Abstract
Background
Necrotizing soft tissue infections (NSTI) are rapidly progressing and life-threatening conditions that require prompt diagnosis. However, differentiating NSTI from other non-necrotizing skin and soft tissue infections (SSTIs) remains challenging. We aimed to evaluate the diagnostic value of the biochemical analysis of soft tissue infectious fluid in distinguishing NSTIs from non-necrotizing SSTIs.
Methods
This cohort study prospectively enrolled adult patients between May 2023 and April 2024, and retrospectively included patients from April 2019 to April 2023. Patients with a clinical suspicion of NSTI in the limbs who underwent successful ultrasound-guided aspiration to obtain soft tissue infectious fluid for biochemical analysis were evaluated and classified into the NSTI and non-necrotizing SSTI groups based on their final discharge diagnosis. Common extravascular body fluid (EBF) criteria were applied.
Results
Of the 72 patients who met the inclusion criteria, 10 patients with abscesses identified via ultrasound-guided aspiration were excluded. Based on discharge diagnoses, 39 and 23 patients were classified into the NSTI and non-necrotizing SSTI groups, respectively. Biochemical analysis revealed significantly higher albumin, lactate, lactate dehydrogenase (LDH), and total protein levels in the NSTI group than in the non-necrotizing SSTI group, and the NSTI group had significantly lower glucose levels and pH in soft tissue fluids.
In the biochemical analysis, LDH demonstrated outstanding discrimination (area under the curve (AUC) = 0.955; p < 0.001) among the biochemical markers. Albumin (AUC = 0.884; p < 0.001), lactate (AUC = 0.891; p < 0.001), and total protein (AUC = 0.883; p < 0.001) levels also showed excellent discrimination. Glucose level (AUC = 0.774; p < 0.001) and pH (AUC = 0.780; p < 0.001) showed acceptable discrimination. When the EBF criteria were evaluated, the total scores of Light’s criteria (AUC = 0.925; p < 0.001), fluid-to-serum LDH ratio (AUC = 0.929; p < 0.001), and fluid-to-serum total protein ratio (AUC = 0.927; p < 0.001) demonstrated outstanding discrimination.
Conclusion
Biochemical analysis and EBF criteria demonstrated diagnostic performances ranging from acceptable to outstanding for NSTI when analyzing soft tissue infectious fluid. These findings provide valuable diagnostic insights into the recognition of NSTI. Further research is required to validate these findings.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-024-05146-0.
Keyword: Soft tissue infectious fluid; Biochemical analysis; Necrotizing soft tissue infection; Skin and soft tissue infections
Background
Skin and soft tissue infections (SSTIs) result from the microbial invasion of the skin, subcutaneous tissue, fascia, or muscles, leading to a spectrum of conditions ranging from simple superficial infections to severe necrotizing infections [1]. SSTIs are common infectious diseases with an incidence of approximately 77.5 cases per 1000 person-years [2]. Most SSTIs can be effectively managed using antibiotic treatment or drainage alone [1, 3]. By contrast, necrotizing soft tissue infection (NSTI), a rare but severe subtype of SSTI, has a high mortality rate, with 20–30% of affected patients dying during hospitalization [1, 4]. Timely diagnosis and early surgical debridement are crucial for reducing NSTI mortality. Recent meta-analyses have shown that surgical intervention within 6 h of presentation can reduce mortality by approximately 50% [5].
Various diagnostic tools have been developed to identify NSTIs accurately, including laboratory tests, scoring systems, ultrasonography, imaging, and tissue biopsies [1, 6]. However, no biomarker, scoring system, or imaging modality has proven sufficiently sensitive to definitively exclude NSTIs. Consequently, differentiating early NSTI from severe non-necrotizing SSTIs remains challenging, as more than half of the patients are initially misdiagnosed owing to the non-specific clinical symptoms of early NSTI, such as erythema, swelling, pain, and fever [6, 7]. Therefore, additional diagnostic tools are warranted to assist physicians in the timely recognition of NSTI.
Extravascular body fluids (EBF) refers to all body fluids outside the bloodstream, including pleural effusion, ascites, and synovial fluid [8, 9]. Examining EBFs can provide valuable insights, particularly in differential diagnosis. Several well-known EBF criteria, such as Light’s criteria, are widely used in clinical practice to differentiate exudates from transudates [9, 10]. However, soft tissue infectious fluid, which accumulates along the fascia in severe soft tissue infections, has rarely been investigated, and its diagnostic value remains undetermined [6]. This cohort study hypothesized that soft tissue infectious fluids in patients with NSTI differ from those in patients with non-necrotizing SSTI, owing to more severe tissue damage, infection, and disease severity in NSTI. To evaluate the diagnostic performance in distinguishing NSTI from severe non-necrotizing SSTI, the biochemical characteristics of soft tissue infectious fluids were analyzed and evaluated using the EBF criteria.
Methods
Study design and participants
This cohort study was conducted at Chang Gung Memorial Hospital (Chiayi branch), a tertiary referral hospital in Taiwan. Adult patients who presented to the emergency department (ED) with clinical suspicion of necrotizing soft tissue infection of the limbs and underwent successful ultrasound-guided aspiration for biochemical analysis of soft tissue infectious fluid were enrolled. Patients with suspected NSTI of the cephalic region, trunk, or pelvis (including the groin) were excluded. Because of the geographical location of Chiayi County (bordering the Taiwan Strait to the west) and the large population of fishermen in the area, our hospital treats more NSTI patients than other hospitals in Taiwan, making NSTI a specialized area of care in our hospital. Resultantly, we have a standard clinical management for patients with suspected NSTI who present to the ED. The primary ED physician established the clinical suspicion of NSTI based on symptoms and signs such as limb erythema, swelling, localized heat, disproportionate pain, septic shock, fever, and hemorrhagic bullae, in addition to the results of laboratory tests and imaging studies such as point-of-care ultrasound of the infection site. Consequently, broad-spectrum antibiotics are administered, and emergency surgeon consultation is sought for surgical intervention assessment. Simultaneously, ultrasound-guided aspiration may be performed to provide additional diagnostic information and fluids for culture. The decision to perform ultrasound-guided aspiration is made by the primary attending physician in the emergency department. Once collected, the soft tissue fluid is sent to our hospital’s clinical laboratory by medical courier. Medical technologists who are not part of the research team perform tests on soft tissue infectious fluids as part of their professional duties. The results are subsequently reported in the electronic medical system of our hospital. Patients who received antibiotic treatment before fluid aspiration; those who had chronic or recurrent infections (such as osteomyelitis), deep trauma, a history of surgery, burns, or skin neoplasms at the lesion site of the infected limb; and those who declined surgical intervention recommended by the surgeon despite receiving a clinical diagnosis of NSTI. Patients with abscesses, defined as the accumulation of pus in the dermis or subcutaneous tissue, identified through ultrasound-guided aspiration, were also excluded because the diagnosis of a skin abscess was clear when pus formation was noted during aspiration. Patients were prospectively enrolled between May 2023 and April 2024 and retrospectively included between April 2019 and April 2023. This study was approved by the hospital’s Institutional Review Board (IRB) (prospective cohort IRB No. 202300399B0 and retrospective cohort IRB No. 202301747B0).
Data collection and outcome measurement
Clinical data of all enrolled patients were extracted from electronic medical records. Data on age, sex, comorbidities (hypertension; diabetes; liver cirrhosis; chronic kidney disease; malignancy; autoimmune diseases, including systemic lupus erythematosus, rheumatoid arthritis, and Sjögren’s syndrome; solid organ transplantation history; and alcohol use disorder), vital signs (including hypotension), physical examination (e.g., hemorrhagic bullae), laboratory results of blood and soft tissue fluids, and final discharge diagnosis were obtained. Based on the final discharge diagnosis, the patients were subsequently classified into NSTI and non-necrotizing SSTI groups. Typically, the diagnosis of NSTI at discharge is based on surgical findings and pathological reports. Patients who lacked supporting surgical findings or were successfully managed with medical treatment alone were classified into the non-necrotizing SSTI group.
The primary outcomes were the diagnostic performance of biochemical markers for NSTI, including lactate dehydrogenase (LDH), total protein, albumin, lactate, pH, and glucose, and the diagnostic performance of extravascular body fluid (EBF) criteria for NSTI using soft tissue infectious fluid. The biochemical markers and criteria investigated in this study were based on the common tests and previously established criteria [8–20]. After a narrative review of EBF, the potential biomarkers and EBF criteria related to inflammation and cell damage were identified. Additional file 1 summarizes the biochemical tests and common EBF criteria used in this study. Based on the original Light’s criteria, pleural effusion is classified as exudative when at least one of the three criteria is met. In this study, the total score for the Light’s criteria, which represents the number of criteria met, was calculated and used as an additional independent parameter.
A subgroup analysis was planned to better understand whether biochemical tests and the EBF criteria could be utilized in patients without clear signs of NSTI. A recently published systematic review and meta-analysis identified two clinical signs as high-risk indicators for NSTI: hemorrhagic bullae and the presence of hypotension (defined as systolic blood pressure of ≤ 90 mmHg), with positive likelihood ratios of 5.97 and 9.20, respectively [21]. Thus, the subgroup analysis compared NSTI patients who did not exhibit hemorrhagic bullae or hypotension at presentation with those diagnosed with non-necrotizing SSTIs.
Statistical analysis
Statistical analyses were performed using SPSS software (IBM Corp., Armonk, NY, USA). Student’s t-tests were used to compare continuous variables between the NSTI and non-necrotizing SSTI groups, whereas chi-square or Fisher’s exact tests were used to compare categorical variables, as appropriate. The Mann–Whitney U test was used to compare ordinal data. A p value of < 0.05 was considered statistically significant. Continuous variables were expressed as the mean (± standard deviation), whereas categorical variables were expressed as counts and percentages. Receiver operating characteristic (ROC) curves were generated to assess the diagnostic performance of the biochemical markers and criteria for NSTI, and the area under the ROC curve (AUC) was calculated. The optimal cutoff points were determined using the Youden index [22]. Generally, AUC values of 0.5–0.7 indicate poor discrimination, 0.7–0.8 indicate acceptable discrimination, 0.8–0.9 indicate excellent discrimination, and > 0.9 indicate outstanding discrimination [23]. Additionally, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated to assess the diagnostic performance of the biochemical markers and criteria for NSTI.
A power analysis was conducted to estimate the minimum sample size. We used input values with 80% power and a two-sided significance level of 5% for the calculation, with adjustments for the t-distribution (Additional file 2).
Results
Patient characteristics
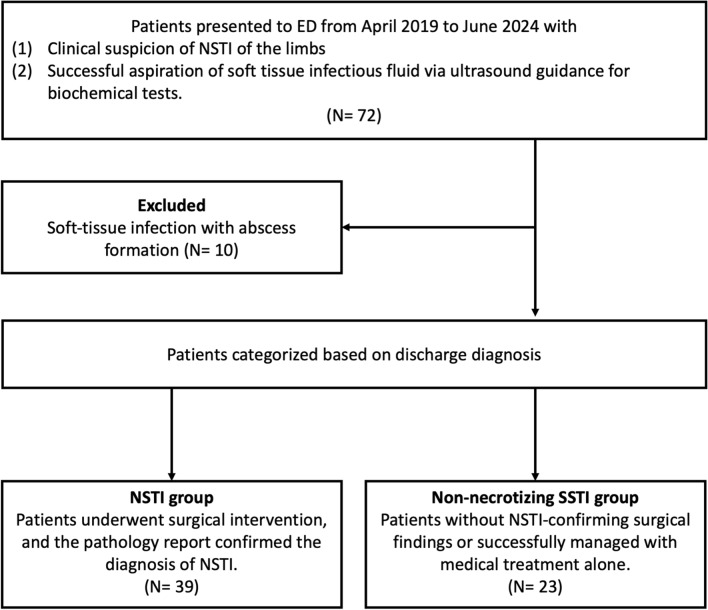
Of the 72 patients who met the inclusion criteria, ten patients with abscesses identified via ultrasound-guided aspiration were excluded. Based on discharge diagnoses, 39 and 23 patients were classified into the NSTI and non-necrotizing SSTI groups, respectively (Fig. 1). Table 1 presents a comparison of patient characteristics between the NSTI and non-necrotizing SSTI groups. Statistical analysis revealed that laboratory data showed significantly higher white blood cell counts, rates of bandemia, and creatinine, C-reactive protein, and lactate levels in the NSTI group than in the non-necrotizing SSTI group.
Fig. 1.
Flowchart of the participant selection process. ED, emergency department; NSTI, necrotizing soft tissue infection; SSTI, skin and soft tissue infection
Table 1.
General characteristics and blood test results in the NSTI and non-necrotizing SSTI groups
| NSTI | Non-necrotizing SSTI | p | |||
|---|---|---|---|---|---|
| n | Mean ± SD or Number (%) | n | Mean ± SD or Number (%) | ||
| General characteristics | |||||
| Age (years) | 39 | 69.0 ± 12.65 | 23 | 63.5 ± 14.77 | 0.124 |
| Sex | 39 | 23 | 0.338 | ||
| Male | 29(74.4%) | 20(87.0%) | |||
| Female | 10(25.6%) | 3(13.0%) | |||
| Comorbidity | |||||
| Hypertension | 39 | 24(61.5%) | 23 | 12(52.2%) | 0.470 |
| Diabetes mellitus | 39 | 16(41%) | 23 | 7(30.4%) | 0.404 |
| Liver cirrhosis | 39 | 9(23.1%) | 23 | 6(26.1%) | 0.789 |
| Chronic kidney disease | 39 | 16(41%) | 23 | 6(26.1%) | 0.235 |
| Malignancy | 39 | 2(5.1%) | 23 | 5(21.7%) | 0.090 |
| Autoimmune disease | 39 | 1(2.6%) | 23 | 1(4.3%) | 0.701 |
| Solid organ transplantation | 39 | 0(0%) | 23 | 1(4.3%) | 0.371 |
| Alcohol use disorder | 39 | 2(5.1%) | 23 | 3(13%) | 0.350 |
| Hypotension | 39 | 7(17.9%) | 23 | 0(0%) | 0.040* |
| Hemorrhagic bullae | 39 | 10(25.6%) | 23 | 0(0%) | 0.010* |
| Laboratory data in blood | |||||
| White blood counts (103/μL) | 39 | 13.8 ± 6.47 | 23 | 10.4 ± 4.26 | 0.016* |
| Segment (%) | 39 | 78.9 ± 12.99 | 23 | 74.2 ± 12.66 | 0.168 |
| Band (%) | 39 | 4.9 ± 6.97 | 23 | 0.1 ± 0.31 | < 0.001* |
| Hemoglobin (g/dL) | 39 | 12.4 ± 2.09 | 23 | 12.6 ± 2.64 | 0.784 |
| Platelet counts (103/μL) | 39 | 184.6 ± 86.28 | 23 | 206.8 ± 90.50 | 0.341 |
| Glucose (mg/dL) | 39 | 166 ± 94.6 | 23 | 154 ± 60.2 | 0.601 |
| Creatinine (mg/dL) | 37 | 1.56 ± 0.86 | 23 | 1.20 ± 0.37 | 0.032* |
| ALT (U/L) | 39 | 37 ± 22.7 | 23 | 41 ± 44.6 | 0.599 |
| Sodium (mEq/L) | 39 | 137 ± 5.3 | 23 | 136 ± 3.3 | 0.714 |
| Potassium (mEq/L) | 39 | 3.7 ± 0.62 | 23 | 3.8 ± 0.54 | 0.842 |
| C-reactive protein (mg/L) | 36 | 169.3 ± 125.53 | 22 | 83.3 ± 80.63 | 0.002* |
| Albumin (serum) (g/dL) | 38 | 3.7 ± 0.50 | 23 | 3.8 ± 0.53 | 0.543 |
| Lactate (serum) (mg/dL) | 37 | 29.5 ± 19.81 | 22 | 15.2 ± 7.13 | < 0.001* |
| Lactate dehydrogenase (serum) (U/L) | 37 | 258.8 ± 119.17 | 22 | 240.1 ± 100.86 | 0.539 |
| Total protein (serum) (g/dL) | 36 | 6.5 ± 0.85 | 19 | 6.8 ± 0.52 | 0.119 |
Continuous variables are expressed as the mean (± SD), whereas categorical variables are expressed as counts (percentages)
ALT, alanine transaminase; NSTI, necrotizing soft tissue infection; SD, standard deviation; SSTI, skin and soft tissue infection
*p < 0.05, considered significant
Biochemical analysis and common extravascular fluid criteria in soft tissue infectious fluids
The biochemical characteristics of soft tissue infectious fluids were analyzed, and common EBF criteria were assessed using these parameters. Table 2 presents the results of this study. The analysis revealed significantly higher levels of albumin (p < 0.001), lactate (p < 0.001), LDH (p < 0.001), and total protein (p < 0.001) in the NSTI group than in the non-necrotizing SSTI group. Additionally, the NSTI group had significantly lower soft tissue fluid glucose (p = 0.001) and pH levels (p = 0.001) than the non-necrotizing SSTI group.
Table 2.
Biochemical data and scores on the extracellular body fluid criteria of soft tissue infectious fluids in the NSTI and SSTI groups
| NSTI | Non-necrotizing SSTI | p | |||
|---|---|---|---|---|---|
| n | Mean ± SD or Number (%) | n | Mean ± SD or Number (%) | ||
| Laboratory data in soft tissue infectious fluid | |||||
| Albumin (fluid) (g/dL) | 38 | 2.21 ± 0.89 | 23 | 1.16 ± 0.49 | < 0.001 |
| Lactate (fluid) (mg/dL) | 37 | 124.99 ± 101.20 | 22 | 26.54 ± 14.01 | < 0.001 |
| Lactate dehydrogenase (fluid) (U/L) | 38 | 4816.10 ± 7205.90 | 23 | 213.5 ± 224.019 | < 0.001 |
| Glucose (fluid) (mg/dL) | 35 | 74.32 ± 84.16 | 23 | 151.35 ± 83.36 | 0.001 |
| Total protein (fluid) (g/dL) | 37 | 3.52 ± 1.58 | 23 | 1.42 ± 0.84 | < 0.001 |
| pH (fluid) | 36 | 8.13 ± 0.46 | 20 | 8.54 ± 0.32 | 0.001 |
| Common EBF criteria using soft tissue infectious fluid | |||||
| Light’s criteria (at least one criterion is met) | 34 | 34(100%) | 19 | 10(52.6%) | < 0.001 |
| Light’s criteria (total score) | 34 | 19 | < 0.001 | ||
| 0 | 0(0%) | 9(47.3%) | |||
| 1 | 2(5.9%) | 1(5.3%) | |||
| 2 | 3(8.8%) | 8(42.1%) | |||
| 3 | 29(85.3%) | 1(5.3%) | |||
| Serum-to-fluid albumin gradient (g/L) | 37 | 1.49 ± 0.78 | 23 | 2.62 ± 0.51 | < 0.001 |
| Fluid-to-serum LDH ratio | 36 | 20.73 ± 35.22 | 22 | 1.12 ± 1.46 | 0.002 |
| Fluid-to-serum total protein ratio | 34 | 0.95 ± 0.43 | 19 | 0.35 ± 0.14 | < 0.001 |
Continuous variables are expressed as the mean (± SD), whereas categorical variables are expressed as counts (percentages)
ALT, alanine transaminase; EBF, extravascular body fluid; LDH, lactate dehydrogenase; NSTI, necrotizing soft tissue infection; SD, standard deviation; SSTI, skin and soft tissue infection
*p < 0.05, considered statistically significant
In the evaluation of the common EBF criteria, the NSTI group had a higher proportion of patients who met Light’s criteria (p < 0.001) and a higher total score on Light’s criteria (p < 0.001) compared to the non-necrotizing SSTI group. Moreover, the NSTI group exhibited higher serum-to-fluid albumin gradients (p < 0.001), fluid-to-serum LDH ratios (p = 0.002), and fluid-to-serum total protein ratios (p < 0.001) compared to the non-necrotizing SSTI group.
Diagnostic performance of biochemical tests and extravascular fluid criteria
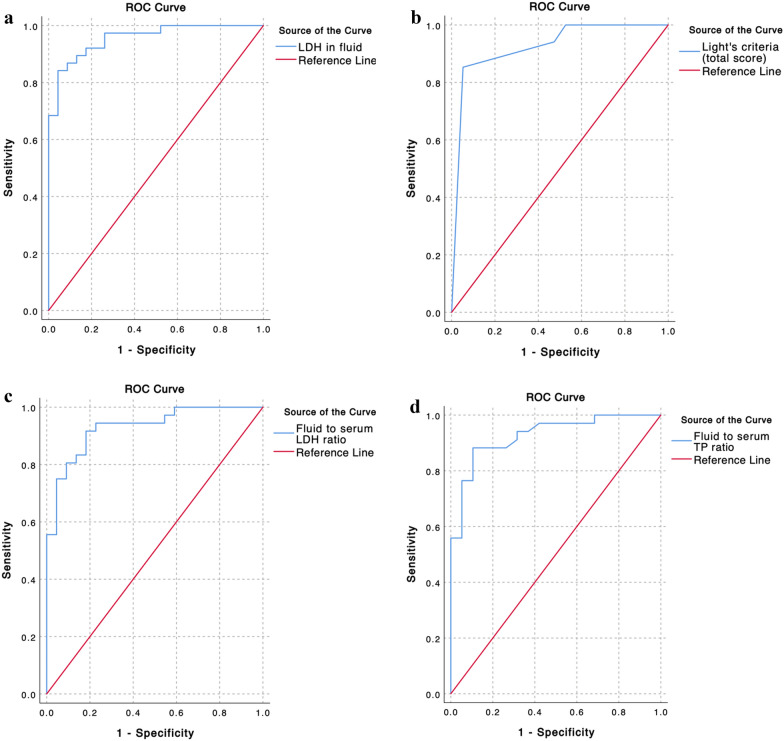
The diagnostic performances of biochemical tests and EBF criteria for NSTI using soft tissue infectious fluid were investigated. The results of the statistical analyses are presented in Table 3. Among the biochemical tests, LDH level demonstrated outstanding discrimination (area under the curve, 0.955; p < 0.001). Albumin (AUC = 0.884; p < 0.001) and lactate levels (AUC = 0.891; p < 0.001) also showed excellent discrimination. Additionally, glucose (AUC = 0.774; p < 0.001) and pH (AUC = 0.780; p < 0.001) showed acceptable discrimination. In the evaluation of the EBF criteria, the total scores on Light’s criteria (AUC = 0.925; p < 0.001), fluid-to-serum LDH ratio (AUC = 0.929; p < 0.001), and fluid-to-serum total protein ratio (AUC = 0.927; p < 0.001) demonstrated outstanding discrimination. The serum-to-fluid albumin gradient exhibited excellent discrimination (AUC = 0.881; p < 0.001). In addition, Light’s criteria (at least one criterion met) showed acceptable discrimination (AUC = 0.737; p = 0.003). Figure 2 displays the four ROC curve plots and the corresponding AUC values for predicting the diagnosis of NSTI, demonstrating the overall outstanding discriminatory ability for identifying NSTI. Additional ROC curve plots for other biochemical tests and EBF criteria are presented in Additional file 3.
Table 3.
Diagnostic performance of biochemical tests and extravascular body fluid criteria for NSTI using soft tissue infectious fluids
| AUC | 95% CI | p value | Optimal cut-off-point | Sensitivity | Specificity | PPV | NPV | LR + | LR- | |
|---|---|---|---|---|---|---|---|---|---|---|
| Biochemical tests | ||||||||||
| Albumin (fluid) (g/dL) | 0.884 | 0.790–0.978 | < 0.001* | 1.25 | 81.6% | 91.3% | 93.9% | 75.0% | 9.4 | 0.2 |
| Lactate (fluid) (mg/dL) | 0.891 | 0.808–0.973 | < 0.001* | 64.25 | 75.7% | 100.0% | 100.0% | 71.0% | – | 0.2 |
| Lactate dehydrogenase (fluid) (U/L) | 0.955 | 0.910–1.001 | < 0.001* | 493.85 | 86.8% | 91.3% | 94.3% | 80.8% | 10.0 | 0.1 |
| Glucose (fluid) (mg/dL) | 0.774 | 0.654–0.894 | < 0.001* | 109.5 | 71.4% | 82.6% | 86.2% | 65.5% | 4.1 | 0.3 |
| Total protein (fluid) (g/dL) | 0.883 | 0.789–0.977 | < 0.001* | 1.95 | 86.5% | 91.3% | 94.1% | 80.8% | 9.9 | 0.1 |
| pH (fluid) | 0.780 | 0.654–0.906 | < 0.001* | 8.4 | 69.4% | 70.0% | 80.6% | 56.0% | 2.3 | 0.4 |
| Common extravascular body fluid criteria | ||||||||||
| Light’s criteria (at least one criterion met) | 0.737 | 0.580–0.893 | 0.003* | > 0 | 100.0% | 47.4% | 77.3% | 100.0% | 1.9 | 0.0 |
| Light’s criteria (total score) | 0.925 | 0.847–1.003 | < 0.001* | > 2 | 85.3% | 94.7% | 96.7% | 78.3% | 16.1 | 0.2 |
| Serum-to-fluid albumin gradient (g/L) | 0.881 | 0.800–0.963 | < 0.001* | 2.00 | 70.3% | 91.3% | 92.9% | 65.6% | 8.1 | 0.3 |
| Fluid-to-serum LDH ratio | 0.929 | 0.865–0.993 | < 0.001* | 1.51 | 91.7% | 81.8% | 89.2% | 85.7% | 5.0 | 0.1 |
| Fluid-to-serum total protein ratio | 0.927 | 0.858–0.996 | < 0.001* | 0.47 | 88.2% | 89.5% | 93.8% | 81.0% | 8.4 | 0.1 |
(1) The optimal cutoff points were determined using the Youden index. (2) The positive likelihood ratio (LR +) is theoretically infinite when the specificity is 100%
Note: Bold formatting highlights key statistical values (p-values and AUC)
AUC, area under the curve; CI, confidence interval; LDH, lactate dehydrogenase; LR + , positive likelihood ratio; LR − , negative likelihood ratio; NPV, negative predictive value; NSTI, necrotizing soft tissue infection; PPV, positive predictive value
*p < 0.05, considered significant
Fig. 2.
Representation of the ROC curves and AUC for predicting the diagnosis of NSTI. a Lactate dehydrogenase (LDH) in fluids (AUC = 0.955), b Light’s criteria (total score) (AUC = 0.925), c fluid-to-serum LDH ratio (AUC = 0.929), and d fluid-to-serum total protein ratio (AUC = 0.927) Additional plots of ROC curves for other biochemical tests and extravascular body fluid criteria are shown in Additional file 3. AUC, area under the receiver operating characteristic curve; ROC, receiver operating characteristic; NSTI, necrotizing soft tissue infection
The optimal cutoff points were calculated using the Youden index and the diagnostic performance of each cutoff point was evaluated. The results showed that LDH (cutoff point: 493.85 U/L; sensitivity = 86.8%; specificity = 91.3%), the total score on Light’s criteria (cutoff point: more than 2; sensitivity = 85.3%; specificity = 94.7%), fluid-to-serum LDH ratio (cutoff point: 1.51; sensitivity = 91.7%; specificity = 81.8%), and fluid-to-serum total protein ratio (cutoff point: 0.47; sensitivity = 88.2%; specificity = 89.5%) demonstrated high sensitivity and specificity for diagnosing NSTI. Additionally, lactate levels exhibited the highest specificity (100%) and PPV (100%) at the optimal cutoff point of 64.25 mg/dL.
Subgroup analysis
In the subgroup analysis, 24 NSTI patients without high-risk clinical signs (hemorrhagic bullae and hypotension) were compared with 23 non-necrotizing SSTI patients. Consistent with our previous findings, the subgroup analysis demonstrated significant differences in all six biochemical parameters and five EBF criteria examined in this study using soft tissue infectious fluid between the NSTI subgroup and the non-necrotizing SSTI group. Table 4 presents the results of the study.
Table 4.
Subgroup analysis of biochemical tests and EBF criteria using soft tissue infectious fluids in NSTI patients without high-risk signs compared to non-necrotizing SSTI patients
| NSTI without high-risk signs+ | Non-necrotizing SSTI | p value | Diagnostic performance for NSTI | |||||
|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD or Number (%) | n | Mean ± SD or Number (%) | AUC | 95% CI | p value | ||
| Laboratory data in soft tissue infectious fluid | ||||||||
| Albumin (fluid) (g/dL) | 24 | 2.12 ± 0.86 | 23 | 1.16 ± 0.49 | < 0.001* | 0.874 | 0.766–0.982 | < 0.001* |
| Lactate (fluid) (mg/dL) | 23 | 120.80 ± 102.58 | 22 | 26.54 ± 14.01 | < 0.001* | 0.904 | 0.814–0.995 | < 0.001* |
| Lactate dehydrogenase (fluid) (U/L) | 24 | 4956.50 ± 7883.53 | 23 | 213.59 ± 224.01 | 0.007* | 0.966 | 0.922–1.000 | < 0.001* |
| Glucose (fluid) (mg/dL) | 21 | 93.30 ± 97.55 | 23 | 151.35 ± 83.36 | 0.039* | 0.697 | 0.531–0.863 | 0.026* |
| Total protein (fluid) (g/dL) | 23 | 3.37 ± 1.62 | 23 | 1.42 ± 0.84 | < 0.001* | 0.876 | 0.766–0.986 | < 0.001* |
| pH (fluid) | 22 | 8.10 ± 0.50 | 20 | 8.54 ± 0.32 | 0.002* | 0.782 | 0.643–0.920 | 0.002* |
| Common EBF criteria using soft tissue infectious fluid | ||||||||
| Light’s criteria (at least one criterion is met) | 20 | 20(100%) | 19 | 10(52.6%) | < 0.001* | 0.737 | 0.574–0.900 | 0.004* |
| Light’s criteria (total score) | 20 | 19 | < 0.001* | 0.926 | 0.840–1.013 | < 0.001* | ||
| 0 | 0(0%) | 9(47.3%) | ||||||
| 1 | 1(5%) | 1(5.3%) | ||||||
| 2 | 2(10%) | 8(42.1%) | ||||||
| 3 | 17(85%) | 1(5.3%) | ||||||
| Serum-to-fluid albumin gradient (g/L) | 23 | 1.59 ± 0.81 | 23 | 2.62 ± 0.51 | < 0.001* | 0.853 | 0.748–0.959 | < 0.001* |
| Fluid-to-serum LDH ratio | 22 | 17.28 ± 31.32 | 22 | 1.12 ± 1.46 | 0.025* | 0.932 | 0.859–1.005 | < 0.001* |
| Fluid-to-serum total protein ratio | 20 | 0.90 ± 0.47 | 19 | 0.35 ± 0.14 | < 0.001* | 0.904 | 0.808–1.000 | < 0.001* |
Continuous variables are expressed as the mean (± SD), whereas categorical variables are expressed as counts (percentages)
+ High-risk signs of NSTI include two clinical indicators: hemorrhagic bullae and the presence of hypotension (defined as a systolic blood pressure of less than 90 mmHg)
Note: Bold formatting highlights key statistical values (p-values and AUC)
AUC, area under the curve; CI, confidence interval; EBF, extravascular body fluid; LDH, lactate dehydrogenase; NSTI, necrotizing soft tissue infection; SD, standard deviation; SSTI, skin and soft tissue infection
*p < 0.05, considered statistically significant
Consequently, the overall diagnostic performance of biochemical tests and EBF criteria for NSTI in the subgroups were analyzed. In line with our previous findings, among the biochemical tests and EBF criteria, lactate (AUC = 0.904; p < 0.001), LDH (AUC = 0.966; p < 0.001), the total score on Light’s criteria (AUC = 0.926; p < 0.001), fluid-to-serum LDH ratio (AUC = 0.932; p < 0.001), and fluid-to-serum total protein ratio (AUC = 0.904; p < 0.001) demonstrated outstanding discrimination for NSTI. The ROC curve plots for the subgroup analyses are presented in Additional file 4.
Discussion
This cohort study aimed to analyze the biochemical characteristics of soft tissue infectious fluid and evaluate its diagnostic value using both biochemical data and common EBF criteria to differentiate between NSTI and non-necrotizing SSTI. These results demonstrate that the biochemical characteristics of soft tissue infectious fluid are significantly different between NSTIs and non-necrotizing SSTIs. Several diagnostic tests using soft tissue infectious fluid have shown outstanding discrimination for NSTI, including LDH, total score on Light’s criteria, fluid-to-serum LDH ratio, and fluid-to-serum total protein ratio. To the best of our knowledge, this is the first study to explore the diagnostic value of soft tissue infectious fluids by conducting a biochemical analysis and applying the common EBF criteria. These findings may assist physicians in distinguishing NSTI from non-necrotizing SSTI and guide surgeons in determining the need for surgical intervention. For example, in patients with soft tissue infectious fluid demonstrating high levels of lactate, LDH, or a high total score on the Light’s criteria, NSTI is highly suspected because of the high PPV of these tests, and emergent surgery is suggested. On the other hand, if the soft tissue infectious fluid does not meet Light’s criteria, NSTI is less likely to be present because of the high NPV of the test. Furthermore, subgroup analysis demonstrated similar diagnostic performance among the biochemical tests and EBF criteria in patients with NSTIs without high-risk signs, highlighting their potential diagnostic value in early stage NSTI. However, the optimal clinical decision pathway using soft tissue infectious fluids remains unclear, and further studies are needed to develop a decision-making process that potentially incorporates scoring systems or machine learning approaches.
The diagnosis of NSTIs depends mainly on clinical evaluation [1, 6]. However, early symptoms like pain, swelling, and erythema often mimic those of non-necrotizing SSTIs, making differentiation challenging. Several clinical tests have been developed to diagnose NSTI. The LRINEC score, a widely used clinical diagnostic tool for NSTI based on blood laboratory tests, indicates a 75% risk of NSTI when the score is 8 or higher [24]. However, recent studies have shown conflicting results regarding its diagnostic accuracy and its utility in clinical practice remains controversial [6, 21]. In imaging tests, a systematic review and meta-analysis published in 2019 reported that computed tomography (CT) has a high sensitivity of 94.3% but an insufficient specificity of 76.6% for detecting NSTI [21]. MRI is one of the most effective imaging modalities for diagnosing NSTI [1, 6]. However, it can be challenging to conduct in emergency situations and is not recommended as the first-line imaging technique. Our study demonstrates that biochemical tests using soft tissue infectious fluid can potentially provide valuable diagnostic information that may influence clinical decision-making. The advantages of using soft tissue infectious fluid include its ability to be collected upon patient arrival at the emergency department or bedside, making it a quick and readily available test in critical situations. Additionally, these biochemical tests are accessible in most hospitals, and their examination turnaround times are rapid. However, further research is required to validate these findings.
Most SSTIs result from microbial invasion of the skin through a breach or transient bacteremia following non-penetrating tissue injury [3, 25]. This invasion leads to the release of endotoxins, triggering an inflammatory response that causes fluid extravasation, which becomes trapped between the layers of infected fascia and adjacent tissues [25, 26]. In theory, these soft tissue infectious fluids may contain higher levels of inflammatory markers in patients with NSTI than in those with non-necrotizing SSTI, as NSTIs are generally more severe than non-necrotizing SSTIs. Previous studies have demonstrated that fluid accumulation alone is not sufficiently specific to differentiate NSTIs from non-necrotizing SSTIs [27, 28]. This raises the question of whether the biochemical characteristics of these soft tissue infectious fluids could provide further diagnostic information.
EBF analysis can provide valuable information for the differential diagnosis and is widely used to assess pleural effusion, ascites, synovial fluid, and pericardial fluid [8, 9]. For example, pleural effusions are typically classified as transudates or exudates [10, 29, 30]. Exudates result from increased capillary permeability, allowing proteins and other serum constituents to leak into the fluid, usually due to infections or inflammatory conditions. Conversely, transudative effusions, which are often associated with non-inflammatory conditions, result from increased hydrostatic pressure or decreased plasma oncotic pressure, leading to the accumulation of fluids with low protein content. Several biomarkers have been identified as useful in EBF analysis for distinguishing exudative fluids from transudative fluids, as well as between infectious fluids from non-infectious fluids: (1) The levels of LDH, a marker of cellular injury or inflammation, are typically higher in exudates. An LDH level of > 1000 IU/L was considered to indicate complicated parapneumonic effusion or empyema [14, 15], (2) Exudative fluids often exhibit significantly lower glucose and pH levels, especially in the presence of bacterial infection [14, 31], (3) Total protein and albumin levels are typically elevated in exudative fluids due to increased capillary permeability and inflammatory conditions [15, 32], and (4) The levels of lactate, a product of anaerobic glycolysis, are often elevated in infection-related conditions [13, 33, 34]. However, studies investigating the biochemical characteristics or diagnostic value of soft tissue infectious fluids are limited. The present study evaluated these biochemical markers in soft tissue infectious fluids, and the results demonstrated significant differences in the levels of albumin, lactate, LDH, glucose, total protein, and pH between the NSTI and non-necrotizing SSTI groups. These findings support the hypothesis that soft tissue infectious fluids in patients with NSTI differ from those in patients with non-necrotizing SSTI, possibly due to differences in infection severity and tissue damage.
Based on biochemical markers identified in published studies, several criteria have been developed to effectively differentiate exudative from transudative fluids. Light’s criteria, developed by Dr. Richard Light in 1972, are used to differentiate transudates and exudates through biochemical analysis [10]. They are known for their high sensitivity (97.5%) and acceptable specificity (80%) for detecting exudative pleural effusion [35]. Light’s criteria have also been applied to evaluate other EBFs such as pericardial fluid and ascites to distinguish exudative from transudative body fluids [36]. The serum-ascites albumin gradient is another parameter used to categorize ascites more effectively than the exudate-transudate concept [18, 19]. A low gradient (< 1.1 g/dL) indicates that the causes of ascites are not related to increased portal pressure and significantly reduces the likelihood ratio (LR = 0.06, 95% confidence interval = 0.02–0.20) of portal hypertension compared with bacterial peritonitis [18, 19, 37]. These criteria are widely used for various extravascular body fluids, but their diagnostic performance and optimal cutoff points can vary depending on the type of specimen, such as pleural effusion, pericardial effusion, and ascites [9, 36, 38]. In this study, these EBF criteria were applied to soft tissue infectious fluids to differentiate NSTIs from non-necrotizing SSTIs, and the optimal cutoff points were calculated. The results demonstrated that several criteria provided excellent-to-outstanding diagnostic discrimination for NSTI. Further research is required to validate these findings.
Soft tissue infectious fluids have rarely been studied, and most studies have focused on evaluating imaging characteristics, such as the presence of fluid or the depth of fluid accumulation, to differentiate NSTIs from other SSTIs [39]. This study aimed to better understand the characteristics of soft tissue infectious fluids using biochemical analyses and EBF criteria based on the transudate-exudate concept. However, because both NSTI and non-necrotizing SSTIs are infectious diseases, the fluids they yield are theoretically more likely to be classified as exudative. In this study, many patients in both the non-necrotizing SSTI and NSTI groups met at least one of the Light’s criteria for exudates. This overlap led to a specificity of only 47.4%, which was insufficient to distinguish NSTIs from non-necrotizing SSTIs. Conversely, this study found that the optimal cut-off points for biochemical markers and EBF criteria related to inflammation and cell damage, such as LDH (498.83 U/L) and fluid-to-serum LDH ratio (1.51), were higher than those established in other EBF studies [9, 36]. Together, these findings suggest that both NSTI and non-necrotizing SSTIs are likely to yield exudative fluids. The differences in their biochemical characteristics may reflect the severity of soft tissue infection and damage. Distinguishing between these conditions may depend on markers of inflammation, such as LDH, and the optimal cutoff points for this differentiation might vary from those established in other EBF studies.
In this study, soft tissue infectious fluids were investigated using a biochemical approach, with common biochemical tests employed in other EBF studies. However, several questions that warrant further investigation remain unanswered. First, several clinical biochemical tests related to tissue damage or infection markers, such as serum creatine phosphokinase or myoglobin, may have diagnostic potential for distinguishing NSTIs from non-necrotizing SSTIs when examining soft tissue infectious fluid [40]. Second, molecular diagnostic techniques such as polymerase chain reaction (PCR)-based methods and high-throughput sequencing can be employed to examine the microbial characteristics of soft tissue infectious fluids and facilitate the early identification and quantification of microbial pathogens [41, 42]. Previous studies have shown that metagenomic next-generation sequencing is a promising tool for the etiological diagnosis of SSTIs using skin tissue swabs or pus, and it may also be utilized with soft tissue infectious fluid for pathogen identification [43]. Third, the question remains as to how to use study findings more effectively and straightforwardly in clinical practice. Therefore, a clinical scoring system or machine learning model may be required to make these biochemical results more practical. Fourth, NSTI can also occur in areas other than the limbs; however, the diagnostic performance of soft tissue infectious fluid analysis for NSTI in these regions remains unclear. This study highlights the potential research value of soft tissue infectious fluids. Further studies are needed to achieve a more comprehensive understanding of this type of specimen.
This study had some limitations. First, the cohort study design has inherent limitations, including unmeasured confounding factors and differences in baseline characteristics between the groups. In addition, confounders such as hepatic injury, preexisting malnutrition, or general edema may potentially alter the baseline biochemical results of patients. Second, the number of patients was relatively small owing to the rarity of NSTI and severe SSTIs that mimic NSTI. However, the findings of this study provide valuable insights into this rare condition. Third, this study focused on the biochemical analysis of infectious fluid and did not compare the findings of this analysis with those of other diagnostic tests. Fourth, the location of the soft tissue infectious fluids in our study was fluid accumulation above the fascia. However, it is unclear whether all subtypes of NSTI lead to suprafascial fluid. Furthermore, fluids below the fascia or within the muscle layers were not observed. Further studies are required to address these limitations.
Conclusions
In this study, LDH level, total score on Light’s criteria, fluid-to-serum LDH ratio, and fluid-to-serum total protein ratio demonstrated outstanding discrimination for NSTI. Further research is needed to develop a scoring system or machine learning model that can utilize these findings to make biochemical results more practical.
Supplementary Information
Acknowledgements
The authors would like to express their gratitude to all study participants and acknowledge the support provided by the Chiayi Chang Gung Memorial Hospital.
Author contributions
The study was designed by KH Wu and PJ Chang. Data collection was conducted by KH Wu, PH Wu, HS Wang, HM Shiau, and CP Chang. The analyses were performed by KH Wu, YS Hsu, CY Lee, YT Lin, CT Hsiao, and LC Lin. KH Wu, CP Chang, and PJ Chang draft the manuscript. All the authors contributed to the literature review and approved the final manuscript.
Funding
This study was supported by a grant from the Chang Gung Medical Research Program. (CMRPG6N0141, CMRPG6N0142).
Availability of data and materials
For data requests, please contact the corresponding author.
Declarations
Ethics approval and consent to participate
This study was approved by the hospital’s Institutional Review Board (IRB) (prospective cohort IRB No. 202300399B0 and retrospective cohort IRB No. 202301747B0). Informed consent was obtained from all the patients or their legal guardians before inclusion in the prospective cohort.
Consent for publication
Not applicable.
Competing interests
The authors have disclosed no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chia-Peng Chang, Email: giovanni850730@gmail.com.
Pey-Jium Chang, Email: eilrahc142@gmail.com.
References
- 1.Sartelli M, Coccolini F, Kluger Y, Agastra E, Abu-Zidan FM, Abbas AES, et al. WSES/GAIS/WSIS/SIS-E/AAST global clinical pathways for patients with skin and soft tissue infections. World J Emerg Surg. 2022;17(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vella V, Derreumaux D, Aris E, Pellegrini M, Contorni M, Scherbakov M, et al. The incidence of skin and soft tissue infections in the united states and associated healthcare utilization between 2010 and 2020. Open Forum Infect Dis. 2024;11(6):ofae267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramakrishnan K, Salinas RC, Agudelo Higuita NI. Skin and soft tissue infections. Am Fam Phys. 2015;92(6):474–83. [PubMed] [Google Scholar]
- 4.Duane TM, Huston JM, Collom M, Beyer A, Parli S, Buckman S, et al. Surgical infection society 2020 updated guidelines on the management of complicated skin and soft tissue infections. Surg Infect. 2021;22(4):383–99. [DOI] [PubMed] [Google Scholar]
- 5.Nawijn F, Smeeing DPJ, Houwert RM, Leenen LPH, Hietbrink F. Time is of the essence when treating necrotizing soft tissue infections: a systematic review and meta-analysis. World J Emerg Surg. 2020;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hua C, Urbina T, Bosc R, Parks T, Sriskandan S, de Prost N, et al. Necrotising soft-tissue infections. Lancet Infect Dis. 2023;23(3):e81–94. [DOI] [PubMed] [Google Scholar]
- 7.Wu KH, Chang CP. Differentiating lower extremity necrotizing soft tissue infection from severe cellulitis by laboratory parameters and relevant history points. Infect Drug Resist. 2021;14:3563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jokic A, Milevoj Kopcinovic L, Culej J, Kocijan I, Bozovic M. Laboratory testing of extravascular body fluids: National recommendations on behalf of the croatian society of medical biochemistry and laboratory medicine. Part II—synovial fluid. Biochem Med (Zagreb). 2020;30(3):030501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milevoj Kopcinovic L, Culej J, Jokic A, Bozovic M, Kocijan I. Laboratory testing of extravascular body fluids: national recommendations on behalf of the croatian society of medical biochemistry and laboratory medicine. Part I—serous fluids. Biochem Med. 2020;30(1):010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Light RW, Macgregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77(4):507–13. [DOI] [PubMed] [Google Scholar]
- 11.Guarner C, Runyon BA. Chapter 30—Ascites. In: McNally PR, editor. GI/Liver secrets. 4th ed. Philadelphia: Mosby; 2010. p. 217–27. [Google Scholar]
- 12.Mirambeaux Villalona R, Arrieta Narvaez P, Galarza Jimenez MA, Barrios Barreto D, Pérez Rodriguez E. Identifying false exudates by albumin and protein gradients in patients with heart failure and hepatic hydrothorax. Eur Respirat J. 2015;46(suppl 59):PA1827. [Google Scholar]
- 13.Guerra SS, Ferro R, Abrantes T, António C. Pleural fluid lactate: a diagnostic tool in pleural effusion management? J Bras Pneumol. 2022;48(6):e20220350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. Clin Infect Dis. 2007;45(11):1480–6. [DOI] [PubMed] [Google Scholar]
- 15.Mercer RM, Corcoran JP, Porcel JM, Rahman NM, Psallidas I. Interpreting pleural fluid results Clin Med. 2019;19(3):213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Nooijer AH, Pickkers P, Netea MG, Kox M. Inflammatory biomarkers to predict the prognosis of acute bacterial and viral infections. J Crit Care. 2023;78:154360. [DOI] [PubMed] [Google Scholar]
- 17.Houston MC. Pleural fluid pH: diagnostic, therapeutic, and prognostic value. Am J Surg. 1987;154(3):333–7. [DOI] [PubMed] [Google Scholar]
- 18.Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117(3):215–20. [DOI] [PubMed] [Google Scholar]
- 19.Runyon BA. Chapter 91—Ascites and spontaneous bacterial peritonitis. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s gastrointestinal and liver disease. 9th ed. Philadelphia: W.B. Saunders; 2010. p. 1517- 41.e4. [Google Scholar]
- 20.Paramothayan NS, Barron J. New criteria for the differentiation between transudates and exudates. J Clin Pathol. 2002;55(1):69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernando SM, Tran A, Cheng W, Rochwerg B, Kyeremanteng K, Seely AJE, et al. Necrotizing soft tissue infection: diagnostic accuracy of physical examination, imaging, and LRINEC score: a systematic review and meta-analysis. Ann Surg. 2019;269(1):58–65. [DOI] [PubMed] [Google Scholar]
- 22.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–5. [DOI] [PubMed] [Google Scholar]
- 23.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315–6. [DOI] [PubMed] [Google Scholar]
- 24.Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535–41. [DOI] [PubMed] [Google Scholar]
- 25.Stevens DL, Bryant AE. Necrotizing soft-tissue infections. N Engl J Med. 2017;377(23):2253–65. [DOI] [PubMed] [Google Scholar]
- 26.Demicco EG, Kattapuram SV, Kradin RL, Rosenberg AE. Infections of joints, synovium-lined structures, and soft tissue. In: Diagnostic pathology of infectious disease. Elsevier; 2018. p. 404–28. [Google Scholar]
- 27.Demicco EG, Kattapuram SL, Kradin RL, Rosenberg AE. Chapter 14—Infections of joints, synovium-lined structures, and soft tissue. In: Kradin RL, editor. Diagnostic pathology of infectious disease. New York: W.B. Saunders; 2010. p. 377–401. [Google Scholar]
- 28.Ali SZ, Srinivasan S, Peh WC. MRI in necrotizing fasciitis of the extremities. Br J Radiol. 2014;87(1033):20130560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen-Wagner J, Gamble C, MacGilvray P. Pleural effusion: diagnostic approach in adults. Am Fam Physician. 2023;108(5):464–75. [PubMed] [Google Scholar]
- 30.Yu H. Management of pleural effusion, empyema, and lung abscess. Semin Intervent Radiol. 2011;28(1):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzgerald DB, Leong SL, Budgeon CA, Murray K, Rosenstengal A, Smith NA, et al. Relationship of pleural fluid pH and glucose: a multi-centre study of 2,971 cases. J Thorac Dis. 2019;11(1):123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta R, Misra SP, Dwivedi M, Misra V, Kumar S, Gupta SC. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol. 1995;10(3):295–9. [DOI] [PubMed] [Google Scholar]
- 33.Gobelet C, Gerster JC. Synovial fluid lactate levels in septic and non-septic arthritides. Ann Rheum Dis. 1984;43(5):742–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Li C, Wang G, Shi L, Li T, Fan X, et al. Diagnostic accuracy of synovial fluid D-lactate for periprosthetic joint infection: a systematic review and meta-analysis. J Orthop Surg Res. 2021;16(1):606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porcel JM, Peña JM, Vicente de Vera C, Esquerda A. Reappraisal of the standard method (Light’s criteria) for identifying pleural exudates. Med Clin. 2006;126(6):211–3. [DOI] [PubMed] [Google Scholar]
- 36.Kopcinovic LM, Culej J. Pleural, peritoneal and pericardial effusions - a biochemical approach. Biochem Med. 2014;24(1):123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong CL, Holroyd-Leduc J, Thorpe KE, Straus SE. Does this patient have bacterial peritonitis or portal hypertension? How do I perform a paracentesis and analyze the results? JAMA. 2008;299(10):1166–78. [DOI] [PubMed] [Google Scholar]
- 38.Chubb SP, Williams RA. Biochemical analysis of pleural fluid and ascites. Clin Biochem Rev. 2018;39(2):39–50. [PMC free article] [PubMed] [Google Scholar]
- 39.Tso DK, Singh AK. Necrotizing fasciitis of the lower extremity: imaging pearls and pitfalls. Br J Radiol. 2018;91(1088):20180093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simonart T, Nakafusa J, Narisawa Y. The importance of serum creatine phosphokinase level in the early diagnosis and microbiological evaluation of necrotizing fasciitis. J Eur Acad Dermatol Venereol. 2004;18(6):687–90. [DOI] [PubMed] [Google Scholar]
- 41.Procop GW. Molecular diagnostics for the detection and characterization of microbial pathogens. Clin Infect Dis. 2007;45(Suppl 2):S99-s111. [DOI] [PubMed] [Google Scholar]
- 42.Liu Q, Jin X, Cheng J, Zhou H, Zhang Y, Dai Y. Advances in the application of molecular diagnostic techniques for the detection of infectious disease pathogens (Review). Mol Med Rep. 2023. 10.3892/mmr.2023.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Q, Miao Q, Pan J, Jin W, Ma Y, Zhang Y, et al. The clinical value of metagenomic next-generation sequencing in the microbiological diagnosis of skin and soft tissue infections. Int J Infect Dis. 2020;100:414–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For data requests, please contact the corresponding author.