Abstract
Rationale
Pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) cause right ventricular dysfunction, which can impact other solid organs. However, the profiles and consequences of hepatic injury resulting from PAH and CTEPH have not been well studied.
Objectives
We aimed to identify underlying patterns of liver injury in a cohort of patients with PAH and CTEPH enrolled in 15 randomized clinical trials conducted between 1998 and 2014.
Methods
We used unsupervised machine learning to identify liver injury clusters in 13 trials and validated the findings in two additional trials. We then determined whether these liver injury clusters were associated with clinical outcomes or treatment effect heterogeneity.
Measurements and Main Results
Our training dataset included 4,219 patients and our validation dataset included 1,756 patients with serum total bilirubin, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, and albumin data. Using k-means clustering, we identified phenotypes with no liver injury, hepatocellular injury, cholestatic injury, and combined injury patterns. Patients in the cholestatic injury liver cluster had the shortest time to clinical worsening and the highest risk of mortality. The cholestatic injury group also experienced the greatest placebo-corrected treatment effect on 6-minute-walk distance. Randomization to the experimental arm transitioned patients to a healthier liver status.
Conclusions
Liver injury was associated with adverse outcomes in patients with PAH and CTEPH. Randomization to active treatment had beneficial effects on liver health compared with placebo. The role of liver disease (often subclinical) in determining outcomes warrants prospective studies.
Keywords: liver injury phenotypes, pulmonary hypertension, machine learning
At a Glance Commentary
Scientific Knowledge on the Subject
There are few studies of the hepatic consequences of pulmonary arterial hypertension.
What This Study Adds to the Field
Patients with pulmonary arterial hypertension in clinical trials demonstrate patterns of liver function tests that support subclinical liver injury and adverse effects on outcome.
Pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) are characterized by elevations in pulmonary vascular resistance and mean pulmonary arterial pressure leading to right ventricular (RV) dysfunction and eventual failure. It is well established that RV adaptation is the primary determinant of outcomes in PAH; however, the hepatic consequences of RV dysfunction have not been well studied. Specifically, RV dysfunction leads to venous congestion and reduced perfusion, both of which can cause subclinical, acute, and/or chronic solid organ injury (1). In PAH, vena cava backflow is closely related to RV diastolic stiffness and is greatest during right atrial contraction (2). Venous congestion leads to congestive hepatopathy, characterized by elevations in total bilirubin (TB), alkaline phosphatase (ALP), and γ-glutamate transferase (GGT) (3). Furthermore, cholestatic liver injury causes impaired synthetic liver function and abnormal hepatic drug metabolism. Although the 2022 European Respiratory Society guidelines propose routine laboratory testing for liver function in patients with PAH (4), there are no established clinical or subclinical profiles of liver injury or associations with outcome (except TB) for patients with PAH.
We aimed to identify underlying patterns of liver injury using unsupervised machine learning in patients with PAH and CTEPH from randomized clinical trials (RCTs) of PAH and CTEPH therapies. We hypothesized that the presence of liver injury would be associated with a higher risk of adverse clinical outcomes. Furthermore, we hypothesized that treatment would affect liver health and that there would be heterogeneity of treatment effects among baseline liver phenotypes. Some of the results of these studies were previously reported in the forms of an abstract (5) and a preprint (6).
Methods
Additional details are found in the online supplement.
Study and Participant Selection
The U.S. Food and Drug Administration provided individual participant data from 21 RCTs of pulmonary hypertension therapies conducted between 1998 and 2014 (see Table E1 in the online supplement). We included phase III RCTs and limited our analysis to adult patients with PAH or CTEPH with complete data for TB, ALP, aspartate aminotransferase, alanine aminotransferase, and albumin at baseline. We excluded RCTs that were not submitted to the U.S. Food and Drug Administration and RCTs of sitaxentan, which directly caused idiosyncratic severe liver injury (7).
Data and Harmonization
Individual demographic and clinical data were harmonized as described (8, 9). Invasive hemodynamics were captured if they were measured within 90 days of baseline. Clinical worsening was harmonized across trials using a standardized definition.
The main outcomes were change in the 6-minute-walk distance (6MWD) from baseline to 12 or 16 weeks (based on the length of follow-up in the individual trial), time to clinical worsening, and overall survival. Other outcomes included change from baseline to Week 12 or 16 in World Health Organization (WHO) functional class and Medical Outcomes Study 36-item Short Form Health Survey scores.
Statistical Analysis
We split the data into a training set (approximately 70% of subjects, older trials that ended in 2012) and a validation set (approximately 30% of subjects, more recent trials that ended in 2014) to ensure sufficient sample size and generalizability of the clustering results to present-day patients on background therapy. For feature selection, we performed factor analysis with varimax rotation using the most complete baseline liver function tests, including the aminotransferases, ALP, TB, and albumin. Liver function tests were standardized before factor analysis.
We then performed k-means clustering (k-means++ initialization) on data transformed to the factor dimensional space and selected the number of clusters through the elbow method (Figures E1–E3) (10, 11). We derived k-means clusters in the training set and independently rederived the k-means clusters in the validation set. We also predicted clusters in the validation set using the k-means cluster centers from the training set and assessed whether the predicted clusters were similar to the independently derived clusters using a confusion matrix and associated statistics, including accuracy and kappa.
For the longitudinal analyses, we assigned subjects in the validation set and follow-up liver biomarker data for the entire cohort to clusters using the k-means cluster centers from the training set. The overall effect of PAH treatment on the change in liver injury phenotype from baseline to 12 or 16 weeks was assessed using multilevel ordinal regression (cumulative link model) with liver phenotype used as an ordinal variable. This model was adjusted for baseline liver phenotype, age, sex, baseline 6MWD, pulmonary hypertension etiology, and body mass index as fixed effects and trial as a random effect.
We assessed the association between baseline liver cluster and outcomes using one-step multilevel models as described in the online supplement. These models were adjusted for age, sex, baseline 6MWD, body mass index, pulmonary hypertension etiology, and treatment arm assignment defined a priori. In the subset of studies with hemodynamic data within 90 days before the reference start date, we also adjusted for time-varying right atrial pressure and cardiac index.
Results
Study Population and Study Sample
Of the 21 available trials, we included 15 phase III trials (13 phase III trials in the training set and 2 phase III trials in the validation set) in this analysis (Figure 1). Table E1 shows the trials considered for inclusion together with any pertinent liver function exclusion criteria. Of the included trials, 11 trials captured invasive hemodynamics at baseline (excludes TRIUMPH [TReprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension], FREEDOM-C, FREEDOM-M, and FREEDOM-C2). From 6,429 patients in 16 trials, we excluded 57 (0.8%) patients aged less than 18 years and 397 (6%) adult patients missing at least one liver test at baseline (203 [51%] of which were from AIR [Aerosolized Randomized Iloprost] Study, which did not have liver test data), leaving 5,975 patients in the study sample (4,219 patients in the training set and 1,756 patients in the validation set).
Figure 1.
Participant flowchart. FDA = U.S. Food and Drug Administration; PH = pulmonary hypertension.
The baseline demographic and clinical profiles of included and excluded adult patients with pulmonary hypertension were mostly similar (with differences generally related to exclusion of AIR II subjects) (Table E2). Included patients may have had a higher proportion of connective tissue disease and less CTEPH than the excluded patients. The study sample had more WHO functional class II patients and fewer patients in functional class IV. When available, cardiac index was higher and pulmonary vascular resistance lower in the study sample.
The baseline demographic and clinical profiles of the training and validation sets were comparable (Table E3). The mean age of the training sample was 49.7 ± 15.1 years; 77.7% were female, and 58.0% had idiopathic PAH. The mean age of the validation sample was 50.9 ± 15.5 years; 78.6% were female, and 55.6% had idiopathic PAH.
k-Means Clustering
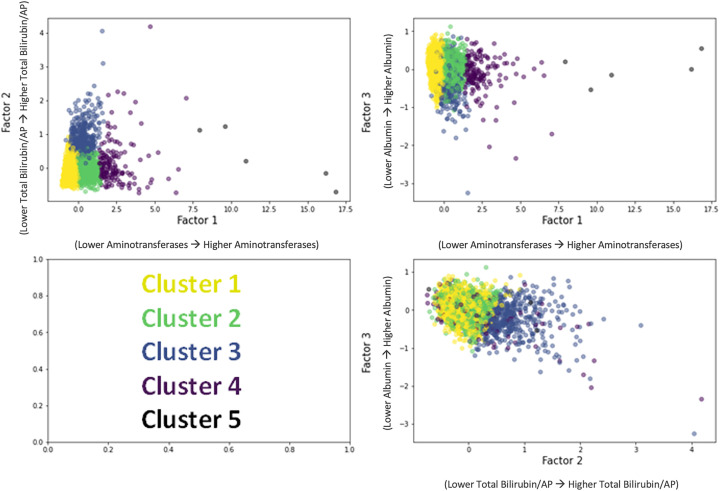
Figure E2 shows the factor analysis with varimax rotation in the derivation sample. There were three distinct factors identified among the five liver tests: Factor 1, alanine aminotransferase and aspartate aminotransferase, considered measures of hepatocellular injury; factor 2, TB and ALP, considered measures of cholestatic injury; and factor 3, albumin, reflected synthetic liver function. Using the three factors, four or five clusters were found to be optimal in k-means clustering (through the elbow method, clusters naturally occur on a sliding scale of each of these factors) (Figure E3). The k-means cluster analysis in the derivation sample is shown within the three-dimensional factor space (Figure 2). Cluster 1 had the most normal values (lowest aminotransferases, lowest TB and ALP, highest albumin), and the phenotype was termed “no liver injury” (Table 1, Figure E4). Cluster 2 only had elevations in aminotransferases, consistent with hepatocellular injury, and the phenotype was termed “hepatocellular injury pattern.” Cluster 3 had higher TB and ALP values (consistent with a cholestatic pattern) and lower albumin concentrations, and the phenotype was termed “cholestatic injury pattern.” Clusters 4 and 5 had the most abnormal values for all tests and were combined because of their proximity in the factor dimensional space and only having five individuals in Cluster 5 and was termed “combined injury pattern.” Figure E5 shows the cumulative distribution plots of liver function tests by cluster.
Figure 2.
k-Means clustering results in factor dimensional space. AP = alkaline phosphatase.
Table 1.
Liver Function Test Results, by Clusters, in Training and Validation Sets
| Characteristic |
n | Overall |
No Liver Injury |
Hepatocellular Injury Pattern |
Cholestatic Injury Pattern |
Combined Injury Pattern |
|---|---|---|---|---|---|---|
| Training data set | N = 4,219 | n = 2,442 | n = 1,145 | n = 474 | n = 158 | |
| Alanine aminotransferase, IU/L | 4,219 | 22.9 ± 18.2 | 15.0 ± 5.4 | 31.9 ± 10.5 | 22.3 ± 8.8 | 81.2 ± 49.3 |
| Albumin, g/dl | 4,219 | 4.1 ± 0.4 | 4.2 ± 0.4 | 4.1 ± 0.4 | 3.9 ± 0.5 | 3.9 ± 0.5 |
| Alkaline phosphatase, IU/L | 4,219 | 87.3 ± 44.2 | 72.6 ± 23.4 | 86.7 ± 27.2 | 152.3 ± 69.3 | 124.7 ± 80.5 |
| Aspartate aminotransferase, IU/L | 4,219 | 25.3 ± 15.2 | 18.1 ± 5.0 | 32.0 ± 7.1 | 30.4 ± 8.8 | 73.9 ± 39.9 |
| Total bilirubin, mg/dl | 4,219 | 0.8 ± 0.6 | 0.6 ± 0.4 | 0.7 ± 0.4 | 1.7 ± 1.0 | 1.0 ± 0.8 |
| γ-Glutamyl transferase, IU/L | 1,081 | 62.7 ± 75.7 | 34.8 ± 35.5 | 72.8 ± 70.8 | 136.2 ± 116.4 | 143.2 ± 134.0 |
| Lactate dehydrogenase IU/L | 1,479 | 237.9 ± 69.3 | 215.1 ± 55.8 | 249.7 ± 63.9 | 273.0 ± 87.3 | 284.0 ± 82.4 |
| Validation data set | N = 1,756 | n = 1,112 | n = 386 | n = 200 | n = 58 | |
|---|---|---|---|---|---|---|
| Alanine aminotransferase, IU/L | 1,756 | 21.3 ± 16.1 | 15.1 ± 5.2 | 32.5 ± 10.0 | 19.3 ± 7.6 | 74.2 ± 46.0 |
| Albumin, g/dl | 1,756 | 4.3 ± 0.4 | 4.4 ± 0.4 | 4.3 ± 0.4 | 4.2 ± 0.4 | 4.2 ± 0.5 |
| Alkaline phosphatase, IU/L | 1,756 | 90.4 ± 43.6 | 76.7 ± 22.2 | 90.4 ± 26.9 | 157.9 ± 71.9 | 121.0 ± 73.6 |
| Aspartate aminotransferase, IU/L | 1,756 | 26.1 ± 17.2 | 20.1 ± 4.4 | 34.0 ± 7.2 | 27.7 ± 8.5 | 83.3 ± 60.9 |
| Total bilirubin, mg/dl | 1,756 | 0.8 ± 0.5 | 0.7 ± 0.3 | 0.8 ± 0.3 | 1.6 ± 0.9 | 0.8 ± 0.4 |
| γ-Glutamyl transferase, IU/L | 606 | 67.6 ± 78.1 | 42.5 ± 42.2 | 78.3 ± 57.8 | 151.7 ± 134.2 | 149.3 ± 140.3 |
| Lactate dehydrogenase, IU/L | — | — | — | — | — | — |
Values are mean ± SD. γ-Glutamyl transferase and lactate dehydrogenase were not used for the clustering because of missing data.
The four phenotypes had similar distributions of age and body mass index, comorbidities, and treatment arm assignment (Table 2). The “no liver injury” phenotype had the highest proportion of females, the lowest prevalence of preexisting liver disease, lowest N-terminal probrain natriuretic peptide (NTproBNP) and brain natriuretic peptide (BNP) values, and the least affected baseline hemodynamics. Compared with the “no liver injury” phenotype, the “hepatocellular injury pattern” phenotype had higher NTproBNP and BNP concentrations, and slightly worse hemodynamics. The “cholestatic injury pattern” phenotype had the lowest proportion of female patients, a higher prevalence of preexisting liver disease, the highest NTproBNP and BNP values, and the worst hemodynamics, including both lower cardiac index and higher mean right atrial pressure. The “cholestatic injury pattern” phenotype also had the shortest 6MWD and the highest Model for End-Stage Liver Disease sodium score.
Table 2.
Baseline Demographic and Clinical Data, by Liver Injury Phenotype (Training Data)
| Characteristic | n | Overall (N = 4,219) | No Liver Injury (n = 2,442) | Hepatocellular Injury Pattern (n = 1,145) | Cholestatic Injury Pattern (n = 474) | Combined Injury Pattern (n = 158) | P Value |
|---|---|---|---|---|---|---|---|
| Age, yr | 4,219 | 49.7 ± 15.1 | 50.2 ± 15.6 | 49.0 ± 14.8 | 49.3 ± 14.1 | 47.7 ± 12.6 | 0.041 |
| Female sex | 4,219 | 3,280 (77.7) | 1,977 (81.0) | 868 (75.8) | 318 (67.1) | 117 (74.1) | <0.001 |
| Race/ethnicity | 4,219 | 0.004 | |||||
| Non-Hispanic White | 2,728 (64.7) | 1,599 (65.5) | 742 (64.8) | 290 (61.2) | 97 (61.4) | ||
| Hispanic White | 98 (2.3) | 43 (1.8) | 31 (2.7) | 20 (4.2) | 4 (2.5) | ||
| Asian | 706 (16.7) | 423 (17.3) | 183 (16.0) | 66 (13.9) | 34 (21.5) | ||
| Non-Hispanic Black | 156 (3.7) | 95 (3.9) | 36 (3.1) | 17 (3.6) | 8 (5.1) | ||
| Other | 36 (0.9) | 15 (0.6) | 12 (1.0) | 9 (1.9) | 0 (0.0) | ||
| Unknown | 461 (10.9) | 249 (10.2) | 131 (11.4) | 66 (13.9) | 15 (9.5) | ||
| Body mass index, kg/m2 | 4,219 | 27.0 ± 5.7 | 26.9 ± 5.7 | 27.2 ± 5.8 | 26.7 ± 5.4 | 27.4 ± 6.0 | 0.20 |
| PH etiology | 4,212 | <0.001 | |||||
| Idiopathic | 2,444 (58.0) | 1,353 (55.5) | 690 (60.3) | 307 (64.9) | 94 (59.9) | ||
| Associated with connective tissue disease | 1,089 (25.9) | 676 (27.7) | 274 (23.9) | 98 (20.7) | 41 (26.1) | ||
| Associated with congenital heart disease | 285 (6.8) | 198 (8.1) | 62 (5.4) | 21 (4.4) | 4 (2.5) | ||
| Drug and toxin induced | 57 (1.4) | 39 (1.6) | 8 (0.7) | 7 (1.5) | 3 (1.9) | ||
| Heritable/familial | 22 (0.5) | 16 (0.7) | 6 (0.5) | 0 (0.0) | 0 (0.0) | ||
| Associated with HIV infection | 44 (1.0) | 13 (0.5) | 21 (1.8) | 7 (1.5) | 3 (1.9) | ||
| Portopulmonary hypertension | 13 (0.3) | 4 (0.2) | 3 (0.3) | 2 (0.4) | 4 (2.5) | ||
| Chronic thromboembolic pulmonary hypertension | 258 (6.1) | 138 (5.7) | 81 (7.1) | 31 (6.6) | 8 (5.1) | ||
| Comorbidities | |||||||
| Diabetes mellitus | 4,219 | 353 (8.4) | 227 (9.3) | 80 (7.0) | 35 (7.4) | 11 (7.0) | 0.086 |
| Hypertension | 4,219 | 1,384 (32.8) | 844 (34.6) | 355 (31.0) | 135 (28.5) | 50 (31.6) | 0.027 |
| Coronary artery disease | 4,219 | 325 (7.7) | 216 (8.8) | 79 (6.9) | 27 (5.7) | 3 (1.9) | 0.001 |
| Preexisting liver condition* | 4,219 | 646 (15.3) | 278 (11.4) | 188 (16.4) | 112 (23.6) | 68 (43.0) | <0.001 |
| Model for End-Stage Liver Disease sodium | 2,049 | 12.0 [8.0, 17.0] | 11.0 [8.0, 16.0] | 11.2 [8.0, 17.0] | 15.0 [11.0, 20.0] | 10.0 [8.0, 16.0] | <0.001 |
| BNP, pg/ml | 357 | 137 [45, 347] | 98 [38, 239] | 143 [55, 322] | 359 [131, 742] | 208 [62, 448] | <0.001 |
| NTproBNP, pg/ml | 963 | 642 [195, 1,788] | 452 [169, 1,342] | 857 [233, 2,099] | 1,777 [894, 3,550] | 644 [173, 1,732] | <0.001 |
| WHO functional class | 4,213 | <0.001 | |||||
| I | 42 (1.0) | 30 (1.2) | 11 (1.0) | 1 (0.2) | 0 (0.0) | ||
| II | 1,318 (31.3) | 829 (34.0) | 328 (28.7) | 117 (24.7) | 44 (27.8) | ||
| III | 2,723 (64.6) | 1,517 (62.2) | 769 (67.3) | 327 (69.1) | 110 (69.6) | ||
| IV | 130 (3.1) | 63 (2.6) | 35 (3.1) | 28 (5.9) | 4 (2.5) | ||
| Six-minute-walk distance, m | 4,216 | 344.9 ± 81.4 | 349.2 ± 82.0 | 347.9 ± 76.3 | 314.4 ± 83.7 | 347.4 ± 81.5 | <0.001 |
| Mean right atrial pressure, mm Hg | 2,512 | 8.0 [5.0, 12.0] | 7.0 [4.0, 10.0] | 8.0 [5.0, 11.0] | 14.0 [10.0, 19.0] | 8.0 [5.2, 12.0] | <0.001 |
| Mean pulmonary artery pressure, mm Hg | 2,530 | 51 [41, 61] | 49 [38, 60] | 52 [43, 61] | 56 [49, 65] | 52 [45, 61] | <0.001 |
| Pulmonary artery wedge pressure, mm Hg | 2,458 | 9.0 [7.0, 12.0] | 9.0 [7.0, 12.0] | 9.0 [6.0, 12.0] | 10.0 [7.0, 12.0] | 8.0 [6.0, 11.0] | 0.006 |
| Cardiac index, L/min/m2 | 2,506 | 2.29 [1.85, 2.80] | 2.40 [1.96, 2.90] | 2.20 [1.77, 2.63] | 2.00 [1.55, 2.47] | 2.06 [1.72, 2.71] | <0.001 |
| Pulmonary vascular resistance, Wood units | 2,459 | 10 [7, 15] | 9 [6, 14] | 11 [8, 15] | 14 [10, 18] | 11 [7, 16] | <0.001 |
| Treatment assignment, n (%) | 4,219 | 0.60 | |||||
| Active | 2,690 (63.8) | 1,567 (64.2) | 712 (62.2) | 309 (65.2) | 102 (64.6) | ||
| Control | 1,529 (36.2) | 875 (35.8) | 433 (37.8) | 165 (34.8) | 56 (35.4) |
Definition of abbreviations: BNP = brain natriuretic peptide; NTproBNP = N-terminal probrain natriuretic peptide; PH = pulmonary hypertension; WHO = World Health Organization.
Data shown as n (%), mean ± SD, or median [interquartile range].
Includes cholecystitis and cholelithiasis (n = 171), hepatitis viral infections (n = 116), hepatocellular damage and hepatitis (n = 94), liver function analyses (n = 48), hepatic fibrosis and cirrhosis (n = 43), hepatobiliary signs and symptoms (n = 34), cholestasis and jaundice (n = 22), hepatobiliary neoplasms (benign) (n = 22), hepatic vascular disorders (n = 9), hepatobiliary neoplasms (malignant) (n = 4), others (n = 83).
Clusters were independently derived in the validation dataset with the same liver function test patterns (Table 1). Summary estimates from the baseline demographic and clinical data across the independently derived phenotypes in the validation dataset were similar to those observed in the training dataset (Table 3). The independently derived clusters in the validation set and the predicted clusters in the validation set using the k-means cluster centers from the training set were very similar (Figure E6). In subsequent analyses, we assigned patients in the validation set and all longitudinal follow-up liver test data for the entire cohort to clusters using the training set k-means cluster centers.
Table 3.
Baseline Demographic and Clinical Profile, by Liver Injury Phenotype (Validation Data)
| Characteristic | n | Overall (N = 1,756) | No Liver Injury (n = 1,112) | Hepatocellular Injury Pattern (n = 386) | Cholestatic Injury Pattern (n = 200) | Combined Injury Pattern (n = 58) | P Value |
|---|---|---|---|---|---|---|---|
| Age, yr | 1,756 | 50.9 ± 15.5 | 51.0 ± 16.0 | 50.9 ± 14.6 | 50.4 ± 14.2 | 50.4 ± 15.4 | 0.95 |
| Female sex | 1,756 | 1,381 (78.6) | 914 (82.2) | 280 (72.5) | 142 (71.0) | 45 (77.6) | <0.001 |
| Race/ethnicity | 1,756 | 0.029 | |||||
| Non-Hispanic White | 1,236 (70.4) | 792 (71.2) | 266 (68.9) | 139 (69.5) | 39 (67.2) | ||
| Hispanic White | 54 (3.1) | 36 (3.2) | 10 (2.6) | 4 (2.0) | 4 (6.9) | ||
| Asian | 257 (14.6) | 162 (14.6) | 54 (14.0) | 30 (15.0) | 11 (19.0) | ||
| Non-Hispanic Black | 65 (3.7) | 47 (4.2) | 14 (3.6) | 2 (1.0) | 2 (3.4) | ||
| Hispanic Black | |||||||
| Other | 1 (0.1) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) | ||
| Unknown | 26 (1.5) | 15 (1.3) | 9 (2.3) | 1 (0.5) | 1 (1.7) | ||
| Body mass index, kg/m2 | 1,756 | 27.5 ± 5.8 | 27.5 ± 5.8 | 27.7 ± 6.0 | 27.8 ± 5.6 | 26.2 ± 5.3 | 0.28 |
| PH etiology | 1,755 | <0.001 | |||||
| Idiopathic | 975 (55.6) | 601 (54.0) | 221 (57.4) | 129 (64.5) | 24 (41.4) | ||
| Associated with connective tissue disease | 549 (31.3) | 349 (31.4) | 120 (31.2) | 52 (26.0) | 28 (48.3) | ||
| Associated with congenital heart disease | 118 (6.7) | 94 (8.5) | 15 (3.9) | 8 (4.0) | 1 (1.7) | ||
| Drug and toxin induced | 50 (2.8) | 31 (2.8) | 12 (3.1) | 4 (2.0) | 3 (5.2) | ||
| Heritable/familial | 43 (2.5) | 30 (2.7) | 11 (2.9) | 2 (1.0) | 0 (0.0) | ||
| Associated with HIV infection | 20 (1.1) | 7 (0.6) | 6 (1.6) | 5 (2.5) | 2 (3.4) | ||
| Comorbidities | |||||||
| Diabetes mellitus | 1,756 | 226 (12.9) | 141 (12.7) | 46 (11.9) | 30 (15.0) | 9 (15.5) | 0.68 |
| Hypertension | 1,756 | 669 (38.1) | 421 (37.9) | 154 (39.9) | 75 (37.5) | 19 (32.8) | 0.73 |
| Coronary artery disease | 1,756 | 192 (10.9) | 120 (10.8) | 40 (10.4) | 26 (13.0) | 6 (10.3) | 0.79 |
| Preexisting liver condition* | 1,756 | 276 (15.7) | 146 (13.1) | 63 (16.3) | 46 (23.0) | 21 (36.2) | <0.001 |
| NTproBNP, pg/ml | 1,736 | 628 [208, 1,750] | 457 [163, 1,278] | 834 [303, 1,906] | 1,947 [1,032, 3,018] | 916 [346, 2,086] | <0.001 |
| WHO functional class | 1,756 | 0.10 | |||||
| I | 9 (0.5) | 7 (0.6) | 1 (0.3) | 1 (0.5) | 0 (0.0) | ||
| II | 719 (40.9) | 478 (43.0) | 150 (38.9) | 65 (32.5) | 26 (44.8) | ||
| III | 1,017 (57.9) | 617 (55.5) | 235 (60.9) | 133 (66.5) | 32 (55.2) | ||
| IV | 11 (0.6) | 10 (0.9) | 0 (0.0) | 1 (0.5) | 0 (0.0) | ||
| Six-minute-walk distance, m | 1,756 | 351.0 ± 84.2 | 356.2 ± 83.3 | 353.1 ± 78.8 | 321.5 ± 91.8 | 338.2 ± 89.2 | <0.001 |
| Mean right atrial pressure, mm Hg | 1,547 | 8.0 [5.0, 11.0] | 7.0 [5.0, 10.0] | 7.5 [5.0, 11.0] | 11.0 [7.0, 16.0] | 8.0 [5.0, 11.0] | <0.001 |
| Mean pulmonary artery pressure, mm Hg | 1,702 | 50 [41, 60] | 49 [40, 58] | 53 [42, 61] | 54 [45, 62] | 51 [43, 58] | <0.001 |
| Pulmonary artery wedge pressure, mm Hg | 1,606 | 9.0 [7.0, 12.0] | 9.0 [7.0, 12.0] | 9.0 [7.0, 12.0] | 10.0 [7.0, 12.0] | 10.0 [6.0, 12.0] | 0.38 |
| Cardiac index, L/min/m2 | 1,636 | 2.40 [1.96, 2.90] | 2.48 [2.02, 2.95] | 2.23 [1.90, 2.70] | 2.12 [1.79, 2.63] | 2.40 [1.92, 2.84] | <0.001 |
| Pulmonary vascular resistance, Wood units | 1,663 | 9.4 [6.7, 13.4] | 8.8 [6.3, 12.5] | 10.9 [7.7, 14.7] | 11.3 [7.8, 15.7] | 10.0 [7.7, 13.6] | <0.001 |
| Treatment assignment, n (%) | 1,756 | 0.70 | |||||
| Active | 876 (49.9) | 556 (50.0) | 199 (51.6) | 93 (46.5) | 28 (48.3) | ||
| Control | 880 (50.1) | 556 (50.0) | 187 (48.4) | 107 (53.5) | 30 (51.7) |
Definition of abbreviations: NTproBNP = N-terminal probrain natriuretic peptide; PH = pulmonary hypertension; WHO = World Health Organization.
Data shown as n (%), mean ± SD, or median [interquartile range].
Includes cholecystitis and cholelithiasis (n = 87), hepatitis viral infections (n = 60), hepatocellular damage and hepatitis (n = 53), hepatic fibrosis and cirrhosis (n = 22), liver function analyses (n = 13), cholestasis and jaundice (n = 10), hepatobiliary neoplasms benign (n = 7), hepatobiliary signs and symptoms (n = 6), hepatic vascular disorders (n = 2), hepatobiliary neoplasms malignant (n = 1), others (n = 15).
Longitudinal Changes in Liver Injury Phenotype by Treatment Assignment
A total of N = 4,932 patients had liver biomarker data available at follow-up (1,838 at Week 4 or 8 and 3,094 at Week 12 and/or 16). Most patients who were excluded from longitudinal analysis of liver injury phenotype had a single value missing and therefore could not be clustered. Randomization to active treatment was associated with a significantly lower odds of transitioning to a “worse” liver injury phenotype at 12 or 16 weeks (odds ratio, 0.67 [95% confidence interval, 0.56, 0.81], P < 0.001) compared with randomization to control after covariate adjustment (Figure 3). More patients in the “cholestatic injury pattern” phenotype treated with active therapy transitioned to the “no liver injury” and “hepatocellular injury pattern” phenotypes than those in the control group, who were more likely to transition from the “hepatocellular injury pattern” phenotype to the “cholestatic injury pattern” phenotype and from the “no liver injury” group to the “hepatocellular injury pattern” group. The interaction between treatment and baseline phenotype in terms of liver injury progression was not significant (P for interaction = 0.62), meaning that the baseline liver pattern phenotype did not determine how much allocation to active treatment versus placebo impacted the transition to other liver injury patterns.
Figure 3.
Changes in liver injury phenotype from baseline to Week 12 or 16, by assignment to active versus control arms. Liver injury phenotype modeled as an ordinal variable (from better to worse): no liver injury > hepatocellular injury pattern > cholestatic injury pattern > combined injury pattern. The overall effect of pulmonary arterial hypertension treatment on the change in liver injury phenotype from baseline to 12 or 16 weeks was assessed using ordinal regression (odds ratio for change to more severe liver injury phenotype for active drug vs. control, 0.67; 95% confidence interval, 0.56, 0.81; P < 0.001). The model was adjusted for baseline phenotype, age, sex, baseline 6-minute-walk distance, pulmonary hypertension etiology, and body mass index as fixed effects and trial as a random effect.
Association between Liver Injury Phenotype and Outcomes
A total of 1,140 patients (249 per 1,000 person-years) had at least one clinical worsening event during follow-up. The “hepatocellular injury pattern,” “cholestatic injury pattern,” and “combined injury pattern” phenotypes had significantly higher likelihood of having a clinical worsening event than the “no liver injury” phenotype using Kaplan-Meier curves and after covariate adjustment (Figure 4, Table 4). These associations persisted when only hospitalization for worsening pulmonary hypertension and death were included as outcomes and with additional adjustment for baseline and (for those who had it available) follow-up right atrial pressure and cardiac index. The “hepatocellular injury pattern” was associated with a higher risk of clinical worsening and hospitalization or death. The results were generally similar when liver injury phenotype was modeled as a time-varying covariate (i.e., accounting for the change in liver injury phenotype from baseline to 12 or 16 wk) (Table E4). Patients with the “cholestatic injury pattern” had an even more pronounced increased risk in this analysis.
Figure 4.
Kaplan-Meier curves for (A) time to clinical worsening and (B) overall survival.
Table 4.
Association between Baseline Liver Injury Phenotype and Clinical Worsening Events and Overall Survival
| Outcome |
Phenotype | Unadjusted Hazard Ratio (95% CI) | P Value | Adjusted Hazard Ratio (95% CI)* | P Value |
|---|---|---|---|---|---|
| Entire sample | |||||
| Clinical worsening | No liver injury | Ref. | Ref. | ||
| Hepatocellular injury pattern | 1.33 (1.14, 1.55) | <0.001 | 1.30 (1.11, 1.51) | <0.001 | |
| Cholestatic injury pattern | 2.25 (1.90, 2.67) | <0.001 | 1.79 (1.50, 2.13) | <0.001 | |
| Combined injury pattern | 1.84 (1.34, 2.53) | <0.001 | 1.67 (1.21, 2.29) | 0.002 | |
| Clinical worsening (death or hospitalization for worsening PH only) | No liver injury | Ref. | Ref. | ||
| Hepatocellular injury pattern | 1.49 (1.23, 1.81) | <0.001 | 1.41 (1.17, 1.71) | <0.001 | |
| Cholestatic injury pattern | 2.62 (2.13, 3.22) | <0.001 | 1.90 (1.54, 2.35) | <0.001 | |
| Combined injury pattern | 2.25 (1.54, 3.28) | <0.001 | 1.97 (1.35, 2.88) | <0.001 | |
| All-cause death | No liver injury | Ref. | Ref. | ||
| Hepatocellular injury pattern | 1.49 (1.07, 2.07) | 0.019 | 1.36 (0.98, 1.88) | 0.068 | |
| Cholestatic injury pattern | 3.06 (2.18, 4.30) | <0.001 | 2.04 (1.44, 2.88) | <0.001 | |
| Combined injury pattern | 3.44 (2.01, 5.89) | <0.001 | 2.71 (1.58, 4.65) | <0.001 | |
| Subset of studies/subjects with baseline (and, if performed, follow-up) hemodynamics | |||||
|---|---|---|---|---|---|
| Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI)*† | ||||
| Clinical worsening | No liver injury | Ref. | Ref. | ||
| Hepatocellular injury pattern | 1.31 (1.11, 1.54) | <0.001 | 1.22 (0.99, 1.55) | 0.066 | |
| Cholestatic injury pattern | 2.24 (1.11, 2.54) | <0.001 | 1.60 (1.22, 2.11) | <0.001 | |
| Combined injury pattern | 1.69 (1.17, 2.42) | 0.005 | 1.66 (1.03, 2.69) | 0.039 | |
| Clinical worsening (death or hospitalization for worsening PH only) | No liver injury | Ref. | Ref. | ||
| Hepatocellular injury pattern | 1.44 (1.18, 1.77) | <0.001 | 1.29 (0.99, 1.68) | 0.056 | |
| Cholestatic injury pattern | 2.66 (2.14, 3.30) | <0.001 | 1.84 (1.34, 2.53) | <0.001 | |
| Combined injury pattern | 2.08 (1.35, 3.19) | <0.001 | 2.16 (1.23, 3.78) | 0.007 | |
| All-cause death | No liver injury | Ref. | Ref. | ||
| Hepatocellular injury pattern | 1.39 (0.96, 2.01) | 0.078 | 1.24 (0.78, 1.97) | 0.364 | |
| Cholestatic injury pattern | 3.20 (2.23, 4.59) | <0.001 | 2.39 (1.42, 4.00) | <0.001 | |
| Combined injury pattern | 2.40 (1.20, 4.81) | 0.013 | 1.70 (0.63, 4.61) | 0.296 | |
Definition of abbreviations: CI = confidence interval; Ref. = referent.
Adjusted for age, sex, body mass index, pulmonary arterial hypertension etiology, baseline 6-minute-walk distance, and treatment assignment.
Additional adjustment for baseline right atrial pressure and cardiac index and (if available) follow-up right atrial pressure and cardiac index as time-varying covariates.
A total of 246 patients (49 per 1,000 person-years) died during follow-up. The “cholestatic injury pattern” phenotype had a significantly higher likelihood of mortality than the “no liver injury” phenotype, which persisted with additional adjustment for baseline and (for those who had them available) follow-up right atrial pressure and cardiac index and when liver injury phenotype was modeled as a time-varying covariate (Figure 4, Tables 4 and E4).
The “cholestatic injury pattern” phenotype had a statistically significantly (but only 10 m) lower change in 6MWD at 12 or 16 weeks than the “no liver injury” phenotype (Figure E7). There was no clinically important association between baseline liver injury phenotype and Medical Outcomes Study 36-item Short Form Health Survey physical component summary and mental component summary scores at 12 or 16 weeks (Figures E8 and E9). There was no association between baseline liver injury phenotype and WHO functional class at 12 or 16 weeks (Table E5).
Heterogeneity of Treatment Effects
There was possible treatment effect heterogeneity by liver injury phenotype (Figure 5). Treatment effects may have been different among types of liver injury pattern in terms of time to clinical worsening (P for interaction = 0.14). Patients with a “cholestatic injury pattern” had a significantly greater treatment effect in terms of 6MWD (P for interaction < 0.001) and possibly WHO functional class (P for interaction = 0.10) than among those in the other liver phenotypes.
Figure 5.
Treatment assignment by liver injury phenotype interaction in terms of (A) change in 6-minute-walk distance at 12 or 16 weeks, (B) time to clinical worsening, (C) time to death, and (D) worsened World Health Organization functional class at 12 or 16 weeks. All models were adjusted for age, sex, body mass index, pulmonary arterial hypertension etiology, and baseline 6-minute-walk distance (except for baseline World Health Organization functional class in that model). aHR = adjusted hazard ratio; aOR = adjusted odds ratio; CI = confidence interval.
Discussion
In a large dataset of harmonized placebo-controlled RCTs, data from five liver tests identified four clusters of patients with liver phenotypes using unsupervised machine learning. Allocation to active treatment significantly reduced the odds of having a worse liver injury phenotype at 12 or 16 weeks. Patients with liver injury had a significantly increased risk of clinical worsening and (for cholestatic liver injury) mortality compared with the “no liver injury” phenotype even after controlling for cardiac function and central filling pressures. The “cholestatic injury pattern” phenotype had a significantly lower change in 6MWD at 12 or 16 weeks than the “no liver injury” phenotype, independent of heart function. The placebo-adjusted treatment effect on the 6MWD was greater in patients with the “hepatocellular injury pattern” or “cholestatic injury pattern” phenotype than in patients with the “no liver injury” phenotype. There were possible treatment by liver phenotype interactions in terms of clinical worsening and WHO functional class, but these were not statistically significant.
Clinical and Research Implications
The clinical impact of these findings include the first (to our knowledge) documentation of subclinical or latent hepatic endotypes, which confirm the clinical importance of systemic effects of PAH and CTEPH even in apparently stable participants entering RCTs. RCTs of novel interventions not only could stratify patients at baseline by liver phenotype to study heterogeneity of treatment effects or to enrich a clinical trial study population but also could use these phenotypes as noninvasive and low-complexity clinical or surrogate outcomes, if validated. Finally, further studies of how even subclinical hepatic injury impacts disease course are needed.
Prior Studies
Some studies have focused on liver evaluation in PAH. Higher bilirubin and lower albumin have been associated with an increased risk of death (12–18). A recent study defining cardiohepatic syndrome in PAH by having at least two of TB, ALP, and GGT above the limit of normal showed an association with increased risk of death but was limited by a small sample size and single-center design (19). Liver imaging has also tracked with RV diastolic function and predicted outcomes in smaller single-center studies in PAH, but large multicenter studies such as this one have not be published, to our knowledge (20–23).
Liver Injury Patterns
Traditionally, abnormalities in liver structure and/or function in congestive heart failure have been attributed to impaired perfusion (i.e., ischemia, usually in the setting of acute critical illness), congestive hepatopathy due to chronically elevated venous pressures, or drug toxicity (24). The traditional blood biochemical profile of impaired perfusion includes dramatic increases in transaminases and lactate dehydrogenase, sometimes followed by TB and prothrombin time. The second largest cluster in this study was characterized by variation in (higher) transaminases even if not frankly elevated and despite some of the trials excluding patients with significantly elevated transaminases, leading to a truncated distribution. We have called this the “hepatocellular injury pattern” phenotype, although we do not have liver biopsies. The profile of this group suggests that cell damage or necrosis (usually seen around the central veins in traditional acute ischemic injury) may occur subclinically in PAH or CTEPH without critical illness. Alternatively, variation in transaminases could be a manifestation of drug toxicity; however, that would be unlikely in almost one-fourth of patients in these RCTs at baseline. Liver congestion could explain the findings, although right atrial pressure was similar between this group and the “no liver injury” cluster. Alternatively, the “hepatocellular injury pattern” phenotype had somewhat lower cardiac index and higher pulmonary vascular resistance than the “no liver injury” phenotype, consistent with hypoperfusion as a mechanism for liver injury.
The third cluster (defined by higher TB and ALP, considered a “cholestatic injury pattern”) was most consistent with congestive hepatopathy, usually attributed to increased hepatic venous pressure, decreased hepatic blood flow, and decreased arterial oxygen saturation (24). Chronically elevated central venous pressure leads to sinusoidal engorgement and degeneration, which can lead to bridging fibrosis, cirrhosis, and even hepatocellular carcinoma. Supporting venous congestion as a key factor, the “cholestatic injury pattern” phenotype had a mean right atrial pressure that was ∼4–6 mm Hg higher than the other groups with lower cardiac index and higher pulmonary vascular resistance. Recent studies have shown that vena cava backflow is predominantly caused by RV diastolic stiffness and decreased right atrial active filling with right atrioventricular uncoupling (25, 26). Pressure–volume loops in the right atrium could more precisely assess the potential for congestive hepatopathy. This cluster had more men, known to have more severe RV dysfunction in PAH, as well as significantly shorter 6MWD and higher BNP concentrations (27, 28).
The final cluster was composed of a very small number of patients defined by variation in albumin and a substantial proportion (but still a minority) with a liver diagnosis at randomization. Our conclusions from the overall study were similar with reanalysis after excluding patients with existing liver disease (data not shown). These injury patterns could serve as a way to “deep phenotype” patients with PAH, providing richer information than a single hemodynamic assessment at a point in time.
Effect of Treatment on Liver Injury Patterns
Short-term PAH treatment mitigated liver injury. Randomization to the active treatment arms resulted in a greater proportion of patients moving from a liver disease cluster to the healthy state or from the cholestatic or other injury phenotypes to a lesser degree of disease (i.e., “hepatocellular injury pattern”) over 3–4 months. Patients randomized to placebo were more likely to move from healthy or less severe liver injury phenotypes to more severe liver injury categories. Investigators have shown that improved RV diastolic stiffness with treatment leads to increased RV active filling and normalization of vena cava backflow, which may in part explain our findings (25). Our results show the clinical impact of PAH therapy on liver health even over the short term, suggesting a novel mechanistic avenue (hepatic function) by which patients improve with current treatments.
Liver Injury Pattern and Outcomes
The presence of the “cholestatic injury pattern” or “hepatocellular injury pattern” was associated with an increased risk of clinical worsening after adjustment for potential confounders. Although liver phenotype could just reflect the hemodynamic severity of disease, this finding persisted despite adjustment for type of pulmonary hypertension and 6MWD at baseline as well as baseline and (when available) follow-up right atrial pressure and cardiac index. The “cholestatic injury pattern” phenotype was also associated with mortality and a significantly lower change in 6MWD. Therefore, it is possible that these liver disease profiles could be linked with other processes, such as nutrition, inflammation, and metabolic syndrome, which may not be adequately addressed by therapies for pulmonary hypertension and could impact short- and long-term outcomes.
Heterogeneity of Treatment Effects on Outcome by Liver Phenotype
There was possible heterogeneity of treatment effect by liver injury phenotype (i.e., greater treatment effect in those with “worse” liver injury type) in terms of clinical worsening (P for interaction = 0.14) and WHO functional class (P for interaction = 0.10), but these were not statistically significant. Patients with the “hepatocellular injury pattern” or “cholestatic injury pattern” phenotypes had a significantly larger treatment effect on 6MWD than patients with “no liver injury.” This could have a major impact on RCT design. Rather than excluding patients with liver injury patterns, investigators may want to enrich a study for these patients who may gain a greater treatment benefit in terms of exercise capacity, functional capacity, and clinical worsening.
Strengths and Limitations
This study had several strengths but also some limitations. We derived and then validated our clustering of patients by patterns of liver tests in a large sample of clinical trial subjects in an unsupervised approach. The clinical characteristics of the clusters in the validation set were very similar to those of the training set, despite the validation set being distinct in terms of time, background therapy, and other features (9). The drugs in these clinical trials are used widely to treat patients with pulmonary hypertension and are approved by regulatory boards on the basis of these studies; our findings should be considered as externally valid as the main results of the therapeutic trials themselves. We focused on efficacy; however, there could also be differences in side effect profile, adverse events, and early discontinuations between the clusters. We chose one approach to clustering. There are certainly alternative approaches that could be useful; however, any approach needs to be validated beyond the derivation set used to establish the clustering.
Some trials did have exclusion criteria pertinent to liver disease, which may have changed the distribution of liver injury patterns and resulted in selection bias. It is therefore likely that more severe or distinct liver injury phenotypes could be observed in clinical practice that were not included in the study sample, although we would expect even stronger association with outcomes with more severe liver injury. Certain liver tests that might have refined the clusters were not routinely drawn in the sample (GGT, direct bilirubin), and we avoided using the international normalized ratio because some of these patients were receiving vitamin K antagonists (and the trials in our validation set did not collect international normalized ratios). We adjusted for confounders (including baseline and follow-up hemodynamics, which were available in a subset of the study sample); however, residual or unmeasured confounding is still possible. We had a focused set of hypotheses and therefore did not account for multiple comparisons, which could result in type I error. Type II error is possible (especially in studying effect modification); however, this is the largest study of liver function tests in patients with PAH, to our knowledge. Although we have assumed that subjects in these studies did not participate in more than one study, we cannot be certain of this. Some studies included participants who were receiving bosentan as background therapy, which could affect liver function tests. However, in the studies that reported specific drugs, the prevalence of bosentan use in the “no liver injury” group was similar to or even greater than in the groups with liver injury (data not shown). Finally, data from recent studies of sotatercept were not available for sharing at this point and could not be included in this analysis.
Conclusions
Distinct clusters of liver health based on patterns of liver tests exist in PAH and CTEPH. Patients with certain injury patterns have an increased risk of clinical worsening and mortality. There may have been a larger treatment effect in patients with liver injury than in others. Future studies need to pursue a better understanding of the contribution of liver disease to PAH and the effect of therapies on outcomes.
Supplemental Materials
Acknowledgments
Acknowledgment
The authors thank the participants and sponsors for generating these data. The authors appreciate the sharing of these data by the U.S. Food and Drug Administration.
Footnotes
Supported by the National Institutes of Health (K24 HL103844) and the Cardiovascular Medical Research and Education Fund (S.M.K.), the American Thoracic Society Early Career Investigator Award in Pulmonary Vascular Disease (J. Minhas), and the National Institutes of Health/National Heart, Lung, and Blood Institute (K23HL141584 [N.A.-N.]).
Author Contributions: J.V.S., J. Moutchia, and S.M.K. designed the study and drafted the manuscript with significant input from R.L.M., N.A.-N., E.W., H.I.P., A.S., S.C.P., and C.E.V. D.K.A. and J. Minhas harmonized the individual participant data. J.V.S., J. Moutchia, and R.L.M. analyzed the data. All authors contributed to interpretation of the results and critical review of the manuscript and approved the final draft.
A data supplement for this article is available via the Supplements tab at the top of the online article.
Originally Published in Press as DOI: 10.1164/rccm.202311-2196OC on May 31, 2024
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Rosenkranz S, Howard LS, Gomberg-Maitland M, Hoeper MM. Systemic consequences of pulmonary hypertension and right-sided heart failure. Circulation . 2020;141:678–693. doi: 10.1161/CIRCULATIONAHA.116.022362. [DOI] [PubMed] [Google Scholar]
- 2. Marcus JT, Westerhof BE, Groeneveldt JA, Bogaard HJ, de Man FS, Vonk Noordegraaf A. Vena cava backflow and right ventricular stiffness in pulmonary arterial hypertension. Eur Respir J . 2019;54:1900625. doi: 10.1183/13993003.00625-2019. [DOI] [PubMed] [Google Scholar]
- 3. Nickel NP, Galura GM, Zuckerman MJ, Hakim MN, Alkhateeb H, Mukherjee D, et al. Liver abnormalities in pulmonary arterial hypertension. Pulm Circ . 2021;11:20458940211054304. doi: 10.1177/20458940211054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. ESC/ERS Scientific Document Group 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J . 2022;43:3618–3731. doi: 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 5. Scott JV, McClelland R, Weinberg E, Al-Namaani N, Baird G, Holmes J, et al. Unsupervised machine learning for identification of cardiohepatic syndrome in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension [abstract] Am J Respir Crit Care Med . 2022;205:A5304. [Google Scholar]
- 6.Scott JV, Moutchia J, McClelland RL, Al-Naamani N, Weinberg E, Palevsky HI, et al. 2023. [DOI]
- 7. Galiè N, Hoeper MM, Gibbs JSR, Simonneau G. Liver toxicity of sitaxentan in pulmonary arterial hypertension. Eur Respir J . 2011;37:475–476. doi: 10.1183/09031936.00194810. [DOI] [PubMed] [Google Scholar]
- 8. Urbanowicz RJ, Holmes JH, Appleby D, Narasimhan V, Durborow S, Al-Naamani N, et al. A semi-automated term harmonization pipeline applied to pulmonary arterial hypertension clinical trials. Methods Inf Med . 2022;61:3–10. doi: 10.1055/s-0041-1739361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Min J, Appleby DH, McClelland RL, Minhas J, Holmes JH, Urbanowicz RJ, et al. Secular and regional trends among pulmonary arterial hypertension clinical trial participants. Ann Am Thorac Soc . 2022;19:952–961. doi: 10.1513/AnnalsATS.202110-1139OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edy U, Jatmiko Endro S, Gunawan SKV. Atlantis Press; 2020. pp. 121–129. [Google Scholar]
- 11. Sesham A, Padmanabham P, Govardhan D. Application of factor analysis to k-means clustering algorithm on transportation data. Int J Comput Appl . 2014;95:40–46. [Google Scholar]
- 12. Xu XQ, Lv ZC, Liu QQ, Zhao QH, Wu Y, Sun K, et al. Direct bilirubin: a new risk factor of adverse outcome in idiopathic pulmonary arterial hypertension. Int J Cardiol . 2017;228:895–899. doi: 10.1016/j.ijcard.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 13. Takeda Y, Takeda Y, Tomimoto S, Tani T, Narita H, Kimura G. Bilirubin as a prognostic marker in patients with pulmonary arterial hypertension. BMC Pulm Med . 2010;10:22. doi: 10.1186/1471-2466-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation . 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 15. Benza RL, Gomberg-Maitland M, Naeije R, Arneson CP, Lang IM. Prognostic factors associated with increased survival in patients with pulmonary arterial hypertension treated with subcutaneous treprostinil in randomized, placebo-controlled trials. J Heart Lung Transplant . 2011;30:982–989. doi: 10.1016/j.healun.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 16. Kawut SM, Horn EM, Berekashvili KK, Garofano RP, Goldsmith RL, Widlitz AC, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol . 2005;95:199–203. doi: 10.1016/j.amjcard.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 17. Haddad F, Peterson T, Fuh E, Kudelko KT, de Jesus Perez V, Skhiri M, et al. Characteristics and outcome after hospitalization for acute right heart failure in patients with pulmonary arterial hypertension. Circ Heart Fail . 2011;4:692–699. doi: 10.1161/CIRCHEARTFAILURE.110.949933. [DOI] [PubMed] [Google Scholar]
- 18. Snipelisky D, Jentzer J, Batal O, Dardari Z, Mathier M. Serum albumin concentration as an independent prognostic indicator in patients with pulmonary arterial hypertension. Clin Cardiol . 2018;41:782–787. doi: 10.1002/clc.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sungur A, Sungur MA, Karagöz A, Sert Şekerci S, Esen Zencirci A, Tanboğa IH, et al. Cardiohepatic syndrome and its prognostic predictive ability in patients with pulmonary arterial hypertension. Scand J Clin Lab Invest . 2023;83:290–298. doi: 10.1080/00365513.2023.2225778. [DOI] [PubMed] [Google Scholar]
- 20. Bogaert J, Claessen G, Dresselaers T, Masci PG, Belge C, Delcroix M, et al. Magnetic resonance relaxometry of the liver – a new imaging biomarker to assess right heart failure in pulmonary hypertension. J Heart Lung Transplant . 2022;41:86–94. doi: 10.1016/j.healun.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 21. Guo J, Wang L, Wang J, Wan K, Gong C, Chen X, et al. Prognostic value of hepatic native T1 and extracellular volume fraction in patients with pulmonary arterial hypertension. J Am Heart Assoc . 2022;11:e026254. doi: 10.1161/JAHA.122.026254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kremer N, Roller FC, Kremer S, Schafer S, Kryvenko V, Rako ZA, et al. Native hepatic T1-time as a non-invasive predictor of diastolic dysfunction and a monitoring tool for disease progression and treatment response in patients with pulmonary hypertension Int J Card 2024;409:132189 [DOI] [PubMed] [Google Scholar]
- 23.Rako ZA, Yogeswaran A, Yildiz S, Weidemann P, Zedler D, Brito da Rocha BR, et al. Liver stiffness is associated with right heart dysfunction, cardiohepatic syndrome, and prognosis in pulmonary hypertension J Heart Lung Transplant 2024;43:1105–1115. [DOI] [PubMed] [Google Scholar]
- 24. Samsky MD, Patel CB, DeWald TA, Smith AD, Felker GM, Rogers JG, et al. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol . 2013;61:2397–2405. doi: 10.1016/j.jacc.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 25. Wessels JN, Mouratoglou SA, van Wezenbeek J, Handoko ML, Marcus JT, Meijboom LJ, et al. Right atrial function is associated with right venticular diastolic stiffness: RA-RV interaction in pulmonary arterial hypertension. Eur Respir J . 2022;59:2101454. doi: 10.1183/13993003.01454-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wessels JN, van Wezenbeek J, de Rover J, Smal R, Llucià-Valldeperas A, Celant LR, et al. Right atrial adaptation to precapillary pulmonary hypertension: pressure-volume, cardiomyocyte, and histological analysis. J Am Coll Cardiol . 2023;82:704–717. doi: 10.1016/j.jacc.2023.05.063. [DOI] [PubMed] [Google Scholar]
- 27.Kawut SM, Al-Naamani N, Agerstrand C, Berman Rosenzweig E, Rowan C, Barst RJ, et al. Determinants of right ventricular ejection fraction in pulmonary arterial hypertension. Chest. 2009;135:752–759. doi: 10.1378/chest.08-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Wezenbeek J, Groeneveldt JA, Llucià-Valldeperas A, van der Bruggen CE, Jansen SMA, Smits AJ, et al. Interplay of sex hormones and long-term right ventricular adaptation in a Dutch PAH-cohort. J Heart Lung Transplant . 2022;41:445–457. doi: 10.1016/j.healun.2021.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.