To the Editor:
Patients with asthma are at risk for structural airway changes that lead to accelerated loss of lung function (1, 2). The identification of modifiable independent risk factors for lung function decline (LFD) is an important goal (3). The Type 2 biomarkers peripheral blood eosinophils and fractional exhaled nitric oxide (FeNO) have been shown to identify different aspects of Type 2 airway inflammation and, collectively, predict asthma exacerbations (4). Evidence exists that they both identify patients who are at risk of future LFD (5–10). However, the role of either biomarker or their combination as prognostic or predictive biomarkers and the effect of treatment have yet to be established definitively. This post hoc analysis of the QUEST (ClinicalTrials.gov ID: NCT02414854) study data was conducted to determine whether FeNO and blood eosinophils are independent prognostic biomarkers for LFD and predictors of dupilumab’s treatment effect on this outcome.
Some of the results of QUEST have been previously reported in the form of abstracts (11–15).
QUEST was a phase-3, randomized, double-blind, placebo-controlled study that assessed the efficacy and safety of dupilumab in patients aged 12 years and older who had uncontrolled, moderate-to-severe asthma despite consistent treatment with inhaled corticosteroids (ICSs) plus one or two additional controllers. Full details of the inclusion and exclusion criteria and the study protocol have been published previously (16). The primary endpoints were the annualized rate of severe asthma exacerbations and the change from baseline to Week 12 in pre-bronchodilator (BD) FEV1. This post hoc analysis took into consideration the adult (⩾18-yr-old) population, and selection was determined according to baseline FeNO or eosinophil levels.
LFD (milliliters per year) and the treatment difference in LFD between dupilumab and placebo were defined as the annual loss of post-BD FEV1, measured by the post-BD FEV1 slope derived from five available measures from Week 8 through Week 52 in patients receiving either placebo or dupilumab across biomarker subgroups. Multivariate regression analyses were conducted to identify factors associated with LFD. Covariates were treatment, age, sex, height, baseline log FeNO, baseline log blood eosinophils, Asthma Control Questionnaire (ACQ-5) score, number of exacerbations during the previous year, age of asthma onset, ICS dose level, baseline post-BD FEV1, time since randomization, time since randomization by treatment, and region. ACQ-5 score, exacerbations in the previous year, and age of asthma onset were not significant and were excluded from the final model. To identify predictive biomarkers associated with LFD and response to dupilumab, the treatment difference between dupilumab and placebo in LFD was assessed across baseline blood eosinophil and FeNO levels.
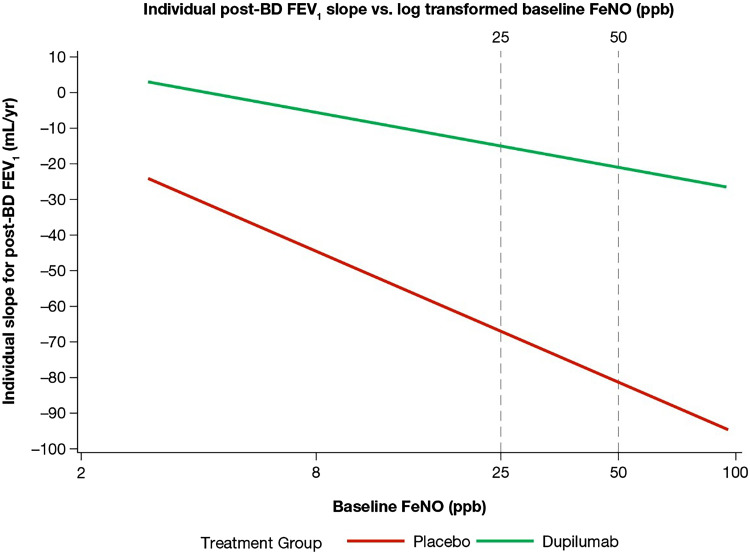
Baseline post-BD FEV1, log blood eosinophils, and log FeNO were significantly associated with post-baseline post-BD FEV1 (P < 0.0001). Lung function declined progressively with increasing baseline FeNO level in patients who received placebo, and the rate of decline was generally attenuated by treatment with dupilumab; the difference of the slope of the two lines was −11.8 (95% confidence interval [CI]: −71.5, 47.8) (Figure 1). In contrast, decline in lung function was similar in patients who received placebo, regardless of baseline blood eosinophils, and patients with higher baseline blood eosinophils had lower LFD attenuation with dupilumab. The treatment difference between dupilumab and placebo increased in populations defined by higher baseline FeNO, with a difference of 39 ml (95% CI: −5, 83) for the population with FeNO ⩾25 parts per billion (ppb), 75 ml (95% CI: 19, 131) for the population with FeNO ⩾35 ppb, and 86 ml (95% CI: 7, 166) for the population with FeNO ⩾50 ppb. The middle cutoff point was established on the basis of an observed “turning point,” whereby treatment differences were more prominent, whereas the lower cutoffs were nonsubstantial, and the higher cutoffs were not clinically meaningfully different.
Figure 1.
Rate of lung function decline across baseline FeNO levels. The slope difference between the two lines is −11.8, with a 95% confidence interval of −71.5, 47.8. BD = bronchodilator; FeNO = fractional exhaled nitric oxide; ppb = parts per billion.
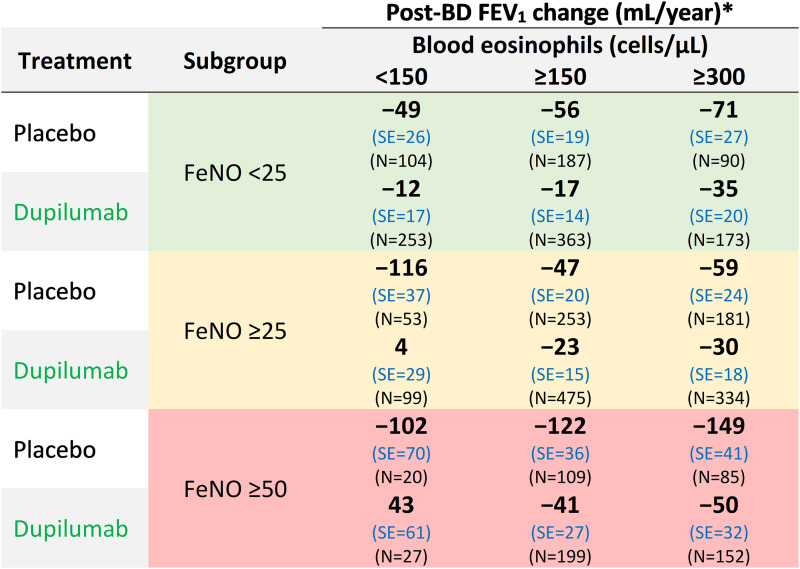
In an analysis of the two biomarkers together, patients who received placebo with elevated baseline FeNO (⩾25 and ⩾50 ppb) showed higher LFD, with a range from 102 ml to 149 ml loss per year, regardless of baseline blood eosinophils (Figure 2). In addition, for patients who were treated with dupilumab, LFD was attenuated across biomarker threshold groups, with a range of 43 ml (n = 27) to 4 ml (n = 99) in the high-FeNO–low-eosinophil groups and −17 ml (n = 363) to −35 ml (n = 172) in the low-FeNO–high-eosinophil groups.
Figure 2.
Lung function decline difference in populations selected by combined baseline FeNO and eosinophil levels. *Estimated from a mixed-effects model with repeated post-BD FEV1 as outcome, and treatment, age, sex, height, region (pooled country), baseline eosinophil strata, baseline ICS dose level, time since randomization, and Treatment × Time interaction and baseline post-BD FEV1 as covariates. Intercept and time since randomization are random effects. BD = bronchodilator; FeNO = fractional exhaled nitric oxide; FEV1 = forced expiratory volume in one second; SE = standard error.
This post hoc analysis supports the use of FeNO as a risk biomarker identifying patients who are at increased risk of LFD, as well as identifying those with greater attenuation in LFD on dupilumab. This is key in identifying patients who might benefit from early specific intervention. The distinct added value of FeNO, compared with blood eosinophils, as a biomarker for prediction of LFD is in contrast to what has been seen for prediction of asthma exacerbations, with previous research indicating that FeNO’s prognostic value was additive in parallel with blood eosinophil counts (17). This finding, coupled with the relationship between baseline FeNO and LFD that was independent of exacerbations, suggests that the mechanisms leading to LFD and exacerbations are somewhat distinct.
The nature of this analysis prompts limitations such as a small sample size in some subgroups, which allows for only mean analysis of limited data. A decline in ICS adherence during QUEST might be expected to have a bigger impact on patients with higher baseline biomarkers and may, therefore, account for some of the observed LFD; however, adherence to background therapy was more than 80% in QUEST, and additional findings have shown that pre-BD FEV1 was consistent across baseline FeNO levels in the placebo group (18); therefore, it is an unlikely confounder. Finally, alternative causes for LFD were not captured. The present data should, therefore, be seen as hypothesis generating while providing a strong basis for further studies of appropriate power and duration to definitively evaluate FeNO as a predictive and prognostic biomarker for LFD.
In conclusion, this analysis provides robust data supporting FeNO as a clinically viable prognostic biomarker for accelerated LFD and predictive of the treatment response to dupilumab. Additional research is needed to establish patterns of LFD in patients with moderate-to-severe asthma, as well as the prognostic and predictive role of FeNO.
Acknowledgments
Acknowledgment
Medical writing and editorial assistance were provided by Sylvia Nkoula, Ph.D., of Excerpta Medica.
QUEST Lung Function Decline Study Group: Nami Pandit-Abid and Paul J. Rowe, Sanofi, Bridgewater, New Jersey; Amr Radwan and Yamo Deniz, Regeneron Pharmaceuticals Inc., Tarrytown, New York.
Footnotes
Supported by Sanofi and Regeneron Pharmaceuticals Inc., according to the Good Publication Practice Guidelines: 2022 update.
Originally Published in Press as DOI: 10.1164/rccm.202310-1751LE on February 8, 2024
Author disclosures are available with the text of this letter at www.atsjournals.org.
Contributor Information
Collaborators: Nami Pandit-Abid, Paul J. Rowe, Amr Radwan, and Yamo Deniz
References
- 1. James AL, Palmer LJ, Kicic E, Maxwell PS, Lagan SE, Ryan GF, et al. Decline in lung function in the Busselton Health Study: the effects of asthma and cigarette smoking. Am J Respir Crit Care Med . 2005;171:109–114. doi: 10.1164/rccm.200402-230OC. [DOI] [PubMed] [Google Scholar]
- 2. Peat JK, Woolcock AJ, Cullen K. Rate of decline of lung function in subjects with asthma. Eur J Respir Dis . 1987;70:171–179. [PubMed] [Google Scholar]
- 3. Matsunaga K, Hirano T, Oka A, Tanaka A, Kanai K, Kikuchi T, et al. Progression of irreversible airflow limitation in asthma: correlation with severe exacerbations. J Allergy Clin Immunol Pract . 2015;3:759–764. doi: 10.1016/j.jaip.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 4. Semprini R, Williams M, Semprini A, McDouall A, Fingleton J, Holweg C, et al. Type 2 biomarkers and prediction of future exacerbations and lung function decline in adult asthma. J Allergy Clin Immunol Pract . 2018;6:1982–1988.e1.5. doi: 10.1016/j.jaip.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 5. Coumou H, Westerhof GA, de Nijs SB, Zwinderman AH, Bel EH. Predictors of accelerated decline in lung function in adult-onset asthma. Eur Respir J . 2018;51:1701785. doi: 10.1183/13993003.01785-2017. [DOI] [PubMed] [Google Scholar]
- 6. Matsunaga K, Hirano T, Oka A, Ito K, Edakuni N. Persistently high exhaled nitric oxide and loss of lung function in controlled asthma. Allergol Int . 2016;65:266–271. doi: 10.1016/j.alit.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 7. van Veen IH, ten Brinke A, Sterk PJ, Sont JK, Gauw SA, Rabe KF, et al. Exhaled nitric oxide predicts lung function decline in difficult-to-treat asthma. Eur Respir J . 2008;32:344–349. doi: 10.1183/09031936.00135907. [DOI] [PubMed] [Google Scholar]
- 8. Bjermer L, Alving K, Diamant Z, Magnussen H, Pavord I, Piacentini G, et al. Current evidence and future research needs for FeNO measurement in respiratory diseases. Respir Med . 2014;108:830–841. doi: 10.1016/j.rmed.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 9. Park HY, Chang Y, Kang D, Hong YS, Zhao D, Ahn J, et al. Blood eosinophil counts and the development of obstructive lung disease: the Kangbuk Samsung Health Study. Eur Respir J . 2021;58:2003823. doi: 10.1183/13993003.03823-2020. [DOI] [PubMed] [Google Scholar]
- 10. Hancox RJ, Pavord ID, Sears MR. Associations between blood eosinophils and decline in lung function among adults with and without asthma. Eur Respir J . 2018;51:1702536. doi: 10.1183/13993003.02536-2017. [DOI] [PubMed] [Google Scholar]
- 11. Pavord ID, Brusselle G, Jackson DJ, Brightling CE, Papi A, Maspero JF, et al. FeNO as a potential prognostic and predictive marker of lung function decline in patients with uncontrolled, moderate-to-severe asthma: LIBERTY ASTHMA QUEST. Am J Respir Crit Care Med . 2022;205:A3418. [Google Scholar]
- 12. Pavord ID, Brusselle G, Jackson DJ, Brightling CE, Papi A, Maspero JF, et al. Biomarkers associated with lung function decline and dupilumab response in patients with moderate-to-severe asthma. Eur Respir J . 2022;60(Supplement):2545. doi: 10.1164/rccm.202310-1751LE. [DOI] [PubMed] [Google Scholar]
- 13. Pavord ID, Brusselle G, Jackson DJ, Brightling CE, Papi A, Maspero JF, et al. FeNO is potentially prognostic of accelerated lung function decline and predictive of dupilumab response in patients with moderate-to-severe asthma. Can J Respir Crit Care Sleep Med . 2023;7(Supplement) [Google Scholar]
- 14.Pavord ID, Brusselle G, Jackson DJ, Brightling CE, Papi A, Maspero JF, et al. 2022.
- 15. Wark P, Pavord ID, Brusselle G, Jackson DJ, Brightling CE, Papi A, et al. FeNO and lung function decline in patients with asthma Respirology 2023. 28 110 246 36617387 [Google Scholar]
- 16. Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med . 2018;378:2486–2496. doi: 10.1056/NEJMoa1804092. [DOI] [PubMed] [Google Scholar]
- 17. Couillard S, Laugerud A, Jabeen M, Ramakrishnan S, Melhorn J, Hinks T, et al. Derivation of a prototype asthma attack risk scale centred on blood eosinophils and exhaled nitric oxide. Thorax . 2022;77:199–202. doi: 10.1136/thoraxjnl-2021-217325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pavord ID, Deniz Y, Corren J, Casale TB, FitzGerald JM, Izuhara K, et al. Baseline FeNO independently predicts the dupilumab response in patients with moderate-to-severe asthma. J Allergy Clin Immunol Pract . 2023;11:1213–1220.e2. doi: 10.1016/j.jaip.2022.11.043. [DOI] [PubMed] [Google Scholar]