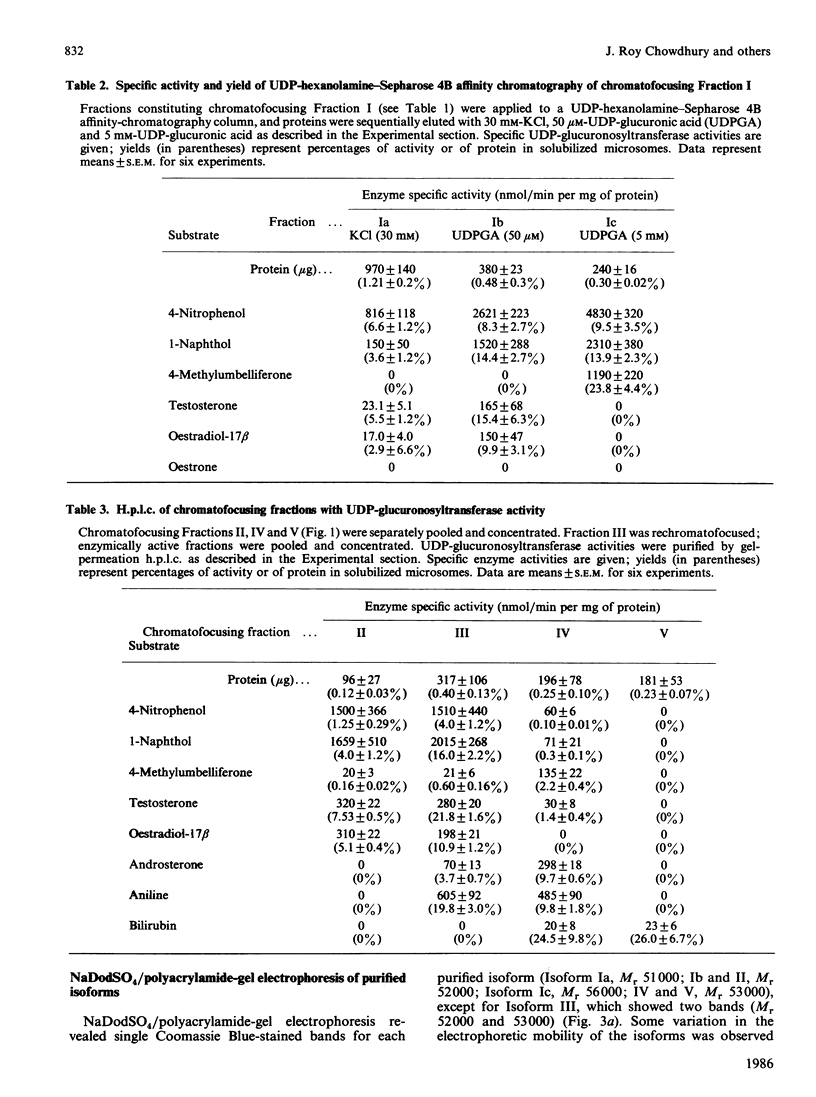
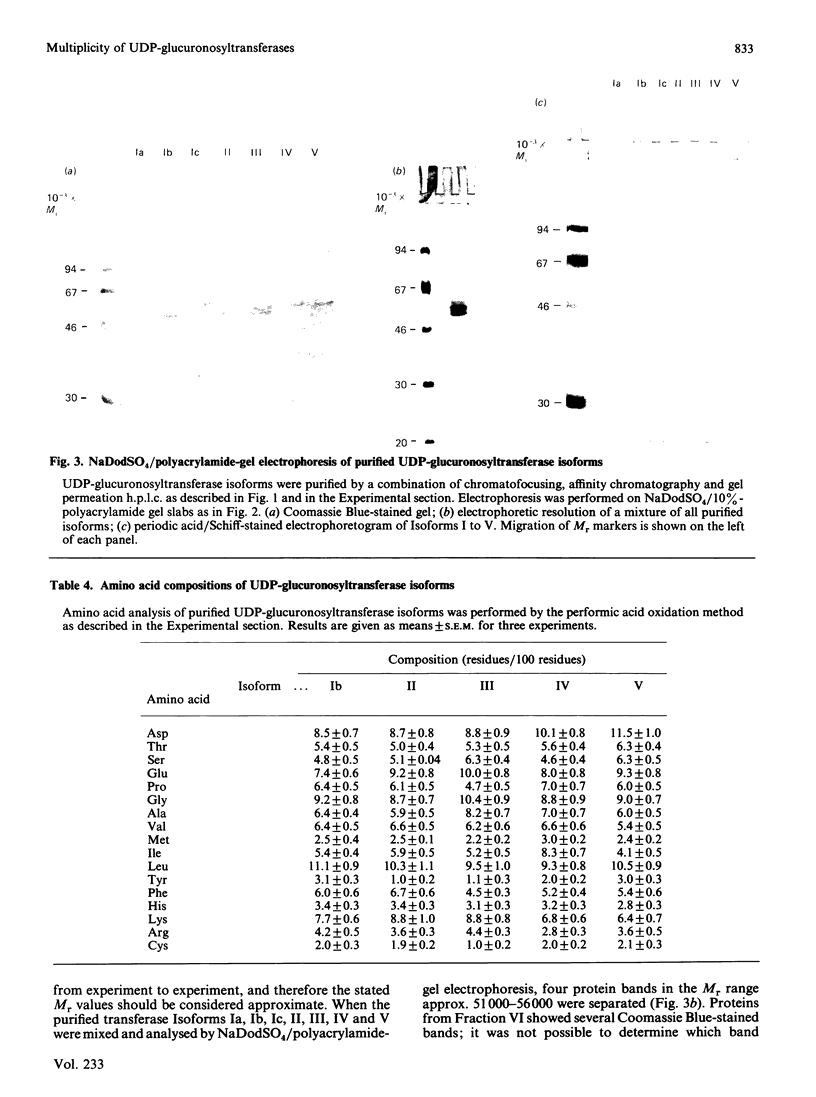
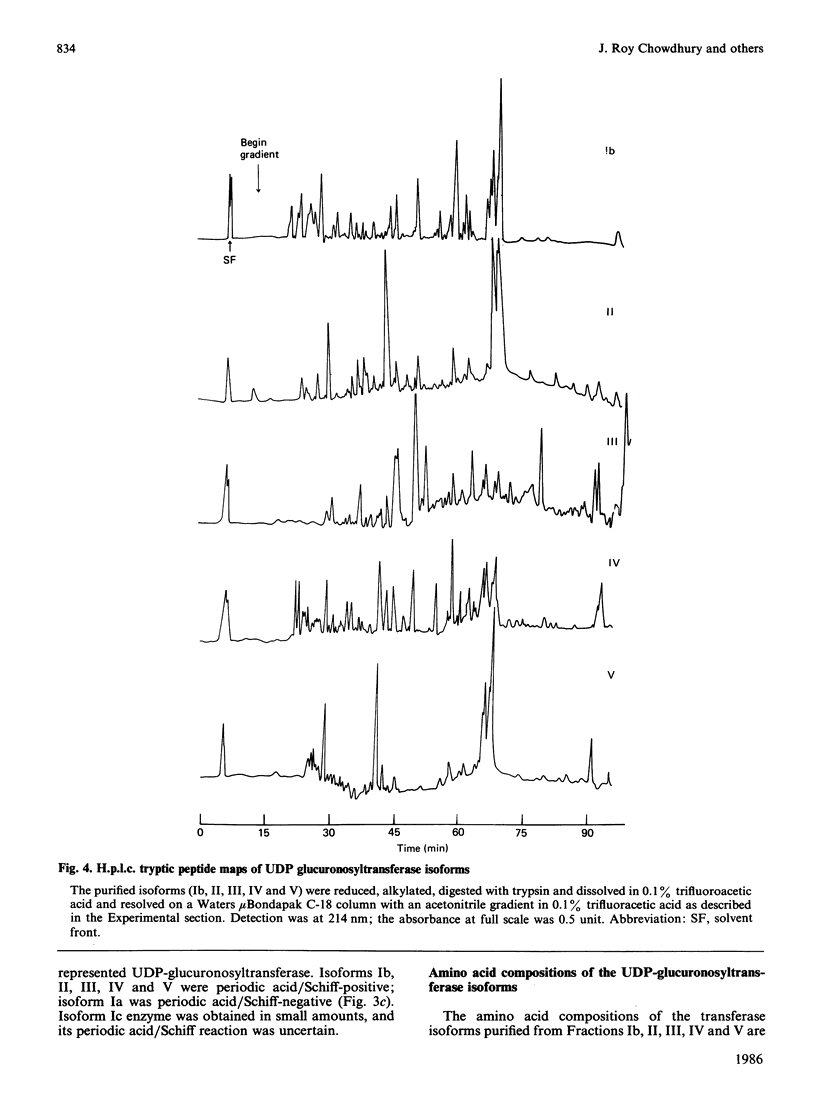
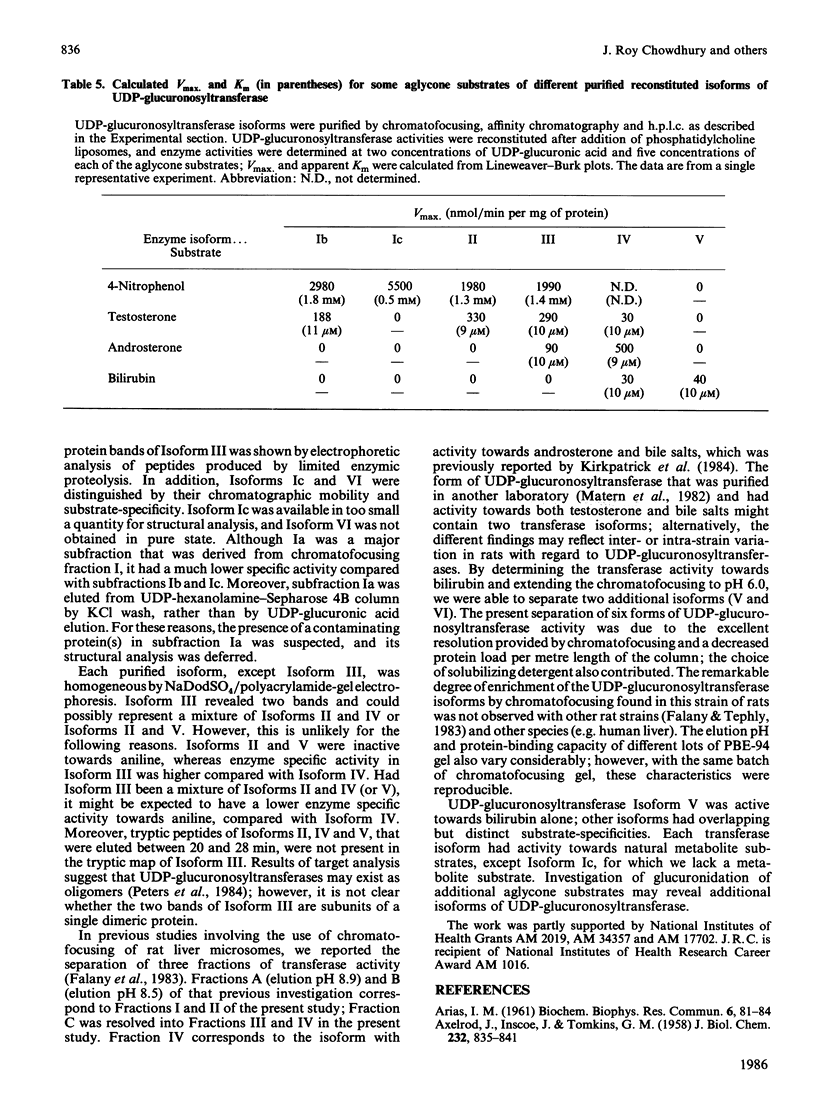
UDP-glucuronosyltransferase (EC 2.4.1.17) activity was solubilized from male Wistar rat liver microsomal fraction in Emulgen 911, and six fractions with the transferase activity were separated by chromatofocusing on PBE 94 (pH 9.4 to 6.0). Fraction I was further separated into Isoforms Ia, Ib and Ic by affinity chromatography on UDP-hexanolamine-Sepharose 4B. UDP-glucuronosyltransferase in Fraction III was further purified by rechromatofocusing (pH 8.7 to 7.5). UDP-glucuronosyltransferases in Fractions IV and V were purified by UDP-hexanolamine-Sepharose chromatography. The transferase isoforms in Fractions II, III, IV and V were finally purified by h.p.l.c. on a TSK G 3000 SW column. Purified UDP-glucuronosyltransferase Isoforms Ia (Mr 51,000), Ib (Mr 52,000), Ic (Mr 56,000), II (Mr 52,000), IV (Mr 53,000) and V (Mr 53,000) revealed single Coomassie Blue-stained bands on sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. Isoform III enzyme showed two bands of Mr 52,000 and 53,000. Comparison of the amino acid compositions by the method of Cornish-Bowden [(1980) Anal. Biochem. 105, 233-238] suggested that all UDP-glucuronosyltransferase isoforms are structurally related. Reverse-phase h.p.l.c. of tryptic peptides of individual isoforms revealed distinct 'maps', indicating differences in primary protein structure. The two bands of Isoform III revealed distinct electrophoretic peptide maps after limited enzymic proteolysis. After reconstitution with phosphatidylcholine liposomes, the purified isoforms exhibited distinct but overlapping substrate specificities. Isoform V was specific for bilirubin glucuronidation, which was not inhibited by other aglycone substrates. Each isoform, except Ia, was identified as a glycoprotein by periodic acid/Schiff staining.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARIAS I. M. Ethereal and N-linked glucuronide formation by normal and Gunn rats in vitro and in vivo. Biochem Biophys Res Commun. 1961 Nov 1;6:81–84. doi: 10.1016/0006-291x(61)90388-6. [DOI] [PubMed] [Google Scholar]
- AXELROD J., INSCOE J. K., TOMKINS G. M. Enzymatic synthesis of N-glucosyluronic acid conjugates. J Biol Chem. 1958 Jun;232(2):835–841. [PubMed] [Google Scholar]
- Bock K. W., Josting D., Lilienblum W., Pfeil H. Purification of rat-liver microsomal UDP-glucuronyltransferase. Separation of two enzyme forms inducible by 3-methylcholanthrene or phenobarbital. Eur J Biochem. 1979 Jul;98(1):19–26. doi: 10.1111/j.1432-1033.1979.tb13155.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burchell B. Substrate specificity and properties of uridine diphosphate glucuronyltransferase purified to apparent homogeneity from phenobarbital-treated rat liver. Biochem J. 1978 Sep 1;173(3):749–757. doi: 10.1042/bj1730749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury J. R., Chowdhury N. R., Moscioni A. D., Tukey R., Tephly T., Arias I. M. Differential regulation by triiodothyronine of substrate-specific uridinediphosphoglucuronate glucuronosyl transferases in rat liver. Biochim Biophys Acta. 1983 Nov 22;761(1):58–65. doi: 10.1016/0304-4165(83)90362-8. [DOI] [PubMed] [Google Scholar]
- Chowdhury J. R., Novikoff P. M., Chowdhury N. R., Novikoff A. B. Distribution of UDPglucuronosyltransferase in rat tissue. Proc Natl Acad Sci U S A. 1985 May;82(9):2990–2994. doi: 10.1073/pnas.82.9.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish-Bowden A. Critical values for testing the significance of amino acid composition indexes. Anal Biochem. 1980 Jul 1;105(2):233–238. doi: 10.1016/0003-2697(80)90450-9. [DOI] [PubMed] [Google Scholar]
- Dutton G. J., Leakey J. E., Pollard M. R. Assays for UDPglucuronyltransferase activities. Methods Enzymol. 1981;77:383–391. doi: 10.1016/s0076-6879(81)77051-4. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Falany C. N., Chowdhury J. R., Chowdhury N. R., Tephly T. R. Steroid 3- and 17-OH UDP-glucuronosyltransferase activities in rat and rabbit liver microsomes. Drug Metab Dispos. 1983 Sep-Oct;11(5):426–432. [PubMed] [Google Scholar]
- Falany C. N., Tephly T. R. Separation, purification and characterization of three isoenzymes of UDP-glucuronyltransferase from rat liver microsomes. Arch Biochem Biophys. 1983 Nov;227(1):248–258. doi: 10.1016/0003-9861(83)90368-5. [DOI] [PubMed] [Google Scholar]
- Jansen P. L., Arias I. M. Delipidation and reactivation of UDPglucuronosyltransferase from rat liver. Biochim Biophys Acta. 1975 May 23;391(1):23–38. [PubMed] [Google Scholar]
- Jansen P. L., Chowdhury J. R., Fischberg E. B., Arias I. M. Enzymatic conversion of bilirubin monoglucuronide to diglucuronide by rat liver plasma membranes. J Biol Chem. 1977 Apr 25;252(8):2710–2716. [PubMed] [Google Scholar]
- Kirkpatrick R. B., Falany C. N., Tephly T. R. Glucuronidation of bile acids by rat liver 3-OH androgen UDP-glucuronyltransferase. J Biol Chem. 1984 May 25;259(10):6176–6180. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lilienblum W., Walli A. K., Bock K. W. Differential induction of rat liver microsomal UDP-glucuronosyltransferase activites by various inducing agents. Biochem Pharmacol. 1982 Mar 15;31(6):907–913. doi: 10.1016/0006-2952(82)90319-7. [DOI] [PubMed] [Google Scholar]
- Lucier G. W., McDaniel O. S. Steroid and non-steroid UDP glucuronyltransferase: glucuronidation of synthetic estrogens as steroids. J Steroid Biochem. 1977 Aug;8(8):867–872. doi: 10.1016/0022-4731(77)90096-6. [DOI] [PubMed] [Google Scholar]
- Mahoney W. C., Hermodson M. A. Separation of large denatured peptides by reverse phase high performance liquid chromatography. Trifluoroacetic acid as a peptide solvent. J Biol Chem. 1980 Dec 10;255(23):11199–11203. [PubMed] [Google Scholar]
- Matern H., Matern S., Gerok W. Isolation and characterization of rat liver microsomal UDP-glucuronosyltransferase activity toward chenodeoxycholic acid and testosterone as a single form of enzyme. J Biol Chem. 1982 Jul 10;257(13):7422–7429. [PubMed] [Google Scholar]
- Peters W. H., Jansen P. L., Nauta H. The molecular weights of UDP-glucuronyltransferase determined with radiation-inactivation analysis. A molecular model of bilirubin UDP-glucuronyltransferase. J Biol Chem. 1984 Oct 10;259(19):11701–11705. [PubMed] [Google Scholar]
- Sadler J. E., Rearick J. I., Paulson J. C., Hill R. L. Purification to homogeneity of a beta-galactoside alpha2 leads to 3 sialyltransferase and partial purification of an alpha-N-acetylgalactosaminide alpha2 leads to 6 sialyltransferase from porcine submaxillary glands. J Biol Chem. 1979 Jun 10;254(11):4434–4442. [PubMed] [Google Scholar]
- Spiess J., Villarreal J., Vale W. Isolation and sequence analysis of a somatostatin-like polypeptide from ovine hypothalamus. Biochemistry. 1981 Mar 31;20(7):1982–1988. doi: 10.1021/bi00510a038. [DOI] [PubMed] [Google Scholar]
- Tukey R. H., Tephly T. R. Purification of properties of rabbit liver estrone and p-nitrophenol UDP-glucuronyltransferases. Arch Biochem Biophys. 1981 Jul;209(2):565–578. doi: 10.1016/0003-9861(81)90314-3. [DOI] [PubMed] [Google Scholar]
- Weatherill P. J., Burchell B. The separation and purification of rat liver UDP-glucuronyltransferase activities towards testosterone and oestrone. Biochem J. 1980 Aug 1;189(2):377–380. doi: 10.1042/bj1890377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherill P. J., Kennedy S. M., Burchell B. Immunochemical comparison of UDP-glucuronyltransferase from Gunn- and Wistar-rat livers. Biochem J. 1980 Oct 1;191(1):155–163. doi: 10.1042/bj1910155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart G. J. Demonstration of functional heterogeneity of hepatic uridine diphosphate glucuronosyltransferase activities after administration of 3-methylcholanthrene and phenobarbital to rats. Biochem J. 1978 Aug 15;174(2):671–672. doi: 10.1042/bj1740671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart G. J. Functional heterogeneity of UDP-glucuronosyltransferase as indicated by its differential development and inducibility by glucocorticoids. Demonstration of two groups within the enzyme's activity towards twelve substrates. Biochem J. 1978 Aug 15;174(2):485–489. doi: 10.1042/bj1740485. [DOI] [PMC free article] [PubMed] [Google Scholar]