Abstract
Background:
Continuous glucose monitor (CGM) use improves type 1 diabetes (T1D) outcomes, yet children from diverse backgrounds and on public insurance have worse outcomes and lower CGM utilization. Using novel CGM data acquisition and analysis of two T1D cohorts, we test the hypothesis that T1D youth from different backgrounds experience disparities in meaningful CGM use following both T1D diagnosis and CGM uptake.
Methods:
Cohorts drawn from a pediatric T1D program were followed for one year beginning at diagnosis (n = 815, 2016-2020) or CGM uptake (n = 1392, 2015-2020). Using chart and CGM data, CGM start and meaningful use outcomes between racial/ethnic and insurance groups were compared using median days, one-year proportions, and survival analysis.
Results:
Publicly compared with privately insured were slower to start CGM (233, 151 days, P < .01), had fewer use-days in the year following uptake (232, 324, P < .001), and had faster first discontinuation rates (hazard ratio [HR] = 1.61, P < .001). Disparities were more pronounced among Hispanic and black compared with white subjects for CGM start time (312, 289, 149, P = .0013) and discontinuation rates (Hispanic HR = 2.17, P < .001; black HR = 1.45, P = .038), and remained even among privately insured (Hispanic/black HR = 1.44, P = .0286).
Conclusions:
Given the impact of insurance and race/ethnicity on CGM initiation and use, it is imperative that we target interventions to support universal access and sustained CGM use to mitigate the potential impact of provider biases and systemic disadvantage and racism. By enabling more equitable and meaningful T1D technology use, such interventions will begin to alleviate outcome disparities between youth with T1D from different backgrounds.
Keywords: continuous glucose monitor, health care disparities, pediatrics, type 1 diabetes
Background
Continuous glucose monitor (CGM) use has revolutionized the care of many children with T1D.1-3 While clinical trials have demonstrated CGM use improves hemoglobin A1c (HbA1c) and decreases hypoglycemia among youth with T1D,4,5 not all populations benefit equally from this technology in clinical practice. 6
Youth from diverse and marginalized backgrounds have worse glycemic outcomes and higher rates of severe hypoglycemia, 7 and yet also have less prevalent CGM use.6,8 Race/ethnicity is a significant predictor of technology use even when multivariable models control for other factors such as socioeconomic status (SES) and health literacy.9,10 In addition to disparities based on race/ethnicity, studies have shown significant gaps in CGM use between patients based on SES both nationally and internationally.11-13
Early research demonstrated the utility of consistent, near-daily CGM use; however, data that characterize subjects’ CGM usage patterns between clinic visits and among vulnerable populations are limited.4,5,14 Published study outcomes include presence of an initial CGM prescription, or electronic medical record (EMR) documented CGM use in clinic. Although the impact of idealized CGM use has been well-described, little is known about time-to-CGM-start after diagnosis, time-to-discontinuation after uptake, and the impact of race/ethnicity and insurance on these measures.
With increasing rates of T1D, particularly among youth of diverse backgrounds, 15 it is imperative to develop a better understanding of the factors that contribute to uptake and sustained CGM use among all populations. We hypothesized that children with diabetes from diverse racial/ethnic backgrounds and/or low SES would demonstrate longer time to CGM initiation and shorter duration of use. Toward this end, our aim was to analyze first initiation in the year following incident T1D and then sustained use of CGM in the year after uptake in two retrospective cohorts drawn from a quaternary care pediatric T1D program. Leveraging time-to-event analyses and clinically shared, cloud-based CGM usage data, we identify differences in meaningful use among vulnerable groups.
Research Design and Methods
Study Cohorts
The source population was people aged 2 to 25 years at diagnosis of T1D and followed at Boston Children’s Hospital (BCH) T1D Program. Individuals with “International” or missing insurance were excluded. From this population, we drew two cohorts to characterize CGM initiation starting from T1D diagnosis (diagnosis cohort) and, separately, duration of meaningful CGM use starting from CGM uptake (uptake cohort).
Diagnosis Cohort
The diagnosis cohort included subjects diagnosed with T1D from January 1, 2016 (BCH T1D registry inception) through December 31, 2020, and followed for at least one year.
Uptake Cohort
The BCH T1D program uses the cloud-based Dexcom (DexCom, Inc, San Diego, CA) Clarity portal to access CGM data from Dexcom users both at and between clinic visits. To further describe CGM utilization following adoption, we defined a separate retrospective cohort of prevalent T1D starting from when their shared CGM data supported uptake. The uptake cohort included subjects followed at BCH for T1D, using Dexcom CGM, with shared CGM data in the database at the time accessed (March 2022), and who met CGM uptake criteria from January 17, 2015 (CGM database inception) through March 17, 2021. CGM data records were matched to BCH EMR subjects. Not all subjects of the uptake cohort were nested within the diagnosis cohort, and not all subjects from the diagnosis cohort entered the uptake cohort. Subjects could be members of both cohorts if they were diagnosed at BCH and met criteria for uptake during the specified time periods.
CGM Measures
Multiple CGM measures were defined to characterize meaningful use from retrospective chart and CGM data. Days to CGM start, the interval from T1D diagnosis to the first clinic visit with a physician or diabetes nurse educator (DNE) where CGM use was documented and glucose data from a CGM device were reported in the visit record was modeled as a survival time (primary endpoint) among subjects in the diagnosis cohort. One-year proportions characterized the number of subjects with CGM start ≤365 days.
Entry into the uptake cohort occurred when Dexcom CGM data sufficiency (%) of expected CGM readings over the prior four-week window was ≥50%. First discontinuation of CGM use occurred when the four-week data sufficiency fell below 50% among those in the uptake cohort. Days meaningful use, the interval from uptake to first discontinuation, was modeled as a failure time (primary endpoint). One-year ever discontinuation was the proportion with a CGM discontinuation event within 365 days of uptake. CGM use-days was the number of days with any CGM data in the year following uptake, regardless of any discontinuation.
Covariates
Demographic data were obtained from retrospective review of the EMR with the most specific race/ethnicity identifier chosen (see Supplemental Figure 1). Diagnosis dates for uptake cohort subjects not diagnosed at BCH were approximated using clinical documentation. Covariates including age, insurance type (public or private), and HbA1c were collected at time of cohort entry. For the diagnosis cohort, additional demographic information was abstracted based on caregiver report at diagnosis. 16
Statistical Analyses
Summary statistics were provided for each cohort for baseline covariates and CGM-related outcomes. Chi-squared test for association between predictors was conducted where indicated. Kruskal-Wallis (multigroup) and Wilcox rank sum (two-group) were performed (rstatix functions kruskal_test and wilcox_test) to compare days of CGM use between groups in the uptake cohort. Pearson’s chi-squared test was used (stats function prop.test) to assess differences in proportions.
Survival curves (ggplot2 3.4.0) and Cox proportional hazards regression (survminer functions survfit and coxph) were used to describe the primary time-to-event outcomes and HR. Survival analyses were limited to subjects with race/ethnicity defined as “white,” “black,” or “Hispanic,” thus reducing sample size of diagnosis (n = 692) and uptake (n = 1245) cohorts. Hypothesis testing to compare survival curves used the log-rank test. Cox proportional HR was assessed for significance using likelihood ratio statistics. Model assumptions were evaluated using visual assessment of proportional hazards and residuals.
Statistical analyses were conducted using R (4.2.2) with tidyverse (1.3.2), tidyquant (1.0.6), stats (4.2.2), rstatix (0.7.2.), and survminer (0.4.9) packages; large data computing was done using the BCH HPC Clusters Enkefalos 2 (installed and configured by BioGrids).
Results
Demographics of the diagnosis cohort (n = 815; Table 1) reflect program demographics during the study period (2016-2020): a slight preponderance of males and a plurality of school age diagnoses, with about a third presenting in diabetic ketoacidosis. The majority were privately insured, English-speaking, with Non-Hispanic white race/ethnicity. Public insurance and diverse race/ethnicity (combining Hispanic and black) were correlated (chi-squared statistic 122.8, P < .0001).
Table 1.
Baseline Characteristics of Diagnosis and Uptake Cohorts.
| Characteristic | Diagnosis | Uptake |
|---|---|---|
| Baseline demographics a | ||
| Number of subjects | 815 | 1391 |
| Sex | ||
| Female | 43.07% (351/815) | 48.89% (680/1391) |
| Male | 56.93% (464/815) | 51.11% (711/1391) |
| Age at diagnosis | ||
| Age (y) | 10.69 (4.24) | 9.07 (4.02) |
| Age group | ||
| Young (<8.0 y) | 25.77% (210/815) | 40.55% (564/1391) |
| School age (8.0-13.9 y) | 51.78% (422/815) | 47.45% (660/1391) |
| Adolescent (14.0-19.9 y) | 21.72% (177/815) | 11.79% (164/1391) |
| Young adult (20-25 y) | 0.74% (6/815) | 0.22% (3/1391) |
| Primary language | ||
| English | 93.99% (766/815) | 94.03% (1308/1391) |
| Spanish | 3.44% (28/815) | 1.65% (23/1391) |
| Other | 0% (0/815) | 1.65% (23/1391) |
| Insurance type | ||
| Private | 76.07% (620/815) | 83.61% (1163/1391) |
| Public | 23.93% (195/815) | 16.39% (228/1391) |
| Race/ethnicity | ||
| White | 70.67% (576/815) | 80.66% (1122/1391) |
| Black | 5.52% (45/815) | 3.02% (42/1391) |
| Hispanic | 8.71% (71/815) | 5.82% (81/1391) |
| Other | 7.24% (59/815) | 5.46% (76/1391) |
| Unknown b | 7.85% (64/815) | 5.03% (70/1391) |
| Insurance + Race/ethnicity (binary) | ||
| Private + white | 59.88% (488/815) | 72.25% (1005/1391) |
| Private + Diverse c | 5.28% (43/815) | 3.59% (50/1391) |
| Public + white | 10.8% (88/815) | 8.41% (117/1391) |
| Public + Diverse c | 8.96% (73/815) | 5.25% (73/1391) |
| Year of diagnosis | ||
| 1990-2010 | 0% (0/815) | 27.46% (382/1391) |
| 2010-2015 | 0% (0/815) | 33.21% (462/1391) |
| 2016-2020 | 100% (815/815) | 39.32% (547/1391) |
| HbA1c at diagnosis (%) | 11.55 (2.13) | 11.14 (2.25) |
| Diagnosis cohort data | ||
| DKA at diagnosis | ||
| No | 60.61% (494/815) | |
| Yes | 38.16% (311/815) | |
| Family structure | ||
| Two parents, one house | 71.41% (582/815) | |
| Two parents, two houses | 12.76% (104/815) | |
| One parent, one house | 7.36% (60/815) | |
| Non-parent primary caregiver | 1.96% (16/815) | |
| Other | 0.86% (7/815) | |
| Unspecified d | 5.64% (46/815) | |
| Uptake cohort data | ||
| Age at uptake | ||
| Age (y) | 13.73 (5.03) | |
| Age group | ||
| Young (<8.0 y) | 13.37% (186/1391) | |
| School age (8.0-13.9 y) | 38.96% (542/1391) | |
| Adolescent (14.0-19.9 y) | 36.38% (506/1391) | |
| Young adult (20-25 y) | 11.29% (157/1391) | |
| Time from diagnosis to uptake (y) | 3.12 [0.44, 7.7] | |
| Year of uptake | ||
| 2015 | 4.39% (61/1391) | |
| 2016 | 12.87% (179/1391) | |
| 2017 | 20.27% (282/1391) | |
| 2018 | 27.89% (388/1391) | |
| 2019 | 30.84% (429/1391) | |
| 2020 | 3.74% (52/1391) | |
| HbA1c at uptake | ||
| % | 8.49 (1.67) | |
| Category e | ||
| Below target | 16.03% (223/1391) | |
| Above target | 79.65% (1108/1391) | |
| CGM device type | ||
| Mobile user | 77.93% (1084/1391) | |
| Non-mobile user | 19.84% (276/1391) | |
Abbreviations: HbA1c, hemoglobin A1c; DKA, diabetic ketoacidosis; CGM, continuous glucose monitor.
Continuous variables are summarized as mean (standard deviation) or median [interquartile range] as appropriate based on underlying distribution. Categorical variables are summarized as % within category.
“Unknown” designates that the field was completed but designed as “Unknown” (in contrast from missing data [aka incomplete] fields).
“Diverse” refers to a combined group of “Hispanic” and “black” subjects.
“Unspecified” designates that the chart was reviewed for the categorical data but data were not present in the available documentation.
HbA1c less than or equal to 7% was defined as below target.
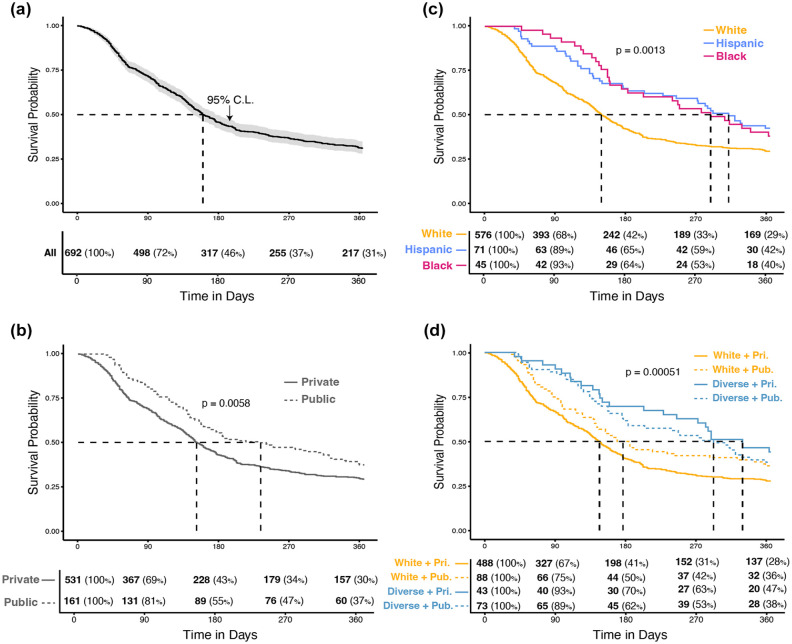
CGM technology was started within six months of diagnosis by more than half of the diagnosis cohort (Table 2). Starting CGM was most rapid in the three months after diagnosis; rates of increase in CGM users gradually slowed after about six months (Figure 1).
Table 2.
CGM Outcomes in Year Following Diagnosis by Demographic Group.
| Characteristic | Days to CGM start (median) | % with CGM start within year (n/N) | P value a |
|---|---|---|---|
| Diagnosis cohort | 160 | 69.08 (563/815) | |
| Sex | |||
| Female | 156 | 70.94 (249/351) | .356 |
| Male | 162 | 67.67 (314/464) | |
| Age group | |||
| Young (<8.0 y) | 139 | 72.86 (153/210) | .00019 |
| School age (8.0-13.9 y) | 156 | 72.99 (308/422) | |
| Adolescent (14.0-19.9 y) | 283 | 55.93 (99/177) | |
| Young adult (20-25 y) | N/A | 50 (3/6) | |
| Primary language b | |||
| English | 157 | 69.71 (534/766) | .2272 |
| Spanish | 320 | 57.14 (16/28) | |
| Other | 249 | 61.9 (13/21) | |
| Insurance type | |||
| Private | 149 | 71.45 (443/620) | 1.16E-02 |
| Public | 233 | 61.54 (120/195) | |
| Race/ethnicity b | |||
| White | 149 | 70.83 (408/576) | 1.41E-01 |
| Black | 289 | 62.22 (28/45) | |
| Hispanic | 312 | 57.75 (41/71) | |
| Other | 226 | 66.1 (39/59) | |
| Unknown c | 137 | 73.44 (47/64) | |
| Race/ethnicity (binary) b | |||
| White | 149 | 70.83 (408/576) | .02144 |
| Diverse d | 299.5 | 59.48 (69/116) | |
| Neither e | 150 | 69.92 (86/123) | |
| Insurance type b + Race/ethnicity (binary) | |||
| Private + white | 146.5 | 72.13 (352/488) | 3.28E-02 |
| Private + Diverse d | 329 | 55.81 (24/43) | |
| Public + white | 176.5 | 63.64 (56/88) | |
| Public + Diverse d | 292 | 61.64 (45/73) | |
| Neither e | 150 | 69.92 (86/123) | |
The P value represents statistical testing based on Pearson’s chi-squared statistic for comparison of proportions.
“Neither,” “Other,” and “Unknown” categories excluded for statistical testing.
“Unknown” designates that the field was completed but designed as “unknown” (in contrast from missing data [aka incomplete] fields).
“Diverse” refers to a combined group of “Hispanic” and “black” subjects.
“Neither” in these categories designates that the subject did not fit into the racial/ethnic category of Hispanic or black and, therefore, could not be classified by this metric.
Figure 1.
Time to start of CGM use in year following diagnosis in pediatric T1D subjects. (a) Diagnosis cohort. (b) By race/ethnicity. (c) By insurance type. (d) By race/ethnicity and insurance type. Kaplan-Meir survival curves of time (days) from diagnosis with T1D to start of CGM as per clinical documentation. Dashed lines indicate median survival time for each group. The P values represent output from log-rank test of comparison between subgroup survival curves. Tables indicate number (%) of the subgroup that remains at risk of the outcome at specified time points. Cohort limited to subjects with race/ethnicity of white, Hispanic, or black (Other and Unknown categories excluded).
Abbreviations: T1D, type 1 diabetes; CI, confidence interval; White + Pri., white and private insurance; White + Pub., white and public insurance; Diverse + Pri., Hispanic or black and private insurance; Diverse + Pub., Hispanic or black and public insurance.
The cohort of subjects defined by having already taken up CGM (uptake cohort n = 1391; Table 1) had a slight preponderance of males, with >80% of subjects described as white, privately insured, and English-speaking. The uptake cohort had similar baseline demographic characteristics to the diagnosis cohort, though there were fewer male, older, publicly insured, non-English-speaking subjects from diverse race/ethnicity groups. Race/ethnicity and insurance were correlated (chi-squared statistic 205, P < .0001). The mean HbA1c was 8.5 ± 1.7% at CGM uptake; 16% of subjects met the American Diabetes Association target for glycemic control.
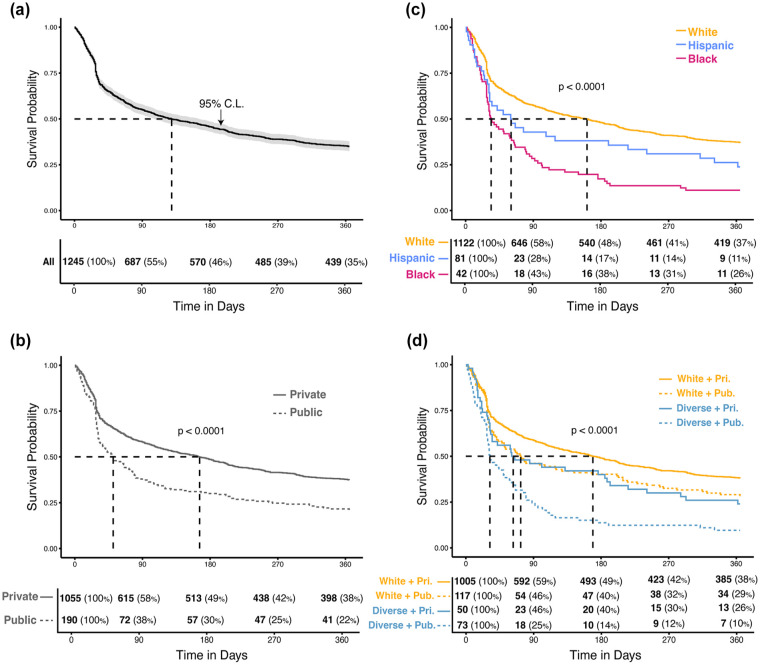
Consistent, meaningful CGM utilization fell to about a third by the end of the year follow-up (Table 3). Median time to the first CGM discontinuation was 129 days (Figure 2). Attrition was more rapid in the early months following CGM uptake among all subgroups. Dropoff rate remained higher among publicly insured and diverse patients following initial decline. Over half of the uptake cohort subjects had meaningful CGM use for over 80% of days in the year following CGM uptake (Table 3).
Table 3.
CGM Meaningful Use Measures in Year Following Uptake by Demographic Group.
| Characteristic | Days to first discontinuation (median) | % with a discontinuation within one year (n/N) | P value a | Days of any use (median [IQR]) | P value b |
|---|---|---|---|---|---|
| Uptake cohort | 132 | 65.42 (910/1391) | 310 [169.5, 359] | ||
| Sex | |||||
| Female | 131.5 | 64.85 (441/680) | .7048 | 303.5 [169.75, 359] | .931 |
| Male | 135 | 65.96 (469/711) | 315 [169.5, 359] | ||
| Age group | |||||
| Young (<8.0 y) | 107 | 65.6 (370/564) | .9865 | 310 [151.75, 360] | .975 |
| School age (8.0-13.9 y) | 158.5 | 65 (429/660) | 311 [176.5, 359] | ||
| Adolescent (14.0-19.9 y) | 173.5 | 66.46 (109/164) | 301.5 [204.25, 358] | ||
| Young adult (20-25 y) | 137 | 66.67 (2/3) | 330 [170, 346.5] | ||
| Language c | |||||
| English | 138.5 | 64.68 (846/1308) | .004094 | 311.5 [172, 359.25] | .000108 |
| Spanish | 32 | 95.65 (22/23) | 148 [89.5, 238] | ||
| Other | 32 | 82.61 (19/23) | 208 [79.5, 336] | ||
| N/A | 200 | 62.16 (23/37) | 337 [277, 362] | ||
| Insurance type | |||||
| Private | 168 | 62.68 (729/1163) | .004094 | 324 [187.5, 360] | 2.81E-09 |
| Public | 51 | 79.39 (181/228) | 231.5 [110, 340.25] | ||
| Race/ethnicity c | |||||
| White | 161.5 | 63.01 (707/1122) | 2.91E-07 | 321 [186.25, 360] | 1.26E-10 |
| Black | 60.5 | 76.19 (32/42) | 265.5 [119.25, 347] | ||
| Hispanic | 34 | 88.89 (72/81) | 160 [74, 254] | ||
| Other | 132.5 | 80.26 (61/76) | 291.5 [175.5, 346.75] | ||
| Unknown d | 231.5 | 54.29 (38/70) | 335 [179.75, 362.75] | ||
| Race/ethnicity (binary) c | |||||
| White | 161.5 | 63.01 (707/1122) | 3.17E-06 | 321 [186.25, 360] | 4.79E-11 |
| Diverse e | 41 | 84.55 (104/123) | 174 [87, 318] | ||
| Neither f | 165.5 | 67.81 (99/146) | 312 [172.5, 358.75] | ||
| Insurance type c + Race/ethnicity (binary) | |||||
| Private + white | 169 | 61.99 (623/1005) | 1.52E-06 | 326 [194, 361] | 2.27E-12 |
| Private + Diverse e | 63 | 76 (38/50) | 249 [107.25, 343.5] | ||
| Public + white | 73 | 71.79 (84/117) | 283 [126, 355] | ||
| Public + Diverse e | 32 | 90.41 (66/73) | 148 [82, 251] | ||
| Neither f | 165.5 | 67.81 (99/146) | 312 [172.5, 358.75] | ||
Abbreviation: IQR, interquartile range.
The P value represents statistical testing based on Pearson’s chi-squared statistic for comparison of proportions.
The P value represents statistical testing for nonparametric distributions (Kruskal-Wallis or Wilcox rank sum depending on number of groups).
“Neither,” “Other,” and “Unknown” categories excluded for statistical testing.
“Unknown” designates that the field was completed but designed as “unknown” (in contrast from missing data [aka incomplete] fields).
“Diverse” refers to a combined group of “black” and “Hispanic” subjects.
“Neither” in these categories designates that the subject did not fit into the racial/ethnic category of Hispanic or black and, therefore, could not be classified by this metric.
Figure 2.
Time to first discontinuation of meaningful CGM use in pediatric T1D subjects. (a) Uptake cohort. (b) By race/ethnicity. (c) By insurance type. (d) By race/ethnicity and insurance type. Kaplan-Meir survival curves of time (days) from uptake of CGM to first discontinuation of meaningful use. Dashed lines indicate median survival time for each group. The P values represent output from log-rank test of comparison between subgroup survival curves. Tables indicate number (%) of the subgroup that remains at risk of the outcome at specified time points. Cohort limited to subjects with race/ethnicity of white, Hispanic, or black (Other and Unknown categories excluded).
Abbreviations: CGM, continuous glucose monitor; T1D, type 1 diabetes; CI, confidence interval; White + Pri., white and private insurance; White + Pub., white and public insurance; Diverse + Pri., Hispanic or black and private insurance; Diverse + Pub., Hispanic or black and public insurance.
Public insurance was associated with reduced utilization of CGM starting from delayed adoption after diagnosis (Table 2) through shorter sustained meaningful use after uptake (Table 3). Those with public insurance started CGM almost three months later than those with private insurance (public median = 233 days, 95% confidence interval [CI] = [169, 230]; private median = 151 days, 95% CI = [141, 169]; Table 4, Figure 1b). By one year following diagnosis, fewer publicly insured subjects had started CGM (61.5%) than privately insured ones (71.5 %, P = .01161; Table 2). Using a Cox proportional hazards model, publicly insured subjects were nearly 30% less likely to start CGM in the year following diagnosis (HR = 0.73, 95% CI = [0.59, 0.92], P = .006; Table 4).
Table 4.
Cox Proportional Hazards for CGM Start in year Following Diagnosis by Group.
| Demographic predictor | At risk (n) | Events (n) | Time to CGM start (d) | 95% CI | HR a | P value b | 95% CI |
|---|---|---|---|---|---|---|---|
| Diagnosis cohort | 692 | 477 | 160 | [149, 177] | — | — | — |
| Insurance type | |||||||
| Private | 531 | 376 | 151 | [141, 169] | — | — | — |
| Public | 161 | 101 | 233 | [169, 320] | 0.73 | .005996903 | [0.59, 0.92] |
| Race/ethnicity | |||||||
| White | 576 | 408 | 149 | [139, 164] | — | — | — |
| Hispanic | 71 | 41 | 312 | [245, NA] | 0.62 | .003045819 | [0.45, 0.85] |
| Black | 45 | 28 | 289 | [183, NA] | 0.64 | .021801477 | [0.44, 0.94] |
| Insurance type + Race/ethnicity (binary) | |||||||
| Private + white | 488 | 352 | 146 | [134, 160] | — | — | — |
| Private + Diverse c | 43 | 24 | 329 | [245, NA] | 0.54 | .00355611 | [0.36, 0.82] |
| Public + white | 88 | 56 | 176 | [142, 356] | 0.76 | .052695147 | [0.57, 1.00] |
| Public + Diverse c | 73 | 45 | 292 | [183, NA] | 0.63 | .004013356 | [0.46, 0.86] |
Abbreviation: CI, confidence interval.
HRs are defined compared with referent group; referent group for each predictor is first listed category.
The P value represents statistical testing based on likelihood ratio for comparison of HRs.
“Diverse” refers to a combined group of “black” and “Hispanic” subjects.
In contrast, those with public insurance first discontinued CGM meaningful use almost four months before those with private insurance (Figure 2b; public median = 51 days, 95% CI = [37, 74]; private median = 166 days, 95% CI = [131, 198]; Table 5). Publicly insured had a 60% higher risk of first discontinuation in the year following uptake (HR = 1.61, 95% CI = [1.35, 1.92], P < .001; Table 5). In addition, the median (interquartile range [IQR]) number of CGM use-days in the year following uptake was three months shorter among the publicly (231.5 [110, 340.25]) compared with the privately (324 [187.5, 360]) insured (P < .001; Table 3). Overall, these results suggest public insurance, a marker of lower SES, was a proxy for and/or repeated cause of disparities via reduced early CGM adoption and, later, decreased and more fragmented use.
Table 5.
Cox Proportional Hazards for First Discontinuation in Year Following Uptake by Group.
| Demographic predictor | At risk (n) | Events (n) | Time to first discontinuation (d) | 95% CI | HR a | P value b | 95% CI |
|---|---|---|---|---|---|---|---|
| Uptake cohort | 1245 | 811 | 129 | [106, 166] | — | — | — |
| Insurance type | |||||||
| Private | 1055 | 661 | 166 | [131, 198] | — | — | — |
| Public | 190 | 150 | 51 | [37, 74] | 1.61 | 1.57E-07 | [1.35, 1.92] |
| Race/ethnicity | |||||||
| White | 1122 | 707 | 161.5 | 125, 192 | — | — | — |
| Black | 42 | 32 | 60.5 | [32, 241] | 1.45 | .03823126 | [1.02, 2.07] |
| Hispanic | 81 | 72 | 34 | [29, 64] | 2.17 | 4.18E-10 | [1.7, 2.77] |
| Insurance type + Race/ethnicity (binary) | |||||||
| Private + white | 1005 | 623 | 169 | [137, 202] | — | — | — |
| Private + Diverse c | 50 | 38 | 63 | [33, 216] | 1.44 | .02860301 | [1.04, 2.00] |
| Public + white | 117 | 84 | 73 | [43, 201] | 1.31 | .02131188 | [1.04, 1.64] |
| Public + Diverse c | 73 | 66 | 32 | [29, 58] | 2.43 | 9.41E-12 | [1.88, 3.14] |
Abbreviation: CI, confidence interval.
HRs are defined compared with referent group; referent group for each predictor is first listed category.
The P value represents statistical testing based on likelihood ratio for comparison of HRs.
“Diverse” refers to a combined group of “black” and “Hispanic” subjects.
Race/ethnicity was associated with multiple meaningful CGM use measures. black (median = 289 days, 95% CI = [183, NA]) and Hispanic (median = 312 days, 95% CI = [245, NA]) subjects initiated CGM in over twice the time of white subjects (median = 149, 95% CI [139, 164]; Table 5). By one year following diagnosis, fewer diverse subjects (59.5%) had started CGM compared with white ones (70.8%, P < .05; Table 2). black (HR = 0.64, P < .05) and Hispanic (HR = 0.62, P < .005) subjects were 40% less likely to start CGM in the year following diagnosis (Table 4).
After CGM uptake, non-white subjects sustained meaningful use for a shorter initial interval (black: 60.5 median days, Hispanic: 34, and white: 161.5; Figure 2c) and had increased risk of discontinuation (black HR = 1.45, 95% CI = [1.02, 2.07], P = .038; Hispanic HR = 2.17, 95% CI = [1.7, 2.8], P < .001; Table 5). Furthermore, median [IQR] CGM use-days for Hispanic subjects (160, [74, 254]) was half that of white subjects (321 [186.25, 360]) while CGM use-days for black subjects (265.5, [119.25, 347]) was in between (P < .001; Table 3).
We defined four strata according to insurance type and dichotomized race/ethnicity (black/Hispanic or “Diverse” vs white). Even among those with private insurance, diverse subjects had a longer time to CGM start and higher proportion not yet started a year after diagnosis (Table 3), as well shorter time to first discontinuation and fewer days use in the year after uptake (Table 4). Similar trends were found among those with public insurance.
In a combined Cox model in the diagnosis cohort, using white privately insured subjects as the referent group, diverse subjects with either insurance were more than a third less likely to start CGM. By a year following diagnosis, less than two-thirds of diverse subjects had started CGM compared with nearly three-quarters of privately insured white subjects (59.5% vs 70.8%, P = .02; Table 2). Similarly, there was an increased risk of discontinuation in the year following uptake among all diverse patients (Table 5). Publicly insured white subjects had median days meaningful use (283) nearly twice that of publicly insured diverse subjects (148) (P < .001; Table 3).
There were significant differences in days until CGM start based on clinical and social factors at diagnosis (Supplemental Table 1). Other clinical factors were also associated with insurance and race/ethnicity with mean HbA1c prior to uptake highest among diverse subjects with public insurance (Supplemental Table 2).
Discussion
We demonstrate a consistent three-month disparity in meaningful use outcomes among vulnerable T1D youth throughout their journey with CGM. These findings are consistent with other published differences of CGM utilization among diverse and low-resourced populations. 6 Our study is, to our knowledge, the first reported comparison of individual-level clinical CGM usage disparities in a large T1D program over the course of the year following CGM uptake. Importantly, our analysis shows that diverse and vulnerable populations experience double risks to “miss-out” and then “drop-out” of CGM-informed care. These same populations endure higher rates of CGM-mitigatable diabetes complications and often experience the combined effects of structural racism and health insurance inequalities. The drive to improve T1D care through meaningful use of diabetes technology must be accompanied by targeted interventions that both limit delays to CGM start and support continued CGM utilization for vulnerable populations.6,12,13,17
Insurance and race/ethnicity-based disparities emerge at diagnosis, with first actual CGM data from publicly insured and non-white youth available for clinician review nearly 100 days after that of privately insured or white youth within our cohort of individuals with new-onset T1D. The care established after T1D diagnosis, for many, sets the course of future T1D management and control.18,19 Initiation of CGM within a year of diagnosis is associated with better glycemic control, fewer acute complications, and improved qualitative experience of diabetes care.20,21 Although clinical care and insurance coverage are shifting to support early CGM adoption in the United States, incentive-based mechanisms to encourage equitable prescription and universal health care coverage for CGM devices (eg, Australian National Diabetes Supply Scheme) 22 and educational supports should be considered to prevent underserved T1D youth from being left behind.
Insurance barriers to CGM-informed care do not fully explain the racial/ethnic disparities in initiation and sustained meaningful use. In our study, diverse populations with private insurance fared worse than their white counterparts. Even in health care systems where there is more universal financial support for diabetes technology, racial/ethnic and SES-based disparities persist, suggesting that financial support is only one piece of the access gap. 23 Health literacy and provider bias are potential mediating factors. Youth with diabetes whose parents have higher levels of health literacy have been found to have better glycemic control than their counterparts.24,25 In the clinical diabetes setting, providers are more likely to offer CGM to those with higher perceived health literacy 26 ; however, studies of CGM use among youth with T1D demonstrate that the disparities in technology use on the basis of race/ethnicity remain even after adjusting for health literacy and SES.9,27 These findings suggest the potential impact of provider bias in perpetuating CGM use disparities. Provider perceptions regarding family competence have been shown to influence decisions about diabetes technology.28,29 In qualitative studies, diverse subjects reported experiencing less “shared decision making” with providers than whites, mistrust of the medical community, and a lack of exposure to diabetes technology, leading to a sense of internalized racism that creates barriers to accessing optimal care.30-32 Further research must assess other factors by characterizing implicit bias in providers and the lived experience of structural racism which makes it more challenging for non-white patients to adopt and then consistently sustain CGM use.33,34 One solution to mitigating such biases could include standardized postdiagnosis pathways whose default option is CGM initiation within two weeks of diagnosis. This access-based solution could be complemented by health literacy-based interventions which consider an individual’s social context. 35
Early studies in CGM efficacy highlighted the importance of sustained sensor use,1,3,6,14,36 which our findings suggest may be more difficult for vulnerable populations to achieve.35,37 Our results indicate that even when insurance access, patient buy-in, and provider “gatekeeping” 9 can be overcome to get patients their first CGM sensors, other sustainable supports will be needed to prevent technology disparities from propagating throughout the lifetime of the CGM user. For example, provision of receiver devices with mobile data plans capable of continuously sharing CGM readings with the clinical team may alleviate the smartphone-based digital health divide. Future studies should investigate the most impactful forms of education and support for diverse, marginalized, and low-resource communities to enable sustained CGM use.38,39
In this era of rapidly developing diabetes technology dependent on real-time CGM data streams (ie, hybrid closed loops), existing gaps in access to CGM and support of sustained use are poised to generate further disparities.40-42 This analysis of the factors related to slower initiation and suboptimal compliance with CGM suggests the importance of universal implementation of CGM complemented by focused education with its initiation and sustained support for use among diverse and under-resourced patients to enable their adoption of advanced diabetes technology and optimize glycemic control and outcomes.
Strengths of this study include its large sample size, representative of the diversity of patients for this source population. Use of chart review with clinically reviewed CGM data improves the validity of CGM start as a measure of clinically impactful CGM use. Our approach to develop meaningful use metrics from actual sensor glucose data shared with providers is also unique among the literature reviewed.
Receiver-based and individual-level differences in CGM data sharing may cause measurement bias. Distrust in digital technology features such as data sharing, if correlated with proxies of vulnerable populations, could induce associations between SES predictors and the measured outcome. 32 Nevertheless, here we conceptualize meaningful CGM use as a measure of shared clinical CGM data, as absent or inconsistent data sharing is of limited clinical benefit in CGM-enhanced decision-making by providers.
The retrospective, observational nature of our cohorts is susceptible to selection and confounding bias. We had limited sample size of black and Hispanic subjects to conduct multivariate analyses and thus limited power to detect effect modification. We did not correct for multiple hypothesis testing, and there remains the potential of unmeasured confounding. In addition, the results of this study may not be generalizable to T1D youth in other clinical settings. We utilized nonparametric measures and multiple CGM metrics to evaluate disparities. However, hazards in the diagnosis cohort subgroups were not proportional, which may limit some of the statistical inferences on the survival curves.
Finally, while our research shows consistent and recurrent disparities for vulnerable subgroups, observational studies cannot attribute causality nor prove mechanisms. Prospective, interventional studies remain essential to define causality and mitigate the sources of disparity.
Conclusion
Our findings point to the need for directed strategies to initiate and sustain meaningful CGM use and advance health equity among diverse pediatric T1D populations. Both the longer time to initiation of CGM and the shorter duration of meaningful use provide important targets for intervention to mitigate existing disparities and improve health equity and outcomes for all children with diabetes as technology continues to expand.
Supplemental Material
Supplemental material, sj-docx-1-dst-10.1177_19322968231183985 for A Retrospective Cohort Study of Racial/Ethnic and Socioeconomic Disparities in Initiation and Meaningful Use of Continuous Glucose Monitoring among Youth With Type 1 Diabetes by Elise Schlissel Tremblay, Allison Bernique, Katherine Garvey and Christina M. Astley in Journal of Diabetes Science and Technology
Supplemental material, sj-docx-2-dst-10.1177_19322968231183985 for A Retrospective Cohort Study of Racial/Ethnic and Socioeconomic Disparities in Initiation and Meaningful Use of Continuous Glucose Monitoring among Youth With Type 1 Diabetes by Elise Schlissel Tremblay, Allison Bernique, Katherine Garvey and Christina M. Astley in Journal of Diabetes Science and Technology
Supplemental material, sj-docx-3-dst-10.1177_19322968231183985 for A Retrospective Cohort Study of Racial/Ethnic and Socioeconomic Disparities in Initiation and Meaningful Use of Continuous Glucose Monitoring among Youth With Type 1 Diabetes by Elise Schlissel Tremblay, Allison Bernique, Katherine Garvey and Christina M. Astley in Journal of Diabetes Science and Technology
Supplemental material, sj-tif-4-dst-10.1177_19322968231183985 for A Retrospective Cohort Study of Racial/Ethnic and Socioeconomic Disparities in Initiation and Meaningful Use of Continuous Glucose Monitoring among Youth With Type 1 Diabetes by Elise Schlissel Tremblay, Allison Bernique, Katherine Garvey and Christina M. Astley in Journal of Diabetes Science and Technology
Acknowledgments
The authors would like to acknowledge the assistance of research assistants: Sarah Clemons and Radhika Joshi as well as data manager James Zavadoski for their chart review and database maintenance. The authors would like to acknowledge Boston Children’s Hospital’s High-Performance Computing Resources BCH HPC Clusters Enkefalos 2 (E2) made available for conducting the research reported in this publication. Software used in the project was installed and configured by BioGrids (cite: eLife 2013;2: e01456, Collaboration gets the most out of software.) We would, in addition, like to acknowledge Grant Tremblay for his figure design and generation.
Footnotes
Abbreviations: BCH, Boston Children’s Hospital; CGM, continuous glucose monitor; CI, confidence interval; DNE, diabetes nurse educator; EMR, electronic medical record; HbA1c, hemoglobin A1c; HR, hazard ratio; IQR, interquartile range; SES, socioeconomic status;T1D, type 1 diabetes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number K12DK094721 (EST), K23DK120899 (CMA) and a Boston Children’s Hospital Health Equity grant (EST).
ORCID iD: Elise Schlissel Tremblay  https://orcid.org/0000-0003-0950-6393
https://orcid.org/0000-0003-0950-6393
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631-1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association Professional Practice Committee. Children and adolescents: standards of medical care in diabetes—2022. Diabetes Care. 2022;45:S208-S231. [DOI] [PubMed] [Google Scholar]
- 4. Beck RW, Weinzimer S, Miller K, et al. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33(1):17-22. doi: 10.2337/dc09-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beck RW, Buckingham B, Miller K, et al. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2009;32(11):1947-1953. doi: 10.2337/dc09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Foster NC, Beck RW, Miller KM, et al. State of Type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. doi: 10.1089/dia.2018.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Willi SM, Miller KM, DiMeglio LA, et al. Racial-ethnic disparities in management and outcomes among children with Type 1 diabetes. Pediatrics. 2015;135(3):424-434. doi: 10.1542/peds.2014-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lipman TH, Hawkes CP, Smith JA, et al. Racial disparities in treatment and outcomes of children with type 1 diabetes. Pediatr Diabetes. 2021;22(2):241-248. doi: 10.1111/pedi.13139. [DOI] [PubMed] [Google Scholar]
- 9. Agarwal S, Schechter C, Gonzalez J, et al. Racial-ethnic disparities in diabetes technology use among young adults with Type 1 diabetes. Diabetes Technol Ther. 2021;23(4):306-313. doi: 10.1089/dia.2020.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lai CW, Lipman TH, Willi SM, et al. Racial and ethnic disparities in rates of continuous glucose monitor initiation and continued use in children with Type 1 diabetes. Diabetes Care. 2021;44:255-257. doi: 10.2337/dc20-1663. [DOI] [PubMed] [Google Scholar]
- 11. Sheikh K, Bartz SK, Lyons SK, et al. Diabetes device use and glycemic control among youth with Type 1 diabetes: a single-center, cross-sectional study. J Diabetes Res. 2018;2018:5162162. doi: 10.1155/2018/5162162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Addala A, Auzanneau M, Miller K, et al. A decade of disparities in diabetes technology use and HBA1c in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care. 2021;44(1):133-140. doi: 10.2337/dc20-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ng SM, Evans ML. Widening health inequalities related to type 1 diabetes care in children and young people in the UK: a time to act now. Diabetic Med. 2021;38:e14620. doi: 10.1111/dme.14620. [DOI] [PubMed] [Google Scholar]
- 14. Wong JC, Foster NC, Maahs DM, et al. Real-time continuous glucose monitoring among participants in the T1D exchange clinic registry. Diabetes Care. 2014;37(10):2702-2709. doi: 10.2337/dc14-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adepoju OE, Bolin JN, Booth EA, et al. Is diabetes color-blind? Growth of Prevalence of diagnosed diabetes in children through 2030. Popul Health Manag. 2015;18(3):172-178. doi: 10.1089/pop.2014.0084. [DOI] [PubMed] [Google Scholar]
- 16. Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3(6):514-522. doi: 10.1370/afm.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes in children and adults with Type 1 diabetes: data from the T1D exchange quality improvement collaborative. Clin Diabetes. 2021;39(3):278-283. doi: 10.2337/cd21-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nirantharakumar K, Mohammed N, Toulis KA, et al. Clinically meaningful and lasting HbA1c improvement rarely occurs after 5 years of Type 1 diabetes: an argument for early, targeted and aggressive intervention following diagnosis. Diabetologia. 2018;61(5):1064-1070. doi: 10.1007/s00125-018-4574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prahalad P, Yang J, Scheinker D, et al. Hemoglobin A1c trajectory in pediatric patients with newly diagnosed Type 1 diabetes. Diabetes Technol Ther. 2019;21(8):456-461. doi: 10.1089/dia.2019.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patton SR, Noser AE, Youngkin EM, et al. Early initiation of diabetes devices relates to improved glycemic control in children with recent-onset Type 1 diabetes mellitus. Diabetes Technol Ther. 2019;21(7):379-384. doi: 10.1089/dia.2019.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanenbaum ML, Zaharieva DP, Addala A, et al. ‘I was ready for it at the beginning’: parent experiences with early introduction of continuous glucose monitoring following their child’s Type 1 diabetes diagnosis. Diabet Med. 2021;38(8):e14567. doi: 10.1111/dme.14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson SR, Holmes-Walker DJ, Chee M, et al. Universal subsidized continuous glucose monitoring funding for young people with Type 1 diabetes: uptake and outcomes over 2 years, a population-based study. Diabetes Care. 2022;45(2):391-397. doi: 10.2337/dc21-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ladd JM, Sharma A, Rahme E, et al. Socioeconomic disparities in pump uptake among children with type 1 diabetes in Canada: findings from Quebec and Manitobaitle. JAMA Netw Open. 2022;5(5):e2210464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pulgarón ER, Sanders LM, Patiño-Fernandez AM, et al. Glycemic control in young children with diabetes: the role of parental health literacy. Patient Educ Couns. 2014;94(1):67-70. doi: 10.1016/j.pec.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Janisse HC, Naar-King S, Ellis D. Brief report: parent’s health literacy among high-risk adolescents with insulin dependent diabetes. J Pediatr Psychol. 2010;35(4):436-440. doi: 10.1093/jpepsy/jsp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Isaacs D, Bellini NJ, Biba U, et al. Health care disparities in use of continuous glucose monitoring. Diabetes Technol Ther. 2021;23(suppl 3):S81-S87. doi: 10.1089/dia.2021.0268. [DOI] [PubMed] [Google Scholar]
- 27. Vrany EA, Hill-Briggs F, Ephraim PL, et al. Continuous glucose monitors and virtual care in high-risk, racial and ethnic minority populations: toward promoting health equity. Front Endocrinol. 2023;14:1083145. doi: 10.3389/fendo.2023.1083145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lawton J, Kirkham J, Rankin D, et al. Who gains clinical benefit from using insulin pump therapy? A qualitative study of the perceptions and views of health professionals involved in the Relative Effectiveness of Pumps over MDI and Structured Education (REPOSE) trial. Diabetic Med. 2016;33(2):243-251. doi: 10.1111/dme.12879. [DOI] [PubMed] [Google Scholar]
- 29. Fredette ME, Zonfrillo MR, Park S, et al. Self-reported insulin pump prescribing practices in pediatric type 1 diabetes. Pediatr Diabetes. 2021;22:758-765. doi: 10.1111/pedi.13213. [DOI] [PubMed] [Google Scholar]
- 30. Peek ME, Odoms-Young A, Quinn MT, et al. Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 2010;71(1):1-9. doi: 10.1016/j.socscimed.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tremblay ES, Ruiz J, Dykeman B, et al. Hispanic caregivers’ experience of pediatric type 1 diabetes: a qualitative study. Pediatr Diabetes. 2021;22(7):1040-1050. doi: 10.1111/pedi.13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mencher SR, Weinzimer SA, Nally LM, et al. Technology utilization in black adolescents with Type 1 diabetes: exploring the decision-making process. Diabetes Technol Ther. 2022;24(4):1-9. doi: 10.1089/dia.2021.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health. 2015;105(12):e60-e76. doi: 10.2105/AJPH.2015.302903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morone JF, Teitelman AM, Cronholm PF, et al. Influence of social determinants of health barriers to family management of type 1 diabetes in black single parent families: a mixed methods study. Pediatr Diabetes. 2021;22(8):1150-1161. doi: 10.1111/pedi.13276. [DOI] [PubMed] [Google Scholar]
- 35. Agarwal S, Kanapka LG, Raymond JK, et al. Racial-ethnic inequity in young adults with Type 1 diabetes. J Clin Endocrinol Metab. 2020;105(8):E2960-E2969. doi: 10.1210/clinem/dgaa236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Group JCMS. Continuous glucose monitoring and intensive treatment of Type 1 diabetes. N Engl J Med. 2008;359(14):1464-1476. doi:10.1016/s0084-3741(09) 79371-0. [DOI] [PubMed] [Google Scholar]
- 37. Addala A, Maahs DM, Scheinker D, et al. Uninterrupted continuous glucose monitoring access is associated with a decrease in HbA1c in youth with type 1 diabetes and public insurance. Pediatr Diabetes. 2020;21(7):1301-1309. doi: 10.1111/pedi.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kraaijeveld SR. Continuous glucose monitoring as a matter of justice. HEC Forum. 2021;33(4):345-370. doi: 10.1007/s10730-020-09413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Monaghan M, Marks B. Personal experiences with COVID-19 and diabetes technology: all for technology yet not technology for all. J Diabetes Sci Technol. 2020;14(4):762-763. doi: 10.1177/1932296820930005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707-1717. doi: 10.1056/NEJMoa1907863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Commissariat PV, Boyle CT, Miller KM, et al. Insulin pump use in young children with type 1 diabetes: sociodemographic factors and parent-reported barriers. Diabetes Technol Ther. 2017;19(6):363-369. doi: 10.1089/dia.2016.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lawton J, Kimbell B, Rankin D, et al. Health professionals’ views about who would benefit from using a closed-loop system: a qualitative study. Diabetic Med. 2020;37(6):1030-1037. doi: 10.1111/dme.14252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dst-10.1177_19322968231183985 for A Retrospective Cohort Study of Racial/Ethnic and Socioeconomic Disparities in Initiation and Meaningful Use of Continuous Glucose Monitoring among Youth With Type 1 Diabetes by Elise Schlissel Tremblay, Allison Bernique, Katherine Garvey and Christina M. Astley in Journal of Diabetes Science and Technology
Supplemental material, sj-docx-2-dst-10.1177_19322968231183985 for A Retrospective Cohort Study of Racial/Ethnic and Socioeconomic Disparities in Initiation and Meaningful Use of Continuous Glucose Monitoring among Youth With Type 1 Diabetes by Elise Schlissel Tremblay, Allison Bernique, Katherine Garvey and Christina M. Astley in Journal of Diabetes Science and Technology
Supplemental material, sj-docx-3-dst-10.1177_19322968231183985 for A Retrospective Cohort Study of Racial/Ethnic and Socioeconomic Disparities in Initiation and Meaningful Use of Continuous Glucose Monitoring among Youth With Type 1 Diabetes by Elise Schlissel Tremblay, Allison Bernique, Katherine Garvey and Christina M. Astley in Journal of Diabetes Science and Technology
Supplemental material, sj-tif-4-dst-10.1177_19322968231183985 for A Retrospective Cohort Study of Racial/Ethnic and Socioeconomic Disparities in Initiation and Meaningful Use of Continuous Glucose Monitoring among Youth With Type 1 Diabetes by Elise Schlissel Tremblay, Allison Bernique, Katherine Garvey and Christina M. Astley in Journal of Diabetes Science and Technology