Abstract
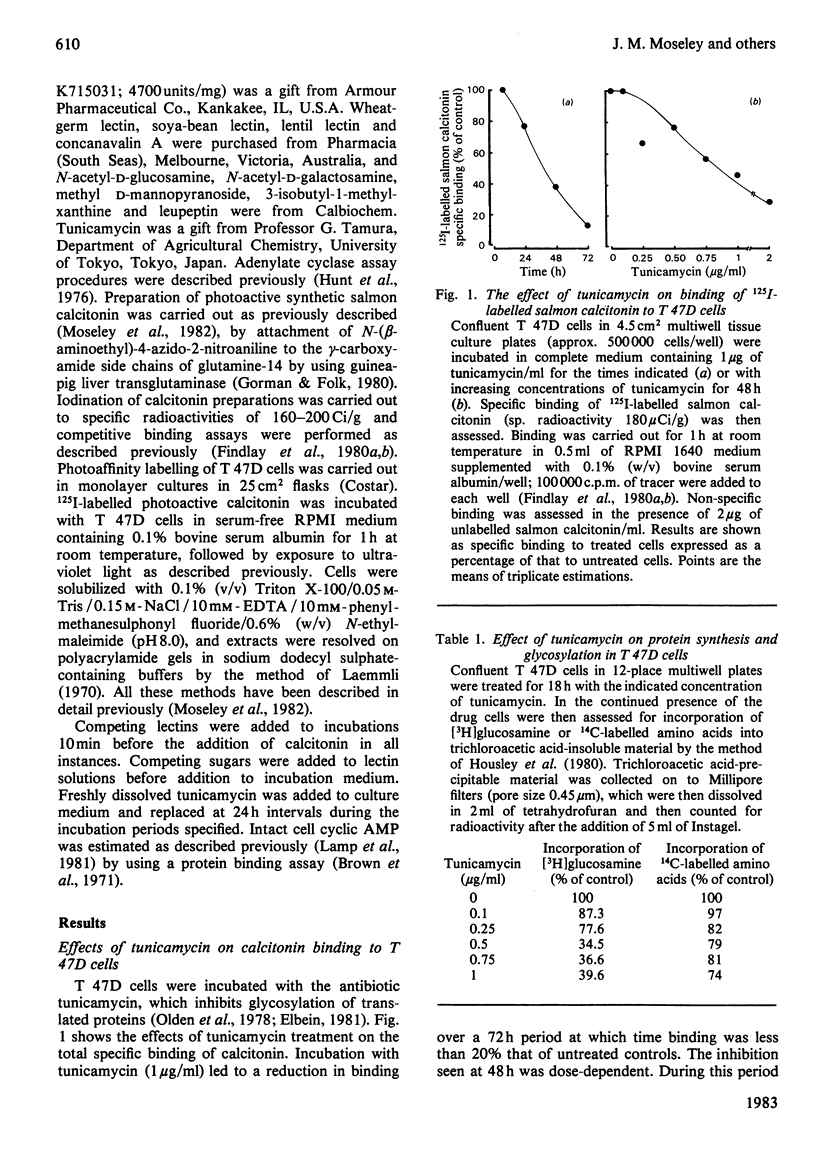
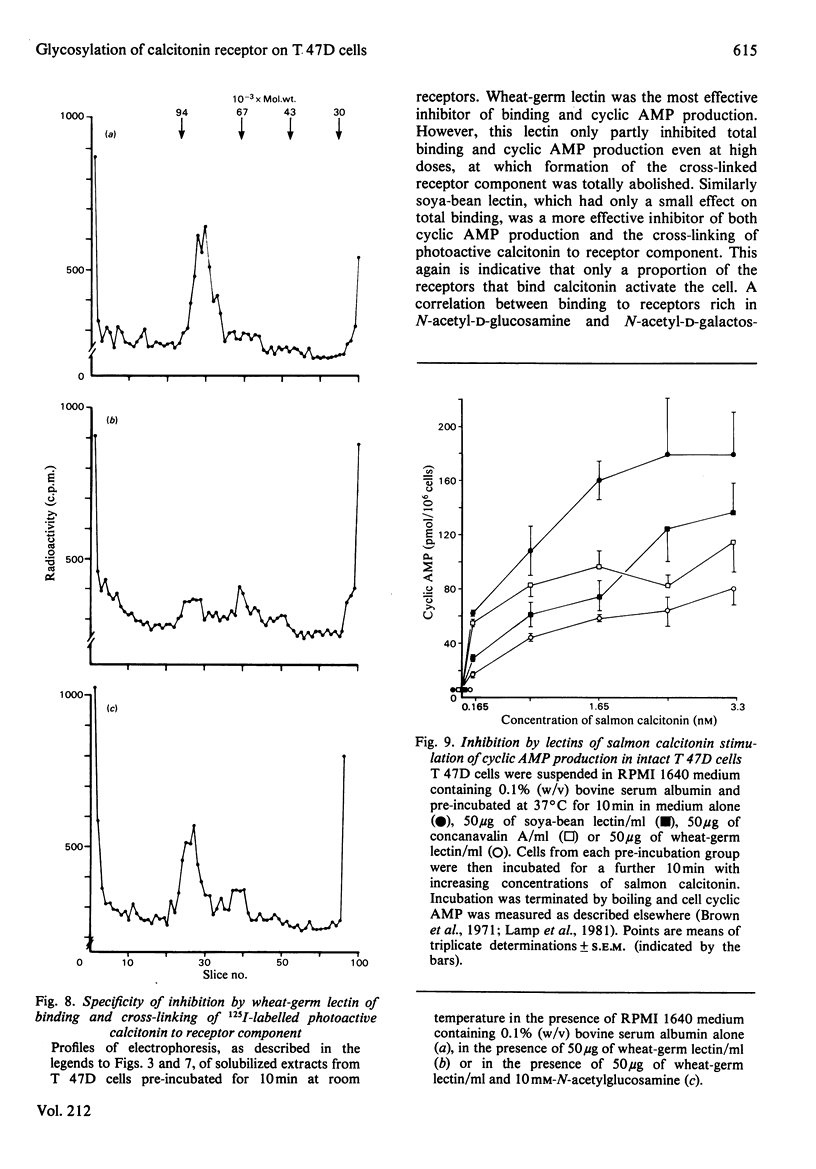
The glycosyl nature of the receptor for the peptide hormone calcitonin has been investigated in a human breast cancer cell line, T 47D. Studies have been carried out to assess the ability of various lectins and of the antibiotic tunicamycin to inhibit specific binding of calcitonin to the cells, to reduce cross-linking of photoactive calcitonin to a macromolecular receptor component and to influence calcitonin stimulation of cyclic AMP. Pre-incubation of cells with low concentrations of tunicamycin for 72 h resulted in a reduction of total specific binding by approx. 80% and a 40% reduction in calcitonin-stimulated adenylate cyclase; formation of the cross-linked receptor component was also inhibited. Wheat-germ lectin showed the most marked inhibition of total specific binding and cyclic AMP production. However, cross-linking of photoactive calcitonin to receptor component was totally inhibited by this lectin. Soya-bean lectin brought about very little reduction in total specific binding but had more profound effects on calcitonin-stimulated cyclic AMP production and cross-linking of photoactive calcitonin. Concanavalin A and lentil lectin showed some inhibition of all parameters. The data indicate that the calcitonin receptor in T 47D cells is associated with glycosyl moieties, the major contributors of which are N-acetyl-D-glucosamine residues, but N-acetyl-D-galactosamine and mannose residues are also associated.
Full text
PDF

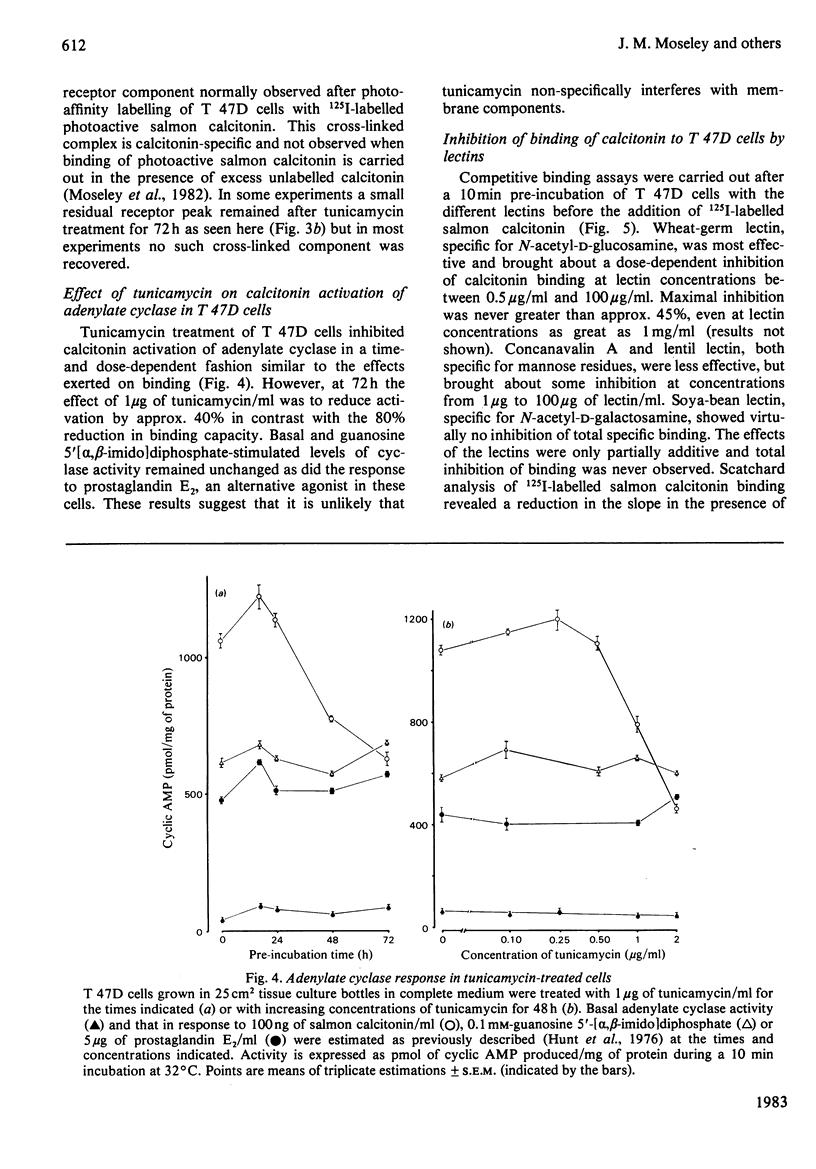

Selected References
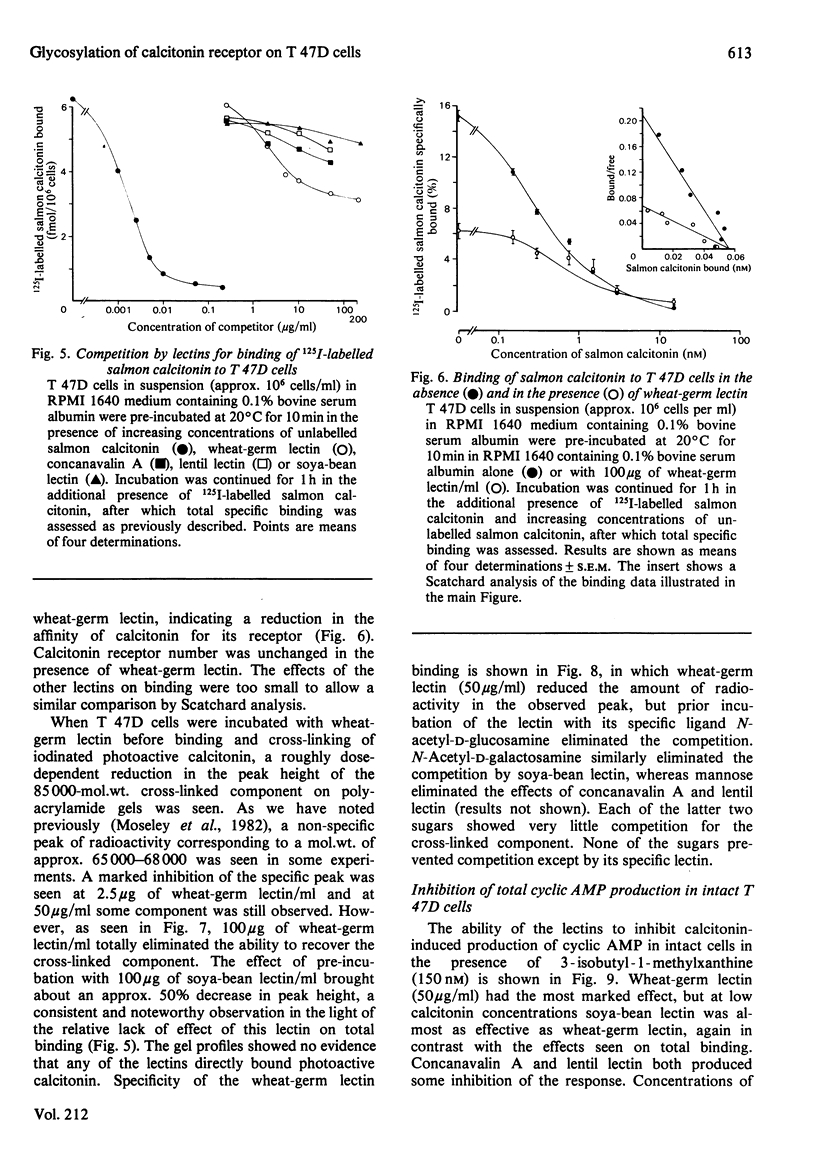
These references are in PubMed. This may not be the complete list of references from this article.
- Azhar S., Menon K. M. Receptor-mediated gonadotropin action in the ovary. Inhibitory actions of concanavalin A and wheat-germ agglutinin on gonadotropin-stimulated cyclic AMP and progesterone responses in ovarian cells. Biochem J. 1981 Oct 15;200(1):153–159. doi: 10.1042/bj2000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
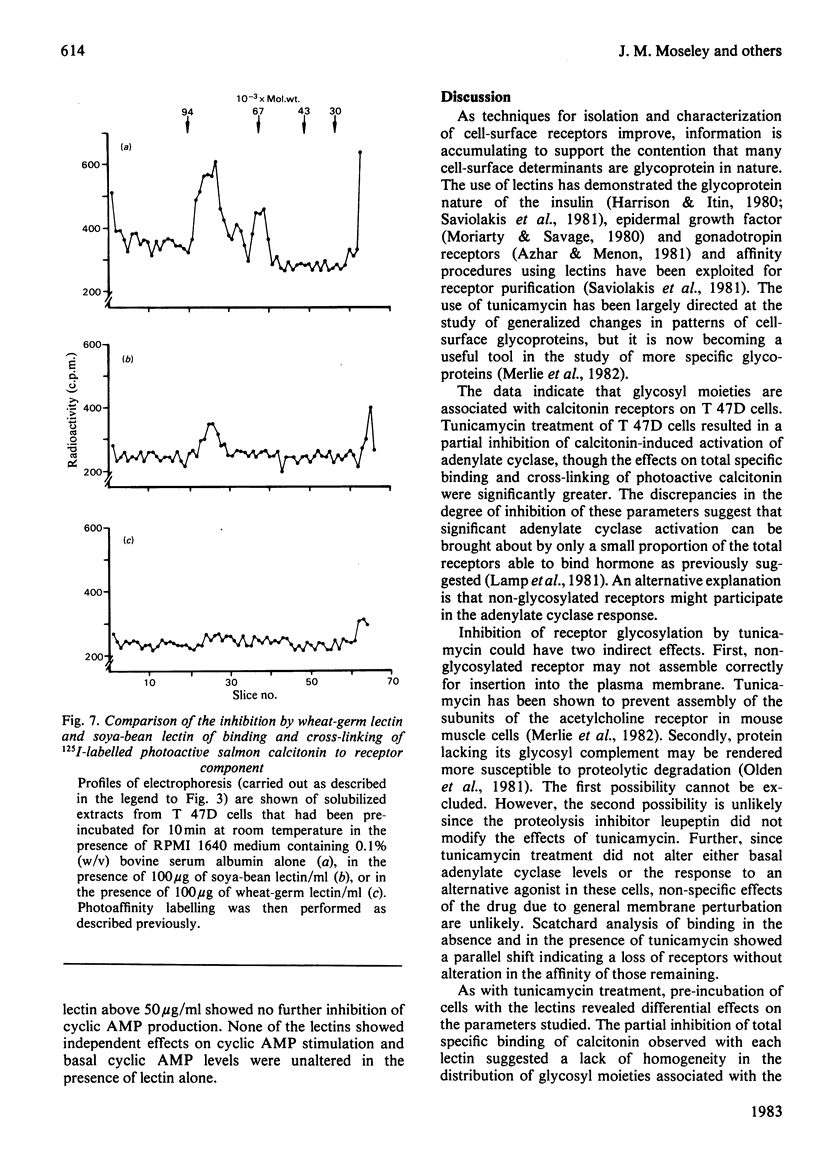
- Beachy J. C., Goldman D., Czech M. P. Lectins activate lymphocyte pyruvate dehydrogenase by a mechanism sensitive to protease inhibitors. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6256–6260. doi: 10.1073/pnas.78.10.6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay D. M., Michelangeli V. P., Eisman J. A., Frampton R. J., Moseley J. M., MacIntyre I., Whitehead R., Martin T. J. Calcitonin and 1,25-dihydroxyvitamin D3 receptors in human breast cancer cell lines. Cancer Res. 1980 Dec;40(12):4764–4767. [PubMed] [Google Scholar]
- Findlay D. M., deLuise M., Michelangeli V. P., Ellison M., Martin T. J. Properties of a calcitonin receptor and adenylate cyclase in BEN cells, a human cancer cell line. Cancer Res. 1980 Apr;40(4):1311–1317. [PubMed] [Google Scholar]
- Gorman J. J., Folk J. E. Transglutaminase amine substrates for photochemical labeling and cleavable cross-linking of proteins. J Biol Chem. 1980 Feb 10;255(3):1175–1180. [PubMed] [Google Scholar]
- Harrison L. C., Itin A. Purification of the insulin receptor from human placenta by chromatography on immobilized wheat germ lectin and receptor antibody. J Biol Chem. 1980 Dec 25;255(24):12066–12072. [PubMed] [Google Scholar]
- Herzberg V., Boughter J. M., Carlisle S., Hill D. E. Evidence for two insulin receptor populations on human erythrocytes. Nature. 1980 Jul 17;286(5770):279–281. doi: 10.1038/286279a0. [DOI] [PubMed] [Google Scholar]
- Housley T. J., Rowland F. N., Ledger P. W., Kaplan J., Tanzer M. L. Effects of tunicamycin on the biosynthesis of procollagen by human fibroblasts. J Biol Chem. 1980 Jan 10;255(1):121–128. [PubMed] [Google Scholar]
- Hunt N. H., Ellison M., Underwood J. C., Martin T. J. Calcitonin-responsive adenylate cyclase in a calcitonin-producing human cancer cell line. Br J Cancer. 1977 Jun;35(6):777–784. doi: 10.1038/bjc.1977.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt N. H., Martin T. J., Michelangeli V. P., Eisman J. A. Effect of guanyl nucleotides on parathyroid hormone-responsive adenylate cyclase in chick kidney. J Endocrinol. 1976 Jun;69(3):401–412. [PubMed] [Google Scholar]
- Klotz I. M. Numbers of receptor sites from Scatchard graphs: facts and fantasies. Science. 1982 Sep 24;217(4566):1247–1249. doi: 10.1126/science.6287580. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Libby P., Goldberg A. L. Leupeptin, a protease inhibitor, decreases protein degradation in normal and diseased muscles. Science. 1978 Feb 3;199(4328):534–536. doi: 10.1126/science.622552. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Sebbane R., Tzartos S., Lindstrom J. Inhibition of glycosylation with tunicamycin blocks assembly of newly synthesized acetylcholine receptor subunits in muscle cells. J Biol Chem. 1982 Mar 10;257(5):2694–2701. [PubMed] [Google Scholar]
- Moseley J. M., Findlay D. M., Martin T. J., Gorman J. J. Covalent cross-linking of a photoactive derivative of calcitonin to human breast cancer cell receptors. J Biol Chem. 1982 May 25;257(10):5846–5851. [PubMed] [Google Scholar]
- Neely A. N., Sitzmann J. V., Kersey J. H. EGTA and proteinase reversal of cellular aggregation of activated lymphocytes. Nature. 1976 Dec 23;264(5588):770–771. doi: 10.1038/264770a0. [DOI] [PubMed] [Google Scholar]
- Olden K., Law J., Hunter V. A., Romain R., Parent J. B. Inhibition of fusion of embryonic muscle cells in culture by tunicamycin is prevented by leupeptin. J Cell Biol. 1981 Jan;88(1):199–204. doi: 10.1083/jcb.88.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Yamada K. M. Role of carbohydrates in protein secretion and turnover: effects of tunicamycin on the major cell surface glycoprotein of chick embryo fibroblasts. Cell. 1978 Mar;13(3):461–473. doi: 10.1016/0092-8674(78)90320-3. [DOI] [PubMed] [Google Scholar]
- Sandra A., Leon M. A., Przybylski R. J. Reversal by insulin of concanavalin A inhibition of myotube formation and evidence for common binding sites. Endocrinology. 1979 Aug;105(2):391–401. doi: 10.1210/endo-105-2-391. [DOI] [PubMed] [Google Scholar]
- Saviolakis G. A., Harrison L. C., Roth J. The binding of 125I-insulin to specific receptors in IM-9 human lymphocytes. Detection of radioactivity covalently linked to receptors. J Biol Chem. 1981 May 25;256(10):4924–4928. [PubMed] [Google Scholar]
- Smith J. D., Liu A. Y. Lectins mimic insulin in the induction of tyrosine aminotransferase. Science. 1981 Nov 13;214(4522):799–800. doi: 10.1126/science.6117128. [DOI] [PubMed] [Google Scholar]


