Abstract
Hypotension following induction of general anaesthesia has been shown to result in increased complications and mortality postoperatively. Patients admitted to the hospital undergoing urgent surgery are often fasted from fluids for significant periods compared to elective patients subject to Enhanced Recovery After Surgery protocols despite guidelines stating that a two-hour fast is sufficient. The aim of this prospective, observational study was to compare fasting times and intravascular volume status between elective surgery patients subject to enhanced recovery protocols and inpatient, urgent surgery patients and to assess differences in the incidence of post-induction hypotension. Fasting data was obtained by questionnaire in the preoperative area in addition to inferior vena cava collapsibility index, a non-invasive measure of intravascular volume. Blood pressure readings and drug administration for the ten minutes following induction were obtained from patients’ charts. Inpatients undergoing urgent surgery were fasted significantly longer than enhanced recovery patients and had lower intravascular volume. However, no difference was found in the incidence of post-induction hypotension.
Keywords: Enhanced recovery, IVC collapsibility, POCUS, Perioperative medicine
This study was designed to compare fasting practices between inpatient and elective surgery patients and assess for subsequent differences in intravascular volume and post-induction hypotension. Enhanced Recovery After Surgery (ERAS) protocols have been shown to improve outcomes postoperatively; however, there is a lack of evidence for their effects on hydration and blood pressure before and during surgery. In addition, this study utilises a non-invasive measure of intravascular volume, ultrasound-measured inferior vena cava (IVC) collapsibility, in the perioperative setting. The results allowed us to assess the utility and accuracy of this bedside test in predicting intraoperative hypotension as has been suggested by previous studies.
Introduction
The drugs used for the induction of general anaesthesia commonly result in decrease in blood pressure due to their vasodilatory and cardiovascular depressive effects (Zhang & Critchley 2016). While these effects are generally well tolerated, they can result in significant changes in mean arterial pressure (MAP) that lead to hypoperfusion in hypovolemic patients, termed post-induction hypotension. There is evidence from multiple studies that MAP readings below 65 mmHg or a 20% reduction from baseline can increase the risk of kidney and myocardial injury (Salmasi et al 2017, Walsh et al 2013). Therefore, it is crucial to identify patients who may be predisposed to post-induction hypotension preoperatively.
Because intravascular volume status has significant implications for changes in blood pressure, perioperative fluid management is critical in mitigating hypotension following induction (Simpao et al 2020, Szabó et al 2023). The American Society of Anesthesiologists’ (ASA 2017) current guidelines allow intake of clear fluids up to two hours prior to the induction of general anaesthesia However, actual fasting times are often greatly extended for inpatients undergoing emergency surgery when the time to surgery is often longer than anticipated and practitioners are conservative with fluid administration (El-Sharkawy et al 2020). Conversely, patients undergoing elective surgery are often encouraged to consume clear liquids up to two hours before surgery through ERAS protocols which involve patients drinking a carbohydrate-rich beverage prior to presenting for surgery.
While identifying patients at risk for post-induction hypotension is clearly important to postoperative outcomes, doing so is often complicated by the invasiveness of traditional techniques, such as central venous or pulmonary artery catheterisation and arterial line pulse pressure variation, to assess intravascular volume status. As a result, measurement of inferior vena cava (IVC) collapsibility index by bedside ultrasound has been proposed as a safer alternative. This metric is compatible with measurements of central venous pressure (CVP) and high IVC collapsibility has been demonstrated to predict hypotension upon induction of general anaesthesia which may make it a valuable screening tool preoperatively (Ciozda et al 2015, Zhang & Critchley 2016).
Despite guidelines encouraging clear fluid intake up to two hours prior to surgery, fasting times continue to be extended, especially for inpatients undergoing emergency surgery (El-Sharkawy et al 2020). However, no evidence could be found in the literature showing differences in intravascular volume status or incidence of post-induction hypotension between patients undergoing emergency surgery and those having elective procedures subjected to ERAS protocols. Therefore, this study aimed to compare fasting times between these groups and intravascular volume status utilising non-invasive, ultrasound-measured IVC collapsibility. Secondarily, we assessed for subsequent episodes of post-induction hypotension and its ability to be predicted by IVC collapsibility as reported in previous studies (Zhang & Critchley 2016).
Methods
Study population and exclusion criteria
Institutional approval was obtained for this study through the University of North Carolina at Chapel Hill IRB (IRB 22-0730) and written, informed patient consent was obtained from all participants. Sample size estimates were based on power analysis of a previously collected dataset from a pilot study of 14 patients comparing IVC collapsibility of inpatient, urgency surgery patients and elective, ERAS patients at Duke University Hospital. Using an α of 0.05 and 80% power, a total of 26 patients were required in each group. This study enrolled 57 patients from 1 June to 1 August 2022 at UNC Hospitals aged 18 or older who had been admitted to the hospital at least 12 hours before surgery or were undergoing an elective procedure utilising the ERAS protocol. Patients were excluded from participation in the study if they: did not follow hospital inpatient nothing by mouth (NPO) guidelines as per standard of care; did not follow ERAS protocol guidelines; lacked the capacity to provide consent; had preexisting severe vascular or valvular disease, had preexisting right-sided heart failure and had taken angiotensin-converting enzyme (ACE) inhibitors within the past 24 hours; or were undergoing cardiac surgery, pregnant, or requiring BiPap, continuous positive airway pressure (CPAP) or mechanical ventilation.
Fluid questionnaire and IVC collapsibility measurements
In the preoperative holding area, patients were asked when their last oral fluid intake was within a one-hour window (two–three hours, three–four hours, four–five hours ago, etc) and if they were currently thirsty. In addition, IV fluid administration for inpatients was recorded for the 12 hours prior to surgery. Ultrasound measurements were made in the preoperative holding area using either a Kosmos handheld or a Sonosite X-Porte ultrasound. Before measuring the IVC, either a parasternal long axis or subxiphoid heart view was obtained to assess heart function qualitatively. A subcostal view of the IVC was then obtained with its junction with the right atrium in view. M-mode was used to record the diameter of the IVC in the long axis approximately 2–3 cm from the right atrial junction as the patient was instructed to take a sniff in through their nose to collapse the IVC, as shown in Figure 1. These measurements were taken three times for each patient and used to calculate mean IVC collapsibility using the formula (IVCmax diameter − IVCmin diameter)/IVCmax diameter. Longitudinal M-mode measurements were confirmed when possible with an additional measurement of collapsibility in the short-axis view to confirm that the IVC was being measured at the widest point.
Figure 1.
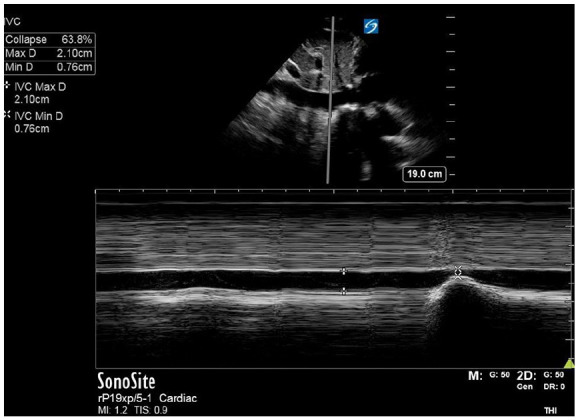
This image shows the M-mode measurement of a highly collapsible IVC. The minimum diameter coincides with the decrease in vessel calibre associated with the patient sniffing in through their nose.
Post-induction measurements
General anaesthesia was induced using UNC Hospitals’ institutional standard of care and was not adjusted for the purposes of this study. Medications and dosages used for the induction of general anaesthesia were recorded. After induction of anaesthesia, patients’ blood pressure was monitored via invasive or non-invasive methods at the discretion of the attending anaesthesiologist and according to the requirements of the surgery. Blood pressure data and drug administration were then pulled from the anaesthesia record for the ten minutes following induction. For the purposes of this study, post-induction hypotension was defined as a drop in MAP below 65 mmHg, a decrease in MAP of 20% compared to the pre-induction baseline, or the administration of vasopressors including phenylephrine, ephedrine or norepinephrine.
Data analysis
The Kolmogorov–Smirnov test was used to compare the distributions of ASA status between ERAS patients and inpatients. T-test was used to compare mean IVC collapsibility and fasting time between the two study groups (inpatient and ERAS). Chi-square test was used to study the association between post-induction hypotension and study groups. Logistic regression was used to evaluate the association between IVC collapsibility and post-induction hypotension. These analyses were conducted using R (version 4.2.2). Finally, a receiver-operating characteristic curve was created to assess the ability of IVC collapsibility to predict post-induction hypotension in Microsoft Excel Version 2304. All outcomes were defined prior to initiation of recruitment.
Results
Recruitment and demographics
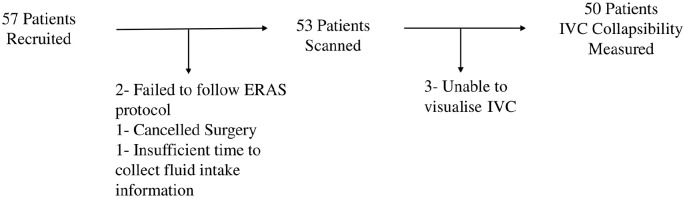
A total of 72 patients were approached for recruitment and ultimately 57 patients consented to participate in the study. Of these 57 patients, four were excluded from all analyses: two patients because of failure to adhere to ERAS protocol fluid intake, one patient because their surgery was cancelled after the consenting process, and one because the patient was unable to answer fluid intake questions or be scanned due to insufficient time available in the preoperative area. Of the remaining 53 patients, three were excluded from comparisons of IVC collapsibility between groups due to poor image quality, but were included in the comparison of fasting times and post-induction hypotension. Additional demographic information is provided in Table 1, and recruitment and exclusion are shown in Figure 2. Finally, we found no significant difference in the distribution of ASA status between groups (p = 1).
Table 1.
Demographic breakdown of study participants
| Age (years) | |
| Mean | 55.3 |
| Standard deviation | 16.5 |
| Sex | |
| Male | 25 (47.2%) |
| Female | 28 (52.8%) |
| Race | |
| African American | 14 (26.4%) |
| Asian | 1 (1.9%) |
| Caucasian | 37 (69.8%) |
| Native American | 1 (1.9%) |
| BMI | |
| Mean | 26.7 |
| Standard deviation | 6.65 |
BMI: body mass index.
Figure 2.
This flowchart illustrates the total recruitment for the study and the reasons for exclusion from certain aspects of data collection.
Fluid questionnaire and IVC collapsibility
The mean fasting time for oral fluid intake was significantly longer for the inpatient group (13.7 hours) compared to the ERAS group (2.56 hours; p < 0.001) and reported thirst was more prevalent in the inpatient group (80.0% versus 28.0%, p < 0.001). The mean IVC collapsibility index in the inpatient, urgent group (56.8%) was found to be significantly higher than that of the ERAS group (40.0%; p < 0.001).
Post-induction hypotension
The incidence of post-induction hypotension (MAP < 65 mmHg, drop in MAP of 20% from baseline, or the use of inotropes or vasopressors) during the ten minutes following the induction of general anaesthesia was higher in the inpatient group (69.2%) compared to the ERAS group (44.4%). However, this difference was not statistically significant (p = 0.123).
Relationship between IVC collapsibility and post-induction hypotension
Logistic regression to test the marginal association of IVC collapsibility and post-induction hypotension yielded an odds ratio of 1.03. However, this relationship was not statistically significant (p = 0.106). A power analysis of marginal association was performed and demonstrated a sample size of 75 subjects was necessary to achieve a power of 90%. In addition, a receiver-operating characteristic curve was created to further assess the ability of IVC collapsibility to predict post-induction hypotension as shown in Figure 3.
Figure 3.
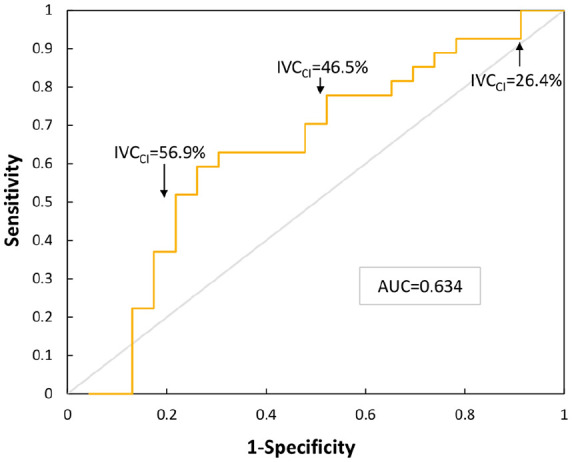
This receiver-operating characteristic curve shows the change in sensitivity of IVC collapsibility for predicting hypotension as a function of 1 − specificity, or false-positive rate. Three IVC collapsibility values are provided for reference on the curve and the area under the curve (AUC) is provided.
Access to research data
The data underlying this research can be accessed through the link to the Figshare repository provided alongside this article.
Discussion
We found that IVC collapsibility was significantly higher in the inpatient, urgent surgery group compared to the ERAS group (p < 0.001). The American Society of Echocardiography endorses the use of IVC collapsibility in the assessment of volume status and clinical studies have demonstrated its utility in estimating CVP (Ciozda et al 2015, Rudski et al 2010). However, the variation in volume status between these groups as measured by IVC collapse is not unexpected due to the drastic difference in fasting times. Despite research as far back as the 1980s demonstrating that prolonged fasting from clear fluids has little to no utility in reducing the incidence of aspiration under general anaesthesia, we found inpatient, urgent surgery patients fasted for 13.7 hours on average compared to only 2.56 hours for ERAS patients (Fawcett & Thomas 2018, Maltby et al 1986).
Not only does prolonged fasting and hypovolemia directly impact patient discomfort, reported thirst was 52% (p < 0.001) more prevalent in the inpatient, urgent group, it has serious implications for patients’ risk of postoperative complications and mortality. A 2013 study of 34 hospitals found that although emergency general surgery cases made up only 11% of procedures, they accounted for 47% of surgical mortality, and a review published just last year suggests that outcomes have not improved reporting that emergency surgeries represent over 50% of surgical mortality nationwide (Ross et al 2022, Smith et al 2013). While preoperative volume status is only one of many variables that may account for the vast disparity in operative outcomes between emergent and elective cases, it represents a concrete area for improvement.
Although the association between IVC collapsibility and post-induction hypotension has been previously described, this relationship and differences in the incidence of post-induction hypotension have not been fully explored between elective surgeries and urgent, inpatient surgeries. While a previous single-centre study of 90 elective surgery patients found increased IVC collapsibility to be a reliable predictor of post-induction hypotension with an odds ratio of 1.17 and area of 0.90 under the receiver-operating characteristic curve, we found IVC collapsibility to be less predictive with an odds ratio of 1.03 that was not statistically significant (p = 0.106) and area under the curve of 0.63 (Zhang & Critchley 2016). This may be due, in part, to the relatively small sample size of our study. Consequently, we performed a retrospective power analysis demonstrating that a sample size of 75 participants would be needed to achieve a statistical power of 90%. While the results of this study may call into question the relative accuracy of IVC collapsibility’s ability to predict post-induction hypotension, we found it to be a relatively simple, time-effective and non-invasive bedside test. Of the total 57 patients recruited, only one patient was unable to be scanned due to time constraints in the preoperative area, and of 53 patients scanned, image quality was insufficient to measure collapsibility in only three patients.
In addition, we found that although hypotension was much more prevalent in the inpatient group, 69.2%, compared to the ERAS group, 44.4%, this was not statistically significant (p = 0.123). Although this difference lacked statistical significance, a greater than 20% difference in incidence suggests there may be a clinically significant difference in the distribution of hypotensive episodes between these groups that warrants further investigation with a larger cohort. While a potential confounder for the comparison of hypotension between these groups could have been a difference in the degree of systemic illness, we found no significant difference in the distribution of ASA status between groups suggesting this was not necessarily the case. Aside from sample size, the definition of post-induction hypotension may have been another limitation to this and other studies on the topic. Despite numerous studies showing the detrimental effect of low blood pressure while under general anaesthesia both before and after surgical incision on postoperative complications and mortality, few agree on what measurements define post-induction and intraoperative hypotension (Bijker et al 2007, Monk et al 2015, Salmasi et al 2017, Walsh et al 2013). To standardise future studies and clarify practice guidelines for anaesthesia providers, a clear threshold needs to be defined for clinically significant hypotension in the operative and perioperative periods.
In conclusion, this study found fasting times to be drastically different between inpatient, urgent surgery patients and patients subject to ERAS protocols. This is likely due in large part to ERAS patients being encouraged to drink a carbohydrate-rich beverage two hours before surgery. In addition, we observed a lower average IVC collapsibility in ERAS patients indicating greater intravascular volume compared to urgent surgery patients. However, no statistically significant difference was observed in the incidence of post-induction hypotension between these groups nor was IVC collapsibility found to be a significant predictor of subsequent hypotension. While this study demonstrates a clear and significant difference in fasting practices between the study groups and intravascular volume status, further investigation is needed to determine whether this plays a role in the prevalence of post-induction hypotension and whether IVC collapsibility can serve as an effective screening tool.
Research Data
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
sj-xlsx-1-ppj-10.1177_17504589231215932 for Comparing preoperative fasting and ultrasound-measured intravascular volume status in elective surgery, enhanced recovery patients versus inpatient, urgent surgery patients and the ability of IVC collapsibility to predict post-induction hypotension by Jacob R Wrobel, Justin C Magin, David Williams, Xinming An, Jacob D Acton, Alexander S Doyal, Shawn Jia, James Krakowski, Ricardo Serrano, Stuart A Grant, David N Flynn and Duncan J McLean in Journal of Perioperative Practice
Footnotes
ORCID iDs: Jacob R Wrobel  https://orcid.org/0009-0007-6572-2636
https://orcid.org/0009-0007-6572-2636
James C Krakowski  https://orcid.org/0000-0003-2820-3419
https://orcid.org/0000-0003-2820-3419
Declarations
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: Funding for this study was provided through the Carolina Medical Student Research Program.
References
- American Society of Anesthesiologists 2017. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: An updated report by the American Society of Anesthesiologists Task Force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration Anesthesiology 126 (3) 376–393 [DOI] [PubMed] [Google Scholar]
- Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KGM, Kalkman CJ. 2007. Incidence of intraoperative hypotension as a function of the chosen definition Anesthesiology 107 (2) 213–220 [DOI] [PubMed] [Google Scholar]
- Ciozda W, Kedan I, Kehl DW, Zimmer R, Khandwalla R, Kimchi A. 2015. The efficacy of sonographic measurement of inferior vena cava diameter as an estimate of central venous pressure Cardiovascular Ultrasound 14(1) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy AM, Daliya P, Lewis-Lloyd C, et al. 2020. Fasting and surgery timing (FaST) audit Clinical Nutrition 40(3) 1405–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett WJ, Thomas M. 2018. Pre-operative fasting in adults and children: Clinical practice and guidelines Anaesthesia 74 (1) 83–88 [DOI] [PubMed] [Google Scholar]
- Maltby JR, Sutherland AD, Sale JP, Shaffer EA. 1986. Preoperative oral fluids Anesthesia & Analgesia 65 (11) 1112–1116 [PubMed] [Google Scholar]
- Monk TG, Bronsert MR, Henderson WG, et al. 2015. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery Anesthesiology 123 (2) 307–319 [DOI] [PubMed] [Google Scholar]
- Ross SW, Reinke CE, Ingraham AM, et al. 2022. Emergency general surgery quality improvement: A review of recommended structure and key issues Journal of the American College of Surgeons 234 (2) 214–225 [DOI] [PubMed] [Google Scholar]
- Rudski LG, Lai WW, Afilalo J, et al. 2010. Guidelines for the echocardiographic assessment of the right heart in adults: A Report from the American Society of Echocardiography Journal of the American Society of Echocardiography 23 (7) 685–713 [DOI] [PubMed] [Google Scholar]
- Salmasi V, Maheshwari K, Yang D, et al. 2017. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: A retrospective cohort analysis Anesthesiology 126 (1) 47–65 [DOI] [PubMed] [Google Scholar]
- Simpao AF, Lezhou W, Nelson O, et al. 2020. Preoperative fluid fasting times and postinduction low blood pressure in children: A retrospective analysis Anesthesiology 133 523–533 [DOI] [PubMed] [Google Scholar]
- Smith M, Hussain A, Xiao J, et al. 2013. The importance of improving the quality of emergency surgery for a regional quality collaborative Annals of Surgery 257 (4) 596–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó M, Pleck AP, Soós SÁ, et al. 2023. A preoperative ultrasound-based protocol for optimisation of fluid therapy to prevent early intraoperative hypotension: A randomised controlled study Perioperative Medicine 12 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M, Devereaux PJ, Garg AX, et al. 2013. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery Anesthesiology 119 (3) 507–515 [DOI] [PubMed] [Google Scholar]
- Zhang J, Critchley LAH. 2016. Inferior vena cava ultrasonography before general anesthesia can predict hypotension after induction Anesthesiology 124 (3) 580–589 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
sj-xlsx-1-ppj-10.1177_17504589231215932 for Comparing preoperative fasting and ultrasound-measured intravascular volume status in elective surgery, enhanced recovery patients versus inpatient, urgent surgery patients and the ability of IVC collapsibility to predict post-induction hypotension by Jacob R Wrobel, Justin C Magin, David Williams, Xinming An, Jacob D Acton, Alexander S Doyal, Shawn Jia, James Krakowski, Ricardo Serrano, Stuart A Grant, David N Flynn and Duncan J McLean in Journal of Perioperative Practice