Abstract
Canonical histone H3 and histone variant H3.3 are posttranslationally modified with the genomic distribution of these marks denoting different features and these modifications may influence transcription. While the majority of posttranslational modifications occur on histone tails, there are defined modifications within the globular domain, such as acetylation of H3K122/H3.3K122. To understand the function of the amino acid H3.3K122 in transcriptional regulation, we attempted to generate H3.3K122A mouse embryonic stem (mES) cells but were unsuccessful. Through multi-omic profiling of mutant cell lines harboring two or three of four H3.3 targeted alleles, we have uncovered that H3.3K122A is neomorphic and results in lethality. This is surprising as prior studies demonstrate H3.3-null mES cells are viable and pluripotent but exhibit a reduced differentiation capacity. Together, these studies have uncovered a novel dependence of a globular domain residue within H3.3 for viability and broadened our understanding of how histone variants contribute to transcription regulation and pluripotency in mES cells.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13072-024-00557-3.
Introduction
Individual canonical histone proteins within a nucleosome can be substituted with histone variants (reviewed in [1]), which play important roles in chromatin dynamics during DNA-templated activities [1, 2]. Variant histone proteins for each of the four canonical histone proteins have been identified, with among the most well studied being histone variant H3.3, which replaces canonical histone H3. In mammals, there are two genes that encode H3.3: H3f3a and H3f3b [2]. H3f3a and H3f3b encode an identical polypeptide sequence, but exhibit allele-specific gene structures, nucleic acid sequences, expression patterns, and have different requirements during development [3–6]. The amino acid sequence of variant H3.3 is very similar to that of canonical H3.1 and H3.2, with only five differing amino acids [1]. H3.3 has a context-specific relationship with transcription, and is found at regions of active transcription such as enhancers and regions of heterochromatin including telomeres and other repetitive regions of the genome [7–10]. The context specific role of H3.3 is mediated by distinct histone chaperone systems and posttranslational modifications: the HIRA complex incorporates H3.3 into euchromatin where it receives transcription-associated modifications, while the nucleosome remodeler ATRX and histone chaperone DAXX incorporate H3.3 into heterochromatic regions where it receives modifications associated with repression [8, 9, 11–13]. At these locations, H3.3 regulates the turnover of nucleosomes to promote proper transcription factor (TF) binding and/or maintenance of histone modifications to preserve the local chromatin state [14–17]. In murine embryonic stem cells (mES cells), H3.3 is dispensable for viability and regulation of pluripotency, but is required for differentiation and transitions between cell states [10, 13, 18, 19].
Active enhancers are characterized by distinct chromatin features, including flanking nucleosomes marked by H3K4me1 and H3K27ac [20, 21]. However, beyond these well-established histone modifications, there are additional modifications that are less well characterized. K122 is a residue found in both H3 and H3.3 at the nucleosome dyad and can be acetylated in mammalian cells and fission yeast (H3K122ac) [22]. In vitro, H3K122ac reduces histone/DNA interactions by destabilizing nucleosomes [23]. Though budding yeast lack H3K122ac, H3K122A, H3K122R, and H3K122Q mutations reduce nucleosome occupancy over highly transcribed genes and result in reduced telomeric, ribosomal, and HML silencing [24, 25]. Finally, the Bickmore lab found that, in mES cells and MCF-7 human cells, H3K122ac marks a subset of enhancers, sometimes without H3K27ac [26]. Together, these data suggest an important role for H3K122 in cells, with the implication that acetylation may stimulate transcription. However, to date, there has been no study dissecting how H3.3 and K122ac may coordinate to regulate transcription.
To examine the role of H3.3K122 in mES cells, we attempted to generate mES cells containing H3f3a and H3f3b K122A homozygous mutations. In addition to altering residue charge and size, a mutation that replaces the lysine residue with an alanine (H3.3K122A) would prevent acetylation (H3.3K122ac) as alanine residues are not readily acetylated. Despite many attempts to generate mES cells where all four H3.3 alleles contain the K122A mutation, we were unable to recover these cell lines, suggesting lethality. We succeeded in generating cell lines containing either two or three of the four H3.3 alleles with the K122A mutation. We were unable to deplete the expression of the remaining wildtype H3.3 within these cell lines, supporting that having H3.3K122A as the sole H3.3 protein results in lethality. Despite this, to investigate the impact of H3.3K122A on mES cell biology, we characterized cell lines containing two or three alleles with the H3.3K122A mutation. We found that with an increasing number of H3.3K122A alleles, the phenotypic, transcriptomic, and epigenomic defects are intensified. Together, these studies suggest that K122 (and perhaps K122ac) is critical to proper H3.3 function in mES cells.
Results
H3.3K122A may be lethal in mES cells
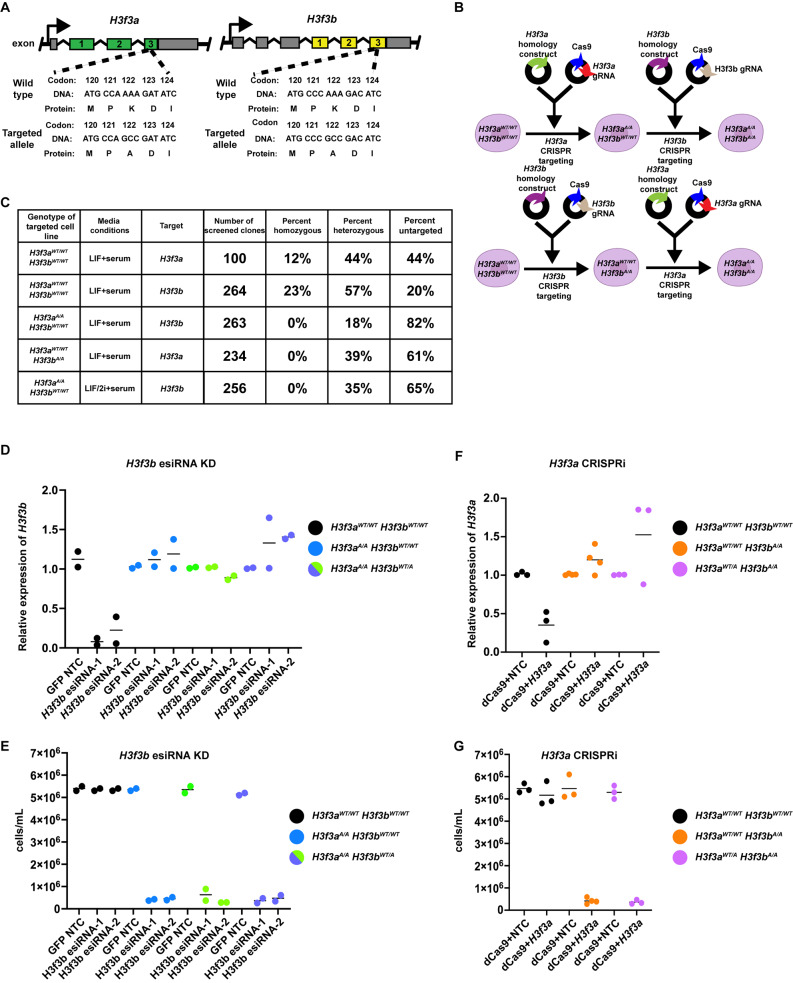
While H3f3a and H3f3b encode the same polypeptide, the gene structures and coding sequences of H3f3a and H3f3b are distinct (Fig. 1A). Therefore, we used a two-step targeting strategy to generate mutations in H3f3a and H3f3b. First, we independently targeted either H3f3a or H3f3b using CRISPR/Cas9-directed homologous recombination to replace the endogenous sequence with a mutation resulting in a single codon change to alter lysine 122 to alanine (K122A; Fig. 1B). We were able to generate homozygous single targeted cell lines (H3f3aA/AH3f3bWT/WT or H3f3aWT/WTH3f3bA/A) with reasonable frequency (Fig. 1C and Supplemental Table 1). Sequence-confirmed H3f3aA/AH3f3bWT/WT and H3f3aWT/WTH3f3bA/A cell lines (referred to as single targeted) were then independently targeted for the opposite H3.3 encoding gene, with the goal of generating cell lines containing all four alleles with the K122A mutation (Fig. 1B and Supplemental Table 1). Despite multiple attempts at generating H3f3aA/AH3f3bA/A cell lines in both LIF/serum and 2i + LIF/serum media, we were unable to generate these clones (Fig. 1C and Supplemental Table 1). However, we did obtain dual targeted (either H3f3aWT/AH3f3bA/A or H3f3aA/AH3f3bWT/A) cell lines where three of the four alleles are mutated to alanine (Fig. 1C and Supplemental Table 1).
Fig. 1.
H3.3K122A mutation may be lethal in ES cells. A. Schematic of H3f3a (left) and H3f3b (right) loci, with wildtype and targeted DNA sequences denoted. B. Schematic of two-step targeting method to generate H3.3K122A cell lines. C. Table summarizing the mES cell clones screened to generate single targeted cell lines (top two rows) and dual targeted cell lines (bottom four rows). “Media conditions” indicates the media formulation used during targeting. A full description of clones screened can be found in the Methods and in Supplemental Table 1. D. RT-qPCR results of esiRNA knockdowns targeting H3f3b or GFP non-template control (NTC) in wildtype cell lines (control for KD efficiency), H3f3aA/AH3f3bWT/WT cell lines, and H3f3aA/AH3f3bWT/A cell lines. Each point is an independent biological replicate. The line represents the average of biological replicates. E. Number of viable cells for esiRNA knockdowns targeting H3f3b or GFP non-template control (NTC) in wildtype cell lines (control for KD efficiency), H3f3aA/AH3f3bWT/WT cell lines (parental), and H3f3aA/AH3f3bWT/A cell lines. Each point is an independent biological replicate. The line represents the average of biological replicates. F. As in D, for RT-qPCR results of CRISPRi targeting of H3f3a or non-template control (NTC) in wildtype cell lines (control for efficiency), H3f3aWT/WTH3f3bA/A cell lines (parental), and H3f3aWT/AH3f3bA/A cell line. G. As in E, for CRISPRi targeting of H3f3a or non-template control (NTC) in wildtype cell lines (control for efficiency), H3f3aWT/WTH3f3bA/A cell lines (parental), and H3f3aWT/AH3f3bA/A cell lines
Given these results, we hypothesized that H3f3aA/AH3f3bA/A may be lethal in mES cells. To test this, we attempted to deplete remaining wildtype H3.3 using esiRNAs or CRISPRi in single targeted (H3f3aWT/WTH3f3bA/A or H3f3aA/AH3f3bWT/WT) and dual targeted (H3f3aWT/AH3f3bA/A or H3f3aA/AH3f3bWT/A) cell lines. We designed and tested two independent sets of esiRNAs targeting H3f3b and transfected these into wildtype, H3f3aA/AH3f3bWT/WT, or H3f3aA/AH3f3bWT/A cell lines using esiRNAs targeting GFP as an unexpressed non-template control (NTC). After 48 h, we observed 85–95% depletion of H3f3b in wildtype cells using either set of H3f3b-targeting esiRNAs, yet no depletion of H3f3b in cell lines harboring H3f3aA/A and H3f3bWT/WT or H3f3bWT/A (Fig. 1D). We believe this is due to lethality upon successful H3f3b depletion in these cell lines, as we observed a ~ 10-fold reduction in viable cells in H3f3aA/A and H3f3bWT/WT or H3f3bWT/A relative to H3f3aWT/WTH3f3bWT/WT and H3f3aWT/AH3f3bWT/WT cell lines (Fig. 1E). Because we were unable to design unique esiRNAs targeting H3f3a that resulted in depletion, we generated sgRNAs targeting H3f3a and performed CRISPRi in wildtype, H3f3aWT/WTH3f3bA/A, and H3f3aWT/AH3f3bA/A cell lines (Fig. 1F). We observed ~ 55% depletion of H3f3a only in wildtype cells, but no depletion of H3f3a in cell lines harboring H3f3bA/A (Fig. 1F). Similar to H3f3b esiRNA targeting, CRISPRi targeting of H3f3a in H3f3aWT/WTH3f3bA/A and H3f3aWT/AH3f3bA/A cells resulted in an approximate 10-fold reduction in abundance of viable cells relative to controls (Fig. 1G). Together with our inability to generate H3f3aA/AH3f3bA/A clones, these results suggest that the H3.3K122A mutation at all four H3.3 alleles may be lethal in mES cells. These results are surprising as it has been previously shown that H3.3 KO mES cells and mES cells depleted of H3.3 are viable [10, 18]. Therefore, to further understand the role of H3.3K122 in mES cells, we characterized cell lines containing either two or three of the H3.3 alleles successfully targeted for H3.3K122A.
H3.3K122A results in reduced pluripotency and slow growth in mES cells
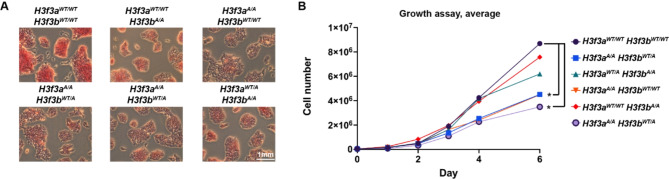
To understand how the H3.3K122A mutation impacts mES cell biology, we assessed whether mES cell pluripotency was disrupted using alkaline phosphatase (AP) staining (Fig. 2A, Supplemental Fig. 1A). AP is a membrane-bound protein highly expressed in mES cells, and is commonly used as a phenotypic proxy for pluripotency [27]. All mutant cell lines displayed reduced AP staining relative to wildtype, with H3f3aA/A or H3f3aWT/A containing cell lines showing slightly less staining relative to H3f3aWT/WT containing cell lines (Fig. 2A, Supplemental Fig. 1A). We next performed a 6-day growth assay with wildtype and mutant cell lines (Fig. 2B, Supplemental Fig. 1B-D). Though all mutant cell lines had a minor reduction in growth by day 6 relative to wildtype, H3f3aA/A containing cell lines had a more consistent and severe growth defect relative to H3f3aWT/WT or H3f3aWT/A containing cell lines (Fig. 2B, Supplemental Fig. 1B-D). These data support a previous study arguing that H3f3a is evolutionarily specialized for transcriptional programs associated with cell proliferation [5]. Together, these observations may suggest allele-specific requirements in the functions of H3.3K122 related to cell proliferation and regulation of pluripotency, with H3f3a having a more prominent role in mES cells.
Fig. 2.
H3.3K122A cell lines have reduced AP staining and growth relative to wildtype cells. A. Alkaline phosphatase (AP) staining of wildtype and H3.3K122A cell lines. Shown are representative images from one of three biological replicates. B. Growth curve for WT and H3.3K122A cell lines with live cell numbers quantified on 1, 2, 3, 4, and 6 days post plating. Graph represents the average of 3 biological replicates
H3.3K122A mES cells have reduced levels of H3.3
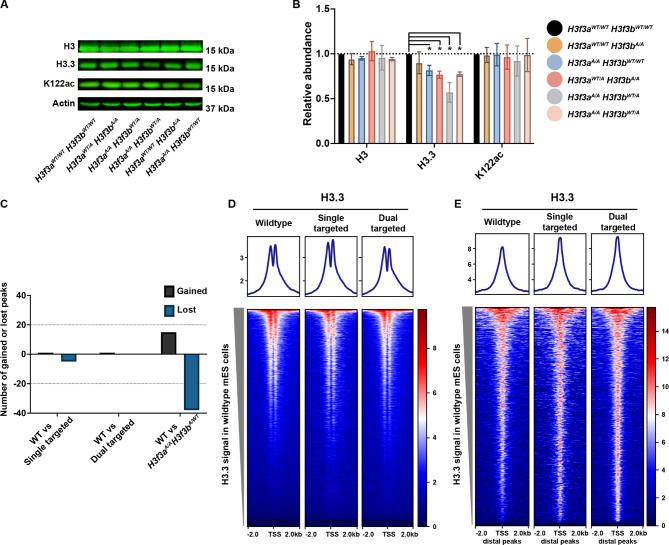
We next examined the protein levels of H3, H3.3, and K122ac (antibody recognizes both H3K122ac and H3.3K122ac) in each cell line and observed little change in H3 and K122ac abundance (Fig. 3A-B). Interestingly, all H3f3a-targeted cell lines (H3f3aWT/A or H3f3aA/A) exhibited a modest reduction in H3.3 abundance (~ 60–70% of WT levels) while the H3f3aWT/WTH3f3bA/A lines had little or no reduction (Fig. 3A-B). We performed total and nascent RT-qPCR and found that all cell lines exhibited similar levels of H3f3a but found the H3f3bA/A cell lines exhibited reduced expression and transcription of H3f3b (Supplemental Fig. 1E-F).
Fig. 3.
H3.3K122A cell lines have reduced H3.3 protein levels relative to wildtype cells but not reduced H3.3 chromatin occupancy. A. Western blot analysis for total H3, H3.3, and K122ac in wildtype and H3.3K122A cell lines. Actin is used as a loading control. B. Quantification of three biological replicates for each western blot in A. *p value ≤ 0.05, two sided T-test. C. Differentially enriched H3.3 CUT&RUN peaks (|LFC|≥0.5 and FDR ≤ 0.05) in the single targeted and dual targeted cell lines relative to wildtype. Black indicates number of gained peaks, and blue indicates number of lost peaks. D. Metaplots and heatmaps visualizing average H3.3 enrichment over TSSs in wildtype (n = 2), single targeted (n = 2), and dual targeted cell lines (n = 3). N = 22,598 loci. E. As in F, for gene distal H3.3 peaks. N = 3,347 loci
H3.3K122A cells display no major changes in H3.3 euchromatic occupancy
Given our finding that total H3.3 protein abundance is reduced in the H3.3K122A mutant cell lines, we examined whether H3.3 chromatin occupancy is altered. To that end, we performed Cleavage Under Target and Release Using Nuclease (CUT&RUN) for H3.3 in wildtype mES cells and each single/dual targeted cell line, with high reproducibility between replicates and across cell lines (Supplemental Fig. 1G). We defined 5,447 H3.3 consensus peaks across the datasets (excluding repetitive regions) yet found less than 60 H3.3 peaks with differential enrichment in all comparisons to wildtype (Fig. 3C). This implies that, while the targeted cell lines have modestly reduced protein abundance of total H3.3 relative to wildtype, these cell lines have maintained similar levels of H3.3 within chromatin. Visualization of H3.3 enrichment at transcription start sites (TSSs) and H3.3 gene distal peaks showed similar enrichment in wildtype, single targeted, and dual targeted cell lines, supporting the peak-based analysis (Fig. 3D-E).
Severity of transcription changes in H3.3K122A mutants increases with number of alleles targeted
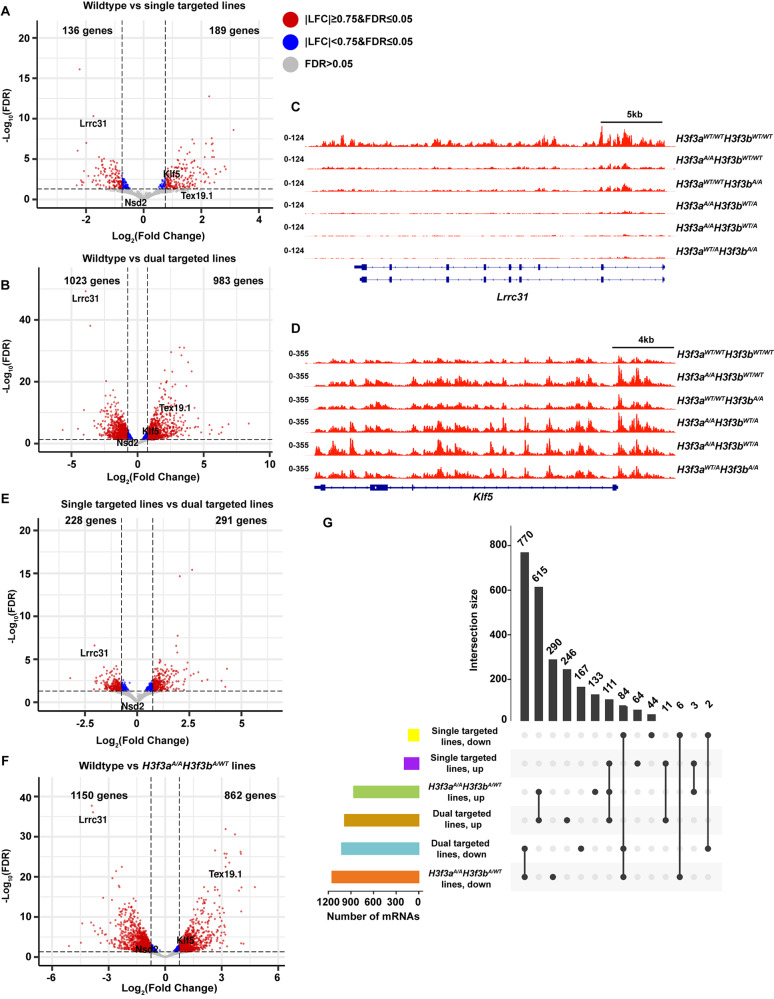
To understand how H3.3K122A impacts the mES cell transcriptome, we performed the nascent RNA sequencing method Transient Transcriptome sequencing (TT-seq [28]) in wildtype and H3.3K122A targeted cell lines with high reproducibility across replicates (Supplemental Fig. 2A). When comparing the single targeted (H3f3aA/AH3f3bWT/WT and H3f3aWT/WTH3f3bA/A) cell lines to wildtype, we observed hundreds of genes with transcriptional changes, with 136 downregulated and 189 upregulated genes (Fig. 4A). However, when comparing dual targeted (H3f3aA/AH3f3bWT/A and H3f3aWT/AH3f3bA/A) cell lines to wildtype, we detected thousands of genes with altered transcription (1023 downregulated and 983 upregulated; Fig. 4B). We selected a panel of genes upregulated (Klf5 and Tex19.1) and downregulated (Nsd2 and Lrcc31) and validated these trends with nascent and total RT-qPCR in all targeted cell lines (Supplemental Fig. 2B-E). Visual comparison of TT-seq data across the Lrrc31 and Klf5 locus in wildtype and mutant cell lines further validated these trends and indicate a relationship between the severity of transcriptional changes and the number of K122A targeted H3.3 alleles in the genetic background (Fig. 4C-D). We compared changes in transcription between the single and dual targeted cell lines, observing an intermediate number of genes with altered transcription, as expected with increasing severity due to increased K122A alleles (Fig. 4E). Since we performed TT-seq on two clones with identical genotypes (H3f3aA/AH3f3bWT/A), we also compared the H3f3aA/AH3f3bWT/A dual targeted lines relative to wildtype and found 1150 downregulated and 862 upregulated genes (Fig. 4F).
Fig. 4.
H3.3K122A results in transcriptomic changes in mES cells. A. Volcano plot of differentially transcribed genes in single targeted H3.3K122A cells (H3f3aA/AH3f3bWT/WT and H3f3aWT/WTH3f3bA/A, n = 2) relative to wildtype (n = 3). Red points are genes |LFC|≥0.75 and FDR ≤ 0.05, blue points are genes |LFC|<0.75 and FDR ≤ 0.05, and grey points are not significantly changed. B. As in A, for dual targeted lines (H3f3aA/AH3f3bA/WT and H3f3aA/WTH3f3bA/A, n = 3) relative to wildtype (n = 3). C. Browser track of nascent RNA-seq (TT-seq) from H3.3K122A cell lines and wildtype cells over the Lrrc31 locus. D. As in E, for the Klf5 locus. E. As in A, for dual targeted (n = 3) relative to single targeted cell lines (n = 2). F. As in A, for H3f3aA/AH3f3bA/WT lines (n = 2) relative to wildtype (n = 3). G. Upset plot showing the number of differentially transcribed genes (log2(FC) ≥ 0.75 and FDR ≤ 0.05) shared between genotypes (upper bar graph, black) and the overall number of differentially transcribed genes in each genotype (lower left bar graph)
We next compared how these differentially transcribed genes compared across single targeted (H3f3aA/AH3f3bWT/WT and H3f3aWT/WTH3f3bA/A) and dual targeted cell lines, where we examined dual targeted as either H3f3aA/AH3f3bWT/A only (two cell lines) or H3f3aA/AH3f3bWT/A and H3f3aWT/AH3f3bA/A (three cell lines; Fig. 4G). Moving forward, we will distinguish these genotypes as dual targeted when referring to results combined from H3f3aA/AH3f3bWT/A and H3f3aWT/AH3f3bA/A and as H3f3aA/AH3f3bWT/A when referring to results from these cell lines only.
When comparing the dual targeted vs. H3f3aA/AH3f3bWT/A differentially transcribed genes we found that the dual targeted vs. wildtype had 246 upregulated and 167 downregulated genes uniquely altered relative to H3f3aA/AH3f3bWT/A (Fig. 4G). These unique differentially transcribed genes therefore likely stem from the H3f3aWT/AH3f3bA/A line, thus allowing an opportunity to examine allele-specific effects. To that end, we performed gene ontology (GO) on the overlapping and unique gene sets from the H3f3aA/AH3f3bWT/A vs. wildtype and dual targeted vs. wildtype comparisons (Supplemental Fig. 2F). Genes upregulated and downregulated in both comparisons were enriched in terms related to metabolism and lineage commitment toward vasculogenesis. Genes uniquely downregulated in the H3f3aA/AH3f3bWT/A lines were enriched for processes related to neuronal lineage commitment, while uniquely upregulated genes were associated with epithelial lineage commitment. Together, these studies further support allele-specific requirements for H3f3a and H3f3b.
Dual targeted cell lines exhibit strong K27ac and subtle K122ac enrichment changes relative to single targeted or wildtype mES cells
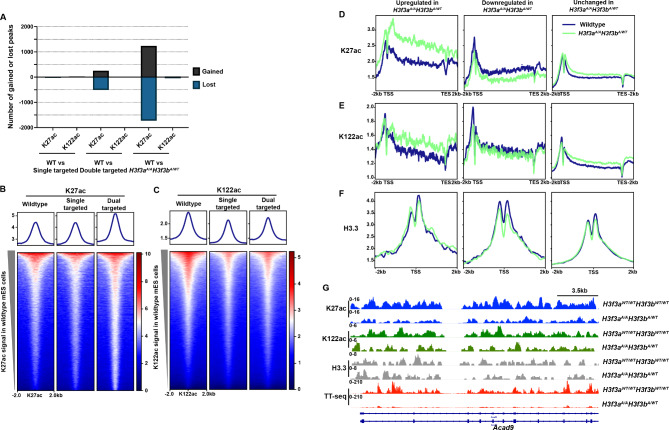
To evaluate how the H3.3K122A mutation influences the epigenome of mES cells, we examined the enrichment of K27ac (antibody recognizes both H3K27ac and H3.3K27ac) and K122ac (antibody recognizes both H3K122ac and H3.3K122ac) using CUT&RUN in wildtype and each single targeted and dual targeted line generating datasets with high reproducibility (Supplemental Fig. 3A, B). For K122ac, we found fewer than 100 peaks with differential enrichment in the single targeted or dual targeted lines relative to wildtype (Fig. 5A). For K27ac, we observed few differentially enriched peaks in the single targeted cell lines, hundreds in dual targeted cell lines relative to wildtype, and thousands when comparing H3f3aA/AH3f3bWT/A lines to wildtype (Fig. 5A). Notably, these data follow a similar trend to the observed transcriptomic changes (Fig. 4), with increasing severity as more targeted alleles are present, especially when H3f3a is mutated at both alleles.
Fig. 5.
H3f3aA/AH3f3bA/WT cell lines exhibit altered patterns of K27ac enrichment relative to wildtype mES cells. A. Bar graph depicting the number of differentially enriched K27ac or K122ac CUT&RUN peaks (|LFC|≥0.5 and FDR ≤ 0.05) for the indicated cell line comparisons. B. Enrichment of K27ac CUT&RUN signal in average wildtype (n = 2), single targeted (n = 2), and dual targeted cell lines (n = 3) over K27ac consensus peaks (this study). C. Enrichment of K122ac CUT&RUN signal over average wildtype (n = 2), single targeted (n = 2), and dual targeted cell lines (n = 3) over K122ac consensus peaks called using previously published H3K122ac ChIP-seq data [26]. D. Metaplots of K27ac enrichment over genes upregulated, downregulated, or unchanged in H3f3aA/AH3f3bA/WT cell lines. E. As in D, for K122ac enrichment. F. As in D, for H3.3 enrichment. G. Browser track showing replicate averaged enrichment of K27ac (blue), K122ac (green), H3.3 (grey), and sense nascent transcription (red) over the 5’ end of the Acad9 locus in H3f3aA/AH3f3bA/WT and wildtype mES cells
We next examined K122ac and K27ac enrichment over our consensus K27ac CUT&RUN peaks and K122ac peaks called from published ChIP-seq datasets [26] in wildtype, single targeted, and dual targeted cell lines. We found that while wildtype and single targeted cell lines had comparable levels of K27ac enrichment, the dual targeted cell lines exhibited higher levels of K27ac over consensus peaks (Fig. 5B). Notably, both single and dual targeted cell lines exhibited lower levels of K122ac over K122ac ChIP-seq peaks relative to wildtype (Fig. 5C). As we predicted that the targeted cell lines would have reduced abundance and chromatin incorporation of H3.3K122ac, but the K122ac antibody does not distinguish H3.3K122ac from H3K122ac, these findings suggest that H3.3K122ac may represent a fraction of the global K122ac found in mES cells.
K27ac, K122ac, and H3.3 levels over promoters and gene bodies reflect transcription changes in targeted cell lines
We next examined how H3K27ac enrichment changes in the H3f3aA/AH3f3bWT/A lines might relate to the transcription defects observed. Specifically, we compared K27ac, K122ac, and H3.3 enrichment in the H3f3aA/AH3f3bWT/A lines and wildtype mES cells at the promoters of genes with significantly changed transcription quantified in our TT-seq datasets. Prior studies demonstrate that H3/H3.3 acetylation at promoters and over protein coding gene bodies generally correlates with transcription levels and changes in gene expression [29–32]. Reflecting the transcription changes, the promoters and gene bodies of upregulated genes exhibited increased K27ac and a modest increase in K122ac in H3f3aA/AH3f3bWT/A lines relative to wildtype, while downregulated genes have decreased enrichment of both marks over promoters and gene bodies (Fig. 5D-E). In H3f3aA/AH3f3bWT/A cell lines, upregulated genes exhibited similar levels in H3.3 enrichment at their promoters relative to wildtype while downregulated genes exhibited a modest reduction in H3.3 enrichment consistent with decreased expression (Fig. 5F). Visual comparison of K27ac, K122ac, H3.3, and nascent transcription in the H3f3aA/AH3f3bWT/A and wildtype mES cells over the Acad9 and Adam19 loci exemplify these trends (Fig. 5G and Supplemental Fig. 3C). Previous studies found that K122ac is enriched at bivalent genes [26]. Using a recently curated list of 8,789 bivalently marked loci in mES cells [57], we compared H3.3, K27ac, and K122ac levels in the single or dual targeted cell lines relative to wildtype (Supplemental Fig. 3D-F). We observe modest differences in these marks. Therefore, the H3.3K122A mutation alters transcription in mES cells and H3 acetylation and H3.3 enrichment at promoters and over protein coding gene bodies reflect these transcription changes.
H3.3K122A leads to epigenetic changes over putative enhancer regions involved in regulation of cell fate
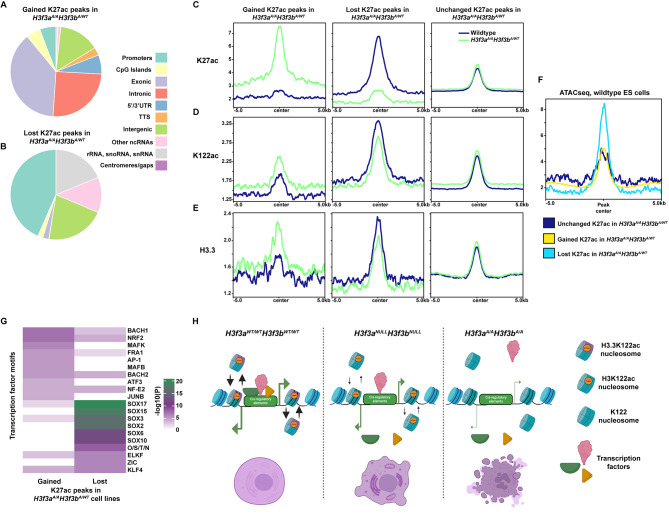
To examine the TSS distal effects of the H3.3K122A mutation, we determined the genomic locations of the gained and lost K27ac peaks in the H3f3aA/AH3f3bWT/A cell lines. Interestingly, while 44% of lost K27ac peaks were associated with promoters, only ~6% of gained K27ac peaks were promoter-associated (Fig. 6A, B). Rather, 38%, 25%, and 14% of gained K27ac peaks were annotated as exonic, intronic, and intergenic, respectively, showing that the majority of regions with increased K27ac in the H3f3aA/AH3f3bWT/A lines are TSS distal (Fig. 6A, B). Therefore, we compared the enrichment of K27ac, K122ac, and H3.3 over gained, lost, and unchanged TSS distal K27ac peaks in wildtype and H3f3aA/AH3f3bWT/A lines (Fig. 6C-E). Gained and lost peaks show enrichment of K27ac, K122ac, and H3.3 in either wildtype or H3f3aA/AH3f3bWT/A mES cells, with lost K27ac exhibiting higher levels of K27ac, K122ac, H3.3, and chromatin accessibility (from a wildtype ATAC-seq dataset [56]) in wildtype mES cells relative to gained or unchanged peaks (Fig. 6C-F). Gained and lost TSS distal K27ac peaks displayed the expected changes in K27ac and K122ac enrichment in H3f3aA/AH3f3bWT/A mES cells (Fig. 6C, D), suggesting that these are putative enhancers with altered activity in the H3f3aA/AH3f3bWT/A mES cells. Interestingly, gained TSS distal K27ac peaks exhibited an increase in H3.3 levels while lost peaks exhibited a reduction but still maintained H3.3 enrichment (at levels similar to gained or unchanged peaks; Fig. 6E). As with the promoter profiles, these changes in enrichment likely reflect changes in transcription activity. Using the K27ac TSS-distal differential peaks as input into motif analysis, we found that peaks lost in the H3f3aA/AH3f3bWT/A cell lines were strongly enriched for motifs associated with regulation of pluripotency (including SOX family motifs and the OCT4/SOX2/NANOG/TCF motif; Fig. 6G). These motifs did not have a robust enrichment at promoter distal K27ac peaks gained in H3f3aA/AH3f3bWT/A cell lines (Fig. 6G). Together, these analyses suggest that the H3.3K122A mutation influences the activity of a subset of putative enhancers in mES cells, and this may contribute to the mRNA transcription changes and pluripotency defects observed in the H3f3aA/AH3f3bWT/A mES cell lines.
Fig. 6.
H3 and H3.3 acetylation dynamics at putative enhancers in targeted and wildtype mES cells. A. Pie chart of the HOMER annotation of gained K27ac peaks in H3f3aA/AH3f3bA/WT mES cells relative to wildtype. Each color corresponds to a genomic feature denoted in the legend (right). B. As in A, for lost K27ac peaks in H3f3aA/AH3f3bA/WT mES cells relative to wildtype. C. K27ac enrichment in wildtype and H3f3aA/AH3f3bA/WT mES cells over gained, lost, and unchanged TSS distal H3K27ac peaks from H3f3aA/AH3f3bA/WT mES cell. D. As in C, for K122ac enrichment. E. As in C, for H3.3 enrichment. F. Wildtype mES cell ATAC-seq signal over gained, lost, and unchanged TSS distal H3K27ac peaks from H3f3aA/AH3f3bA/WT mES cells. G. Top 10 most enriched TF motifs found at TSS distal H3K27ac peaks gained or lost in H3f3aA/AH3f3bA/WT mES cells relative to wildtype. Color indicates -log10(P) as defined by HOMER. H. Model for how H3.3K122A may result in lethality in mES cells
Discussion
The role of H3.3 in mES cells has been extensively characterized, showing that H3.3 and its associated posttranslational modifications are important for the regulation of endogenous retroviruses, heterochromatin, global H3 acetylation, and facilitating differentiation [7, 9, 10, 13, 16, 18, 19, 33, 34]. Past studies also demonstrate that H3.3 is dispensable for proper transcription and regulation of pluripotency in mES cells. Specifically, depletion or deletion of H3.3 is not associated with proliferation defects, reduction in pluripotency, or strong transcriptional phenotypes [10, 13, 18, 33], arguing that the effects we observe are not due to reduced levels of H3.3 and are rather attributed to the H3.3K122A mutants.
Our findings highlight an importance for H3.3K122 in viability and maintenance of mES cells, but the exact mechanism remains elusive. H3f3aA/AH3f3bWT/A mES cells still acquire K27ac and K122ac in a transcription-dependent manner, arguing that this residue does not regulate H3K27 acetylation dynamics as has been shown for H3.3S31P [13]. Our findings indicate that differences in the activity of putative enhancers between H3f3aA/AH3f3bWT/A and wildtype mES cells may play a role in the observed transcriptional defects of the targeted cell lines. Incorporation of H3.3 at regions of active transcription promotes nucleosome turnover, which is important for TF binding, histone modification maintenance, and proper regulation of transcription [1, 2]. Likewise, H3K122ac promotes nucleosome eviction in vitro and is necessary for rapid transcription activation in S. pombe and H3K122 promotes nucleosome occupancy over highly transcribed genes in S. cerevisiae [22, 24]. Therefore, H3.3K122 may play an important role in H3.3 turnover dynamics and the H3.3K122A mutation may disrupt this process. We envision that, whereas mES cells lacking H3.3 have less nucleosome turnover but still enough for viability through incorporation of H3.1/2 in the place of H3.3, in cells expressing H3.3K122A, H3.3K122A is incorporated into nucleosomes but disrupts nucleosome turnover to point that the result is lethality (Fig. 6H). The correlation between the number of targeted H3f3 alleles and severity of phenotypes observed in mutant cell lines supports this model, but further studies are required.
Methods
Cell culture
ES-E14TG2a (E14) mES cells from male Mus musculus origin (RRID: CVCL9108361 [35]) were cultured as previously described [36] at 37 °C and 5% CO2 in DMEM base medium (Sigma Aldrich) supplemented with 10% FBS (Sigma Aldrich), 1X nonessential amino acids (Corning), 2mM L-glutamine (Corning), β-mercaptoethanol (Acros Organics) and LIF on 10 cm plates precoated with 0.2% gelatin. For LIF + 2i media, LIF media was further supplemented with 3 µM CHIR99021 GSK inhibitor (p212121), and 1 µM PD0325091 MEK inhibitor (p212121). Cells were passaged every ~48 h using trypsin and split at a ratio of ~1:8 with fresh medium. Routine anti-mycoplasma TC hood cleaning was conducted (LookOut DNA Erase spray, Sigma Aldrich) and cell lines were screened to confirm no mycoplasma presence. While generating clones, cells were grown in media with the addition of 1X penicillin/streptomycin (Corning) to prevent bacterial contamination.
Guide RNA cloning
Homology constructs for H3f3a and H3f3b (Supplemental Table 3) were designed to mutate residue 122 from lysine to alanine and mutate the PAM sequence (both highlighted in red). Oligos generated for the H3f3a or H3f3b gRNA were phosphorylated and annealed, then ligated to the px330-puro plasmid backbone using a simultaneous Fast Digest Bbs1 (ThermoFisher) digestion and Quick Ligase ligation (NEB). Plasmids were purified using the GenElute HP Plasmid Maxiprep Kit (Sigma Aldrich) following the manufacturer’s instructions and confirmed using Sanger sequencing (Genewiz).
Homology constructs were synthesized using IDT gBlocks Gene Fragments (IDT). The lyophilized construct was resuspended in 5 µL of nuclease-free water and 1 µL was used to clone the construct into the TOPO vector using the Zero Blunt TOPO PCR Cloning Kit for Sequencing (ThermoFisher) according to the manufacturer’s directions. The resulting plasmids were purified using the GenElute HP Plasmid Maxiprep Kit (Sigma Aldrich) and confirmed using Sanger sequencing (Genewiz).
H3.3K122A cell line generation
The targeting strategy used to generate cell lines where all four H3.3 alleles were mutated from lysine 122 to alanine is depicted in Fig. 1A. To generate cell lines, 2 × 105 cells were plated in a single well in a 6 well dish 24 h prior to transfection. One hour prior to transfection, medium was replaced with fresh, antibiotic-free medium. Three µg of pX330-puro Cas9/guide RNA vector and 3 µg of recombination template vector were combined in 100 µL of pre-warmed OptiMEM (Gibco). 24 µL of FuGENE HD (Promega) was added and the mixture was incubated at room temperature for 10 min. The mixture was added in drops evenly around the well of the dish and then gently swirled to ensure even distribution. 14–16 h post-transfection, cells were split using 0.5% trypsin (Gibco) and plated at varying densities on gelatinized 10 cm plates in antibiotic-free media. 48 h post-transfection, the media was replaced with media containing 2 µg/mL puromycin. 72 h post-transfection, the media was replaced with antibiotic-free media. Individual clones were picked 8 days post-transfection. Two days after picking clones, media was replaced with fresh penicillin/streptomycin media. Five days after picking clones, cells were split to 3 identical plates to allow for gDNA extraction and genetic screening and parallel storage for post-screening use. Targeting was performed in LIF and LIF + 2i containing media.
Clone screening
To extract gDNA, cells were washed once in 1xDPBS, lysed with ES cell lysis buffer (10 mM Tris pH 7.5, 10 mM EDTA, 10 mM NaCl, and 0.5% sarkosyl in water) including 1 mg/mL Proteinase K, and incubated overnight at 55 °C. The following day, plates were centrifuged for 2 min at 4 °C and 1000 rpm. DNA was precipitated using ice cold 100% ethanol with 75 mM NaCl and incubated for about 30 min until the solution cleared before centrifuging for 5 min at 4 °C and 3000 rpm. The supernatant was removed and the DNA was washed twice with 70% ethanol, then dried for an hour and resuspended in 50 µL 1X TE. PCR was performed on the extracted DNA in 96-well plates using primers that amplified the targeted H3f3a or H3f3b region (see Supplemental Table 2).
esiRNA generation and knockdown
Endoribonuclease-digested short interfering RNAs (esiRNAs) were generated as previously described [36]. An ideal target DNA sequence was identified within H3f3b and screened for unique nucleotide sequence via DEQOR. Amplified cDNA from wildtype mES cells was in vitro transcribed using T7 RNA polymerase. To generate esiRNAs, IVT products were digested with ShortCut RNase III (NEB) and purified using a PureLink RNA Mini Kit (Invitrogen). Transient transfections to deplete H3.3b were performed on wildtype cells (control for transfection efficiency) or H3f3a K122A clones on 6 wells using 5 µL of Lipofectamine 3000 (ThermoFisher) and 900 ng of esiRNAs for 48 h. Cells were harvested and counted with trypan blue on a BioRad Cell Counter. Following RNA isolation, depletion was quantified using RT-qPCR.
CRISPRi
A guide RNA for H3f3a was cloned into dCas9-KRAB-MeCP2 plasmid (Addgene 110821), as previously described [37]. dCas9-KRAB-MeCP2 was a gift from Alejandro Chavez & George Church (Addgene plasmid #110821; RRID: Addgene 110821). Plasmids were transfected into wildtype mES cells (control for transfection efficiency and CRISPRi activity) or H3f3b K122A clones on 6 wells using 5 µL of Lipofectamine 3000 (ThermoFisher), 5 µL of p3000 reagent, and 5 µg of plasmid for 48 h. Cells were harvested and counted with trypan blue on a BioRad Cell Counter. Following RNA isolation, transcript abundance was quantified using RT-qPCR.
Protein extraction and western blot analysis
For each protein, the following primary/secondary antibodies and dilutions were used: H3 (abcam: ab1791, lot GR300978-2, 1:1000), H3.3 (Millipore: 09-838, lot 3987735, 1:1000), K122ac (Invitrogen: PA5-112508, lot YJ4090633A, 1:1000), and ACTIN (Sigma: A1978, lot 037M4782V, 1:5000), IRDye 800CW Goat anti-rabbit IgG Secondary (LI-COR: 926-32211, 1:10000), and IRDye 800CW Goat anti-mouse IgG Secondary (LI-COR: 926-32210, 1:10000).
Cells were trypsinized and collected in a 15 mL conical tube, then centrifuged at 600 rcf for 5 min at 4 °C. Cell pellets were washed twice with cold 1XPBS and protein was extracted using 200 µL Triton Extraction Buffer (PBS containing 0.5% Triton X 100 (v/v), 2 mM phenylmethylsulfonyl fluoride (PMSF), 0.02% (w/v) NaN3). Concentration was determined using the Pierce BCA protein assay kit (ThermoFisher), and 7.5–10 µg protein per sample was loaded onto 15% Tris-acrylamide gels with Precision Plus ladder (Biorad). Gels were run for 40 V for 1 h, 120 V for 2.5 h, and then transferred at 20 V overnight to a nitrocellulose membrane (Biotrace). Successful transfer assessed using REVERT 700 total protein stain (LI-COR) and membranes were blocked using Intercept blocking buffer (LI-COR). Primary antibody incubations were conducted overnight at 4 °C, and secondary antibody incubations were conducted for 2 h at room temperature. All imaging was performed using a LI-COR Odyssey DLx Imager according to the manufacturers specifications. Protein quantification was conducted using ImageJ [38]. For total protein quantification, identical areas for each lane were selected and the mean pixel intensity was measured and subtracted from the mean pixel background intensity. For target protein quantification, identical size areas immediately surrounding the target band were selected and mean pixel intensity was measured and subtracted from mean background pixel intensity. Target protein quantification was then made relative to total protein quantification, and then made relative to wildtype. Three biological replicates were conducted per target for each cell line.
Cell growth assay
Cells were plated in 6 wells at a density of 50,000 cells per well. At days 1, 2, 3, 4, and 6 post-plating, cells were trypsinized using 0.5 mL of trypsin and stopped with 1.5 mL of ES cell medium. After achieving single cell suspension, cells were counted on a TC20 cell counter (BioRad) using trypan blue stain to distinguish live cells. Three biological replicates were conducted for each cell line.
Alkaline phosphatase staining
Cells were washed twice in 1X Dulbecco’s Phosphate-Buffered Saline (DPBS, Gibco) and crosslinked for five minutes at room temperature using 1% formaldehyde (Fisher) in DPBS. Crosslinking was quenched using 500 mM glycine and cells were washed using 1X DPBS twice. Cells were stained using Vector Red Alkaline Phosphatase Staining Kit (Vector Labs) per manufacturer’s instructions in a 200 mM Tris-HCl buffer, pH 8.4. 2 mL working solution was added to each 6 well and incubated in the dark for 30 min before being washed with 1XPBS and imaged. Three biological replicates were conducted for each cell line.
4sU-labelling and RNA isolation
4sU-labelled RNA was generated and isolated from cells using TT-seq as previously described [28, 39]. Media was aspirated from cell plates and replaced with 10 mL of 500 nM 4-thiouridine (4sU, Carbosynth T4509) containing ES cell media and the plates incubated at 37 °C with 5% CO2 for 5 min. After 5 min, the 4sU-containing media was aspirated and the cells were washed with 1xPBS and 2 mL TRIzol (Invitrogen) was added directly to the plate. Cell lysate were collected, and RNA was extracted according to ThermoFisher’s recommendations. RNA concentration was determined by Qubit with the Qubit RNA broad range quantification kit (ThermoFisher). 100 µg of total RNA was diluted to a concentration of 240 ng/µL at a volume of 416.67 µL in 1XTE and then fragmented with a Diagenode Bioruptor Pico on high power for one 30 s cycle. The fragmented RNA was then combined with 283.33 µL 1XTE, 100 µL 10X Biotinylation buffer (100 mM Tris pH 7.4 and 10 mM EDTA), and 200 µL of 1 mg/mL biotin-HPDP (ThermoFisher) in dimethylformamide (DMF; freshly prepared). Samples were vortexed, then incubated in a thermomixer at 37 °C shaking at 1000 RPM in the dark for 2 h. Samples were chloroform extracted, isopropanol/salt precipitated, and resuspended in 22 µL of nuclease-free water. Sixty uL of Streptavidin C1 beads (Invitrogen) were prepared for RNA separation by washes with 1 mL of 1 M NaOH and 50 mM NaCl and resuspended in 60 µL of TT-seq Binding buffer (10 nM Tris pH 7.4, 300 mM NaCl, 0.1% Triton). Then, 60 µL of prepared streptavidin C1 beads were added to each sample and rotated at room temperature for 20 min. Following incubation, the samples were magnetized for 1 min and the supernatant (containing the unlabeled RNA) was placed in a separate 1.5 mL tube and put on ice. The unlabeled RNA from the supernatant was Phenol: Chroloform: Isopropanol(PCI)/chloroform extracted, isopropanol/salt precipitated, and resuspended in 100 µL of nuclease-free water. The bead-bound labeled nascent RNA was washed twice with 500 µL of High Salt buffer (50 mM Tris pH 7.4, 2 M NaCl, 0.5% Triton), twice with 500 µL of TT-seq Binding buffer, and once with 500 µL of Low Salt buffer (5 mM Tris pH 7.4, 0.1% Triton), rotating for 1 min at room temperature, re-magnetizing and resuspending the beads during each wash. The nascent RNA was recovered from the beads through two rounds of 100 µL of freshly prepared 100 mM DTT and incubating in a thermomixer at 65 °C and 1000 RPM shaking for 5 min. Eluted nascent RNA was recovered with a PCI extraction and an isopropanol/salt/glycogen precipitation. RNA pellets were resuspended in 25 µL of nuclease-free water. The total RNA and nascent RNA from each sample were used for RT-qPCR and TT-seq libraries as described below.
Transient transcriptome sequencing (TT-seq)
For TT-seq, three replicates for wildtype mES cells, and one replicate for each mutant cell line were performed. 4sU labelling for TT-seq was performed as described in 4sU labelling section. RNA pellets were resuspended in 1X TE buffer, at approximately 100 µL per 100 µg of RNA. Strand-specific nascent RNA library building was performed as previously described [39] using the NEBNext Ultra II Directional Library kit with the following changes: The rRNA depleted RNA was fragmented at 94 °C for fifteen minutes following the manufacturer’s instructions for intact RNA, the fragmentation, first strand cDNA synthesis, and second strand cDNA synthesis were performed at double the reaction volume in the manufacturer’s instructions, the adaptors were diluted 1:5 in Adaptor Dilution Buffer, the primers were diluted 1:5 in nuclease-free water, and 7 cycles of PCR were used to amplify the libraries. Finished libraries were quantified by Quibit with the dsDNA High Sensitivity kit and run on a Fragment Analyzer to confirm high quality of each library prior to sequencing. Libraries were sequenced paired-end on an Illumina NextSeq500 to ~ 40 million mapped reads.
TT-seq analysis
Raw paired end fastq files were analyzed with fastQC [40] using default parameters to ensure high data quality and adapter sequences were trimmed using cutadapt [41]. Reads were aligned with the mm10 Gencode annotation (GRCm38.p6, vM23) using STAR [42] with the following parameters: outFilterMismatchNoverReadLmax 0.02, outFilterMultimapNmax (1) samtools [43] was used to filter reads and generate indexed bams with the following parameters: -q 7 -f (2) Feature counts were generated using featurecounts [44] options -B -t “exon” -g “gene_name” -F GTF -p -s 2 with the mm10 (GRCm38.p6, vM23) genome annotation (GTF format). Differential gene expression analysis was conducted using DEseq2 [45] with cutoff as |log2(FC)|≥0.75 and FDR ≤ 0.05. Gene Ontology analysis on gene sets of interest was performed the R package “clusterProfiler” [46] using all Gencode features as background. Upset plots were generated using the R package “upsetR” (https://github.com/hms-dbmi/UpSetR), and volcano plots were generated using R package “EnhancedVolcano” (https://bioconductor.org/packages/release/bioc/html/EnhancedVolcano.html).
Reverse transcription and quantitative PCR (RT-qPCR)
cDNA was generated from 1 µg of total RNA or 100 ng of 4sU labelled RNA with random hexamers (Promega) with homemade reverse transcriptase (RT). Generated cDNA was used as a template in qPCR reactions using 2X SYBR FAST mix (KAPA) and 5 µM target specific primers (see Supplemental Table 2) on a Lightcycler 96 (Roche). Target transcript abundance in samples was determined using the ΔΔCT normalization method relative to wildtype samples, using Gapdh transcript abundance for internal normalization as previously described [47].
CUT&RUN
The following antibodies and dilutions were used for CUT&RUN: H3.3 (Active Motif 91191, lot # 01022005; 1:50), K122ac (Invitrogen PA5-105108, lot # WJ3411065A; 1:50), and K27ac (abcam ab4729, lot # GR3416784-1; 1:100).
CUT&RUN was performed as described [39, 48, 49] using recombinant ProteinA-MNase (pA/G-MN) or Protein A/Protein G-MNase (pA/G-MN), as specified. For each target, two replicates for wildtype and one replicates for each mutant cell line were performed. Briefly, 100,000 nuclei were isolated from cell populations using a hypotonic buffer (20 mM HEPES-KOH, pH 7.9, 10 mM KCl, 0.5mM spermidine, 0.1% Triton X-100, 20% glycerol, freshly added protease inhibitors) and flash frozen. Nuclei were thawed and bound to lectin-coated concanavalin A magnetic beads (50 µL bead slurry per 100,000 nuclei; Polysciences). Immobilized nuclei were pre-blocked with blocking buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 0.5mM spermidine, 0.1% BSA, 2 mM EDTA, fresh protease inhibitors) and washed in wash buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 0.5mM spermidine, 0.1% BSA, fresh protease inhibitors). Nuclei were incubated in wash buffer containing primary antibody (H3.3, K122ac, K27ac) overnight at 4 °C with rotation. Nuclei were incubated in wash buffer containing primary antibody for 1 h at room temperature with rotation. Next, samples were washed twice with wash buffer and incubated in wash buffer containing recombinant pA-MN (for K122ac, K27ac) or pA/G-MN (for H3.3) for 30 min at room temperature with rotation. Controls lacking a primary antibody were subjected to the same conditions but incubated in wash buffer without any primary antibody prior to incubation with pA-MN/pA/G-MN. After incubation, samples were washed twice with was buffer and equilibrated to 0 °C in an ice/water bath. MNase cleavage was activated upon addition of 3 mM CaCl2. Digestion was chelated for samples containing primary antibody K27ac, after 30 min on the ice water bath, using 20 mM EDTA, 4 mM EGTA, 200 mM NaCl, and 1.5 pg MNase-digested S. cerevisiae mononucleosomes were added as a spike-in control. Genomic fragments were released after an RNase A treatment. Released fragments were separated through centrifugation. Digestion was chelated for samples containing primary antibodies K122ac and H3.3 after 30 min on the ice water bath, using a low-salt treatment: 10 mM EDTA, 2 mM EGTA, 150 nM NaCl, 5 mM TritonX, and 1.5 pg MNase-digested S. cerevisiae mononucleosomes were added as a spike-in control. Genomic fragments were released after being incubated for 1 h at 4 °C and released fragments were separated from beads. The salt concentration of low salt treated samples were increased to 500 mM and additional RNase A.
Isolated fragments from all samples were used as input for a library build consisting of end repair and adenylation, adapter ligation, and subsequent purification with AMPure XP beads (Agencourt). Barcoded fragments were then amplified by 15 cycles of high-fidelity PCR and purified using agarose gel extraction. Libraries were pooled and sequenced on an Illumina NextSeq500 to a depth of ~ 10 million mapped reads.
CUT&RUN analysis
Paired-end fastq files were trimmed to 25 bp and mapped to the mm10 genome with bowtie2 [50] (options -q -N 1 -X 1000). Mapped reads were duplicate-filtered using reads with insufficient mapping quality (MAPQ ≥ 10) were removed using samtools [43]. Reads were converted to bigWig files using deepTools [51] with the spike in S. cerevisiae mapped reads used as a scaling factor. Heatmaps and metaplots were generated using deepTools. Peaks were called from CUT&RUN samples using MACS2 [52] with no antibody samples as input. For each target, peaks from all samples were combined to create a consensus peak set. After removing all peaks with less than 10 counts in 50% of samples, differential enrichment peak analysis was performed using edgeR [53] for all available replicates of each condition with RUVseq [54] correction. Differentially enriched peaks were defined as |log2(FC)|≥0.5 and FDR ≤ 0.05. For each target, samples peak annotations and motif enrichment analysis were performed using HOMER [55].
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank members of the Hainer Lab for critical comments, discussion, and feedback regarding this article. This work was supported by the NIH grant R35GM133732 (to S.J. Hainer) and NSF GRFP (to B. J. Patty). This project used the University of Pittsburgh HSCRF Genomics Research Core, RRID: SCR_018301 NextSeq2000, with special thanks to the assistant director, Will MacDonald. This research was supported in part by the University of Pittsburgh Center for Research Computing, RRID: SCR_022735, through the resources provided. Specifically, this work used the HTC cluster, which is supported by NIH award number S10OD028483.
Author contributions
Conceptualization: BJP, SJH; Methodology: BJP, CJ, SML, KT, SJH; Writing – original draft: BJP, SJH; Writing – revised draft: SJH; Funding acquisition: SJH; Supervision: SJH; Project administration: SJH. All authors reviewed the manuscript.
Funding
This work was supported by the NIH grant R35GM133732 (to SJH) and a NSF GRFP (to BJP).
Data availability
We have placed all the data on GEO, accession numbers GSE272575 (TT-seq) and GSE272577 (CUT&RUN).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publishing
The manuscript has been read and approved by all named authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martire S, Banaszynski LA. <ArticleTitle Language=“En”>The roles of histone variants in fine-tuning chromatin organization and function. Nat Rev Mol Cell Biol. 2020;21:522–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talbert PB, Henikoff S. Histone variants at a glance. J Cell Sci. 2021;134:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bramlage B, Kosciessa U, Doenecke D. Differential expression of the murine histone genes H3.3A and H3.3B. Differentiation. 1997;62:13–20. [DOI] [PubMed] [Google Scholar]
- 4.Tang MCW, et al. Contribution of the Two Genes Encoding Histone Variant H3.3 to Viability and Fertility in Mice. PLoS Genet. 2015;11:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muhire BM, Booker MA, Tolstorukov MY. Non-neutral evolution of H3.3-encoding genes occurs without alterations in protein sequence. Sci Rep. 2019;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein RH, Knoepfler PS. Knockout tales: the versatile roles of histone H3.3 in development and disease. Epigenetics Chromatin. 2023;16:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banaszynski LA, et al. Hira-dependent histone H3.3 deposition facilitates prc2 recruitment at developmental loci in ES cells. Cell. 2013;155:107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kori Y, et al. Multi-omic profiling of histone variant H3.3 lysine 27 methylation reveals a distinct role from canonical H3 in stem cell differentiation. Mol Omi. 2022;18:296–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carraro M, et al. DAXX adds a de novo H3.3K9me3 deposition pathway to the histone chaperone network. Mol Cell. 2023;83:1075–e10929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tafessu A, et al. H3.3 contributes to chromatin accessibility and transcription factor binding at promoter-proximal regulatory elements in embryonic stem cells. Genome Biol. 2023;24:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y, Histone. H3.1 and H3.3 Complexes Mediate Nucleosome Assembly Pathways Dependent or Independent of DNA Synthesis. Cell. 2004;116:51–61. [DOI] [PubMed]
- 12.Xue Y et al. The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc. Natl. Acad. Sci. U. S. A. 100, 10635–10640 (2003). [DOI] [PMC free article] [PubMed]
- 13.Martire S, et al. Phosphorylation of histone H3.3 at serine 31 promotes p300 activity and enhancer acetylation. Nat Genet. 2019;51:941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fosslie M, et al. Going low to reach high: Small-scale ChIP-seq maps new terrain. Wiley Interdiscip Rev Syst Biol Med. 2020;12:1–24. [DOI] [PubMed] [Google Scholar]
- 15.Maze I, et al. Critical Role of Histone Turnover in Neuronal Transcription and Plasticity. Neuron. 2015;87:77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deaton AM, et al. Enhancer regions show high histone H3.3 turnover that changes during differentiation. Elife. 2016;5:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraushaar DC et al. Genome-wide incorporation dynamics reveal distinct categories of turnover for the histone variant H3.3. Genome Biol 14, (2013). [DOI] [PMC free article] [PubMed]
- 18.Fang HT et al. Global H3.3 dynamic deposition defines its bimodal role in cell fate transition. Nat Commun 9, (2018). [DOI] [PMC free article] [PubMed]
- 19.Elsässer SJ, Noh KM, Diaz N, Allis CD, Banaszynski LA, Histone. H3.3 is required for endogenous retroviral element silencing in embryonic stem cells. Nature. 2015;522:240–4. [DOI] [PMC free article] [PubMed]
- 20.Claringbould A, Zaugg JB. Enhancers in disease: molecular basis and emerging treatment strategies. Trends Mol Med. 2021;27:1060–73. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Zhang C, Wang Y, Liu X, Zhang Z. Enhancer RNA (eRNA) in Human Diseases. Int J Mol Sci. 2022;23:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tropberger P, et al. Regulation of transcription through acetylation of H3K122 on the lateral surface of the histone octamer. Cell. 2013;152:859–72. [DOI] [PubMed] [Google Scholar]
- 23.Manohar M, et al. Acetylation of histone H3 at the nucleosome dyad alters DNA-histone binding. J Biol Chem. 2009;284:23312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hainer SJ, Martens JA. Identification of histone mutants that are defective for transcription-coupled nucleosome occupancy. Mol Cell Biol. 2011;17:3557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyland EM, et al. Insights into the Role of Histone H3 and Histone H4 Core Modifiable Residues in Saccharomyces cerevisiae. Mol Cell Biol. 2005;25:10060–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pradeepa MM, et al. Histone H3 globular domain acetylation identifies a new class of enhancers. Nat Genet. 2016;48:681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Štefková K, Procházková J, Pacherník J. Alkaline phosphatase in stem cells. Stem Cells Int. 2015, (2015). [DOI] [PMC free article] [PubMed]
- 28.Schwalb B, et al. TT-seq maps the human transient transcriptome. Sci (80-). 2016;352:1225–8. [DOI] [PubMed] [Google Scholar]
- 29.Martin BJE, et al. Transcription shapes genome-wide histone acetylation patterns. Nat Commun. 2021;12:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ALLFREY VG, FAULKNER R, MIRSKY AE. Acetylation and Methylation of Histones and Their Possible Role in the. Proc. Natl. Acad. Sci. United States 51, 786–794 (1964). [DOI] [PMC free article] [PubMed]
- 32.Morgan MAJ, Shilatifard A. Reevaluating the roles of histone-modifying enzymes and their associated chromatin modifications in transcriptional regulation. Nat Genet. 2020;52:1271–81. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, et al. HIRA complex presets transcriptional potential through coordinating depositions of the histone variants H3.3 and H2A.Z on the poised genes in mESCs. Nucleic Acids Res. 2022;50:191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong LH, et al. ATRX interacts with H3.3 in maintaining telomere structural integrity in pluripotent embryonic stem cells. Genome Res. 2010;20:351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Risch N, et al. HPRT -deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Jr Archs gen Psychiat. 1981;20:753–8. [DOI] [PubMed] [Google Scholar]
- 36.Klein DC, Troy K, Tripplehorn SA, Hainer SJ. The esBAF and ISWI nucleosome remodeling complexes influence occupancy of overlapping dinucleosomes and fragile nucleosomes in murine embryonic stem cells. BMC Genomics. 2023;24:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeo NC, et al. An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat Methods. 2018;15:611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson BP, et al. Alpha-arrestins Aly1/Art6 and Aly2/Art3 regulate trafficking of the glycerophosphoinositol transporter Git1 and impact phospholipid homeostasis. Biol Cell. 2022;114:3–31. [DOI] [PubMed] [Google Scholar]
- 39.Klein DC, Lardo SM, McCannell KN, Hainer S. J. FACT regulates pluripotency through distal regulation of gene expression in murine embryonic stem cells. bioRxiv. 2022;454509. 10.1186/s12915-023-01669-0. [DOI] [PMC free article] [PubMed]
- 40.Andrews S. FastQC A Quality control tool for high throughput sequence data. Babraham Bioinfo 3–5 (2018).
- 41.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10. [Google Scholar]
- 42.Dobin A, et al. Ultrafast universal RNA-seq aligner. Bioinf 29. 2013;STAR:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao Y, Smyth GK, Shi W, FeatureCounts. An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–30. [DOI] [PubMed]
- 45.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu T, et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation. 2021;2:100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rao X, Huang X, Zhou Z, Lin X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath. 2013;3:71–85. [PMC free article] [PubMed] [Google Scholar]
- 48.Hainer SJ, et al. Suppression of pervasive noncoding transcription in embryonic stem cells by esBAF. Genes Dev. 2015;29:362–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patty BJ, Hainer SJ. Transcription factor chromatin profiling genome-wide using uliCUT& RUN in single cells and individual blastocysts. Nat Protoc. 2021;16:2633–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramírez F, Dündar F, Diehl S, Grüning BA, Manke T, DeepTools. A flexible platform for exploring deep-sequencing data. Nucleic Acids Res 42, (2014). [DOI] [PMC free article] [PubMed]
- 52.Zhang Y et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol 9, (2008). [DOI] [PMC free article] [PubMed]
- 53.Robinson MD, McCarthy DJ, Smyth GK, edgeR. A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2009;26:139–40. [DOI] [PMC free article] [PubMed]
- 54.Risso D, Ngai J, Speed TP, Dudoit S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat Biotechnol. 2014;32:896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heinz S, et al. Simple Combinations of Lineage-Determining Transcription Factors Prime cis-Regulatory Elements Required for Macrophage and B Cell Identities. Mol Cell. 2010;38:576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patty BJ, Hainer SJ. Widespread impact of nucleosome remodelers on transcription at cis-regulatory elements. bioRxiv [Preprint]. 2024:589208. 10.1101/2024.04.12.589208
- 57.Seneviratne JA, Ho WWH, Glancy E, et al. A low-input high resolution sequential chromatin immunoprecipitation method captures genome-wide dynamics of bivalent chromatin. Epigenetics Chromatin. 2024;17:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We have placed all the data on GEO, accession numbers GSE272575 (TT-seq) and GSE272577 (CUT&RUN).